Abstract
The title chiral imine, C17H15NS, has been obtained via a direct synthesis route. The imine group displays the common E configuration, and is almost coplanar with the thiophene heterocycle; the dihedral angle between the C=N—C group and the thiophene ring is 5.1 (8)°. In contrast, the naphthyl group makes an angle of 83.79 (13)° with the thiophene ring. The observed solid-state molecular conformation is suitable for the use of this molecule as an N,S-bidentate Schiff base ligand. The molecular packing features double C—H⋯π interactions between naphthyl groups of neighboring molecules, which form chains in the [100] direction. The crystal structure is further stabilized by a short C—H⋯π contact involving the methyl group and one ring of a naphthyl group. The resulting two-dimensional network is completed by a weak intermolecular C—H(imine)⋯π(thiophene) interaction.
Related literature
For background to direct synthesis, see: Tanaka & Toda (2000 ▶); Jeon et al. (2005 ▶); Tovar et al. (2007 ▶). For the configuration and conformation of imines derived from thiophene, see: Arjona et al. (1986 ▶).
Experimental
Crystal data
C17H15NS
M r = 265.36
Orthorhombic,

a = 5.5274 (14) Å
b = 7.990 (2) Å
c = 31.517 (8) Å
V = 1392.0 (6) Å3
Z = 4
Mo Kα radiation
μ = 0.22 mm−1
T = 298 K
0.50 × 0.36 × 0.04 mm
Data collection
Siemens P4 diffractometer
Absorption correction: ψ scan (XSCANS; Siemens, 1996 ▶) T min = 0.802, T max = 0.991
4462 measured reflections
2446 independent reflections
1280 reflections with I > 2σ(I)
R int = 0.045
2 standard reflections every 48 reflections intensity decay: 1.8%
Refinement
R[F 2 > 2σ(F 2)] = 0.059
wR(F 2) = 0.174
S = 1.56
2446 reflections
174 parameters
H-atom parameters constrained
Δρmax = 0.31 e Å−3
Δρmin = −0.39 e Å−3
Absolute structure: Flack (1983 ▶), 946 Friedel pairs
Flack parameter: 0.2 (2)
Data collection: XSCANS (Siemens, 1996 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809022375/wn2331sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809022375/wn2331Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C9—H9B⋯CgAi | 0.96 | 2.85 | 3.682 (6) | 145 |
| C6—H6A⋯CgBii | 0.93 | 3.03 | 3.891 (5) | 155 |
| C13—H13A⋯CgCiii | 0.93 | 3.54 | 4.399 (6) | 155 |
| C15—H15A⋯CgAiii | 0.93 | 3.22 | 4.030 (6) | 147 |
Symmetry codes: (i)  ; (ii)
; (ii) 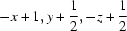 ; (iii)
; (iii)  . CgA is the centroid of ring C10–C14/C19, CgB is the centroid of the thiophene ring and CgC is the centroid of ring C14–C19.
. CgA is the centroid of ring C10–C14/C19, CgB is the centroid of the thiophene ring and CgC is the centroid of ring C14–C19.
Acknowledgments
Partial support from VIEP-UAP (GUPJ-NAT08-G) is acknowledged.
supplementary crystallographic information
Comment
Nowadays, there is an increasing interest in the use of environmentally benign reagents and conditions, leading particularly to solvent-free procedures. Avoiding organic solvents during the reactions in organic synthesis affords clean, efficient and economical features: safety is largely increased, working is considerably simplified, cost is reduced, increased amounts of reactants can be used, etc. (Tanaka & Toda, 2000; Jeon et al., 2005).
On the other hand, imines continue to attract much attention, mainly due to their versatile coordination behavior and the interesting properties of their metal complexes. Continuing our work on the synthesis of chiral imines (Tovar et al., 2007), we synthesized the title compound under solvent-free conditions (see Experimental) and report here its X-ray crystal structure.
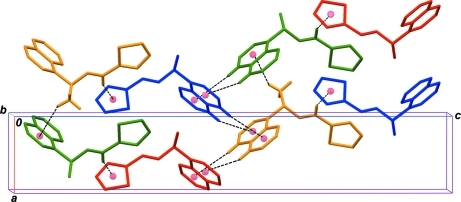
The molecule is stabilized in the solid state as an E-trans aldimine (Fig. 1), which has been shown to be the preferred configuration for imine systems derived from thiophene (Arjona et al., 1986). By conjugation, the imine group C6/N7/C8 is almost coplanar with the thiophene ring S1/C2/C3/C4/C5, with a dihedral angle of 5.1 (8)°. In contrast, the naphthyl group is almost normal to the thiophene ring, at 83.79 (13)°. The crystal packing features a number of intermolecular C—H···π contacts (Fig. 2), the strongest involving the methyl group and a naphthyl group of a symmetry-related molecule. Naphthyl systems aggregate through double C—H···π interactions, forming chains along the [100] direction. The set of contacts results in a two-dimensional framework of efficiently stacked molecules.
Experimental
Under solvent-free conditions, (S)-(-)-(1-naphthyl)ethylamine (213 mg, 1.24 mmol) and 2-thiophenecarboxaldehyde (139 mg, 1.24 mmol) were mixed at 298 K, giving a white solid. The crude product was recrystallized from CH2Cl2, affording colorless crystals of the title compound. Yield 87%; m.p. 345 K. Analytical data are in agreement with the structure determined by X-ray diffraction (see archived CIF).
Refinement
The title molecule crystallizes as thin plates, and the selected crystal was a poorly diffracting sample, limiting data resolution. All H atoms were placed in idealized positions and refined as riding on their carrier C atoms, with bond lengths fixed to 0.93 (aromatic CH), 0.96 (methyl CH3), and 0.98 Å (methine CH). Isotropic displacement parameters were calculated as Uiso(H) = 1.5Ueq(carrier atom) for the methyl group and Uiso(H) = 1.2Ueq(carrier atom) otherwise. The absolute configuration was assigned by refinement of a Flack parameter, and agrees with the chirality expected from the synthetic route.
Figures
Fig. 1.
The title molecule with displacement ellipsoids for non-H atoms shown at the 30% probability level. Hydrogen atoms are shown as spheres of arbitrary radius.
Fig. 2.
A part of the crystal structure of the title compound, viewed down [010]. The color scheme is used for the sake of clarity. Dashed lines represent C—H···π interactions in the crystal structure, and the centroids of involved π systems have been represented by red spheres. H atoms not involved in the network of intermolecular contacts have been omitted.
Crystal data
| C17H15NS | Dx = 1.266 Mg m−3 |
| Mr = 265.36 | Melting point: 345 K |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 68 reflections |
| a = 5.5274 (14) Å | θ = 4.9–11.5° |
| b = 7.990 (2) Å | µ = 0.22 mm−1 |
| c = 31.517 (8) Å | T = 298 K |
| V = 1392.0 (6) Å3 | Plate, colourless |
| Z = 4 | 0.50 × 0.36 × 0.04 mm |
| F(000) = 560 |
Data collection
| Siemens P4 diffractometer | 1280 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.045 |
| graphite | θmax = 25.0°, θmin = 2.6° |
| ω scans | h = −6→6 |
| Absorption correction: ψ scan (XSCANS; Siemens, 1996) | k = −9→9 |
| Tmin = 0.802, Tmax = 0.991 | l = −37→37 |
| 4462 measured reflections | 2 standard reflections every 48 reflections |
| 2446 independent reflections | intensity decay: 1.8% |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.059 | w = 1/[σ2(Fo2) + (0.05P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.174 | (Δ/σ)max < 0.001 |
| S = 1.56 | Δρmax = 0.31 e Å−3 |
| 2446 reflections | Δρmin = −0.39 e Å−3 |
| 174 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.046 (6) |
| 0 constraints | Absolute structure: Flack (1983), 946 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.2 (2) |
| Secondary atom site location: difference Fourier map |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.0890 (3) | 0.77472 (18) | 0.23563 (4) | 0.0851 (5) | |
| C2 | 0.0387 (11) | 0.7930 (7) | 0.28848 (16) | 0.0838 (16) | |
| H2A | −0.0852 | 0.7377 | 0.3027 | 0.101* | |
| C3 | 0.1958 (10) | 0.8951 (7) | 0.30739 (17) | 0.0808 (16) | |
| H3A | 0.1920 | 0.9189 | 0.3363 | 0.097* | |
| C4 | 0.3675 (11) | 0.9631 (6) | 0.27948 (16) | 0.0765 (15) | |
| H4A | 0.4906 | 1.0359 | 0.2875 | 0.092* | |
| C5 | 0.3292 (8) | 0.9077 (6) | 0.23880 (16) | 0.0637 (12) | |
| C6 | 0.4666 (10) | 0.9518 (6) | 0.20103 (16) | 0.0709 (14) | |
| H6A | 0.5865 | 1.0331 | 0.2030 | 0.085* | |
| N7 | 0.4278 (8) | 0.8830 (5) | 0.16565 (12) | 0.0732 (11) | |
| C8 | 0.5830 (11) | 0.9318 (6) | 0.12949 (14) | 0.0743 (14) | |
| H8A | 0.6648 | 1.0374 | 0.1362 | 0.089* | |
| C9 | 0.7706 (10) | 0.7968 (8) | 0.12312 (17) | 0.0982 (19) | |
| H9A | 0.8835 | 0.7989 | 0.1462 | 0.147* | |
| H9B | 0.8550 | 0.8161 | 0.0970 | 0.147* | |
| H9C | 0.6924 | 0.6896 | 0.1221 | 0.147* | |
| C10 | 0.4174 (10) | 0.9587 (6) | 0.09162 (14) | 0.0679 (13) | |
| C11 | 0.3745 (10) | 0.8354 (6) | 0.06322 (15) | 0.0771 (14) | |
| H11A | 0.4564 | 0.7344 | 0.0662 | 0.093* | |
| C12 | 0.2109 (10) | 0.8542 (7) | 0.02938 (16) | 0.0837 (17) | |
| H12A | 0.1858 | 0.7662 | 0.0106 | 0.100* | |
| C13 | 0.0910 (12) | 0.9986 (7) | 0.02414 (17) | 0.0835 (16) | |
| H13A | −0.0180 | 1.0099 | 0.0019 | 0.100* | |
| C14 | 0.1289 (10) | 1.1329 (7) | 0.05212 (16) | 0.0757 (14) | |
| C15 | 0.0159 (12) | 1.2883 (8) | 0.04640 (19) | 0.101 (2) | |
| H15A | −0.0913 | 1.3014 | 0.0239 | 0.121* | |
| C16 | 0.0570 (16) | 1.4195 (9) | 0.0723 (2) | 0.110 (2) | |
| H16A | −0.0224 | 1.5205 | 0.0677 | 0.132* | |
| C17 | 0.2162 (13) | 1.4043 (8) | 0.1058 (2) | 0.0980 (19) | |
| H17A | 0.2446 | 1.4950 | 0.1236 | 0.118* | |
| C18 | 0.3321 (11) | 1.2564 (7) | 0.11269 (16) | 0.0850 (16) | |
| H18A | 0.4378 | 1.2477 | 0.1355 | 0.102* | |
| C19 | 0.2965 (10) | 1.1158 (6) | 0.08620 (14) | 0.0702 (14) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0830 (9) | 0.0941 (10) | 0.0780 (9) | −0.0071 (9) | −0.0027 (8) | −0.0051 (8) |
| C2 | 0.092 (4) | 0.093 (4) | 0.066 (3) | 0.000 (4) | 0.010 (3) | 0.006 (3) |
| C3 | 0.093 (4) | 0.083 (4) | 0.067 (3) | 0.008 (4) | −0.008 (3) | −0.003 (3) |
| C4 | 0.085 (4) | 0.074 (3) | 0.071 (3) | −0.009 (3) | −0.001 (3) | −0.005 (3) |
| C5 | 0.058 (3) | 0.063 (3) | 0.070 (3) | −0.006 (2) | −0.003 (2) | −0.002 (3) |
| C6 | 0.069 (4) | 0.071 (3) | 0.073 (3) | −0.002 (3) | −0.005 (3) | 0.000 (3) |
| N7 | 0.075 (3) | 0.082 (3) | 0.062 (2) | 0.001 (3) | 0.003 (2) | 0.001 (2) |
| C8 | 0.078 (3) | 0.079 (3) | 0.066 (3) | 0.000 (3) | 0.003 (3) | 0.008 (3) |
| C9 | 0.086 (4) | 0.119 (5) | 0.090 (4) | 0.029 (4) | 0.004 (3) | 0.007 (4) |
| C10 | 0.073 (3) | 0.069 (3) | 0.062 (3) | 0.002 (3) | 0.001 (3) | 0.003 (3) |
| C11 | 0.089 (4) | 0.073 (3) | 0.070 (3) | 0.004 (3) | 0.001 (3) | −0.001 (3) |
| C12 | 0.091 (4) | 0.085 (4) | 0.076 (4) | −0.002 (4) | −0.003 (3) | −0.010 (3) |
| C13 | 0.088 (4) | 0.093 (4) | 0.070 (3) | −0.002 (4) | −0.007 (3) | 0.003 (3) |
| C14 | 0.077 (4) | 0.078 (3) | 0.072 (3) | 0.006 (3) | 0.002 (3) | 0.008 (3) |
| C15 | 0.117 (5) | 0.096 (4) | 0.090 (4) | 0.026 (4) | −0.006 (4) | 0.018 (4) |
| C16 | 0.134 (6) | 0.083 (4) | 0.112 (5) | 0.024 (5) | 0.014 (5) | 0.013 (4) |
| C17 | 0.117 (5) | 0.078 (4) | 0.099 (4) | 0.007 (4) | 0.017 (4) | −0.005 (4) |
| C18 | 0.097 (4) | 0.077 (4) | 0.081 (3) | −0.006 (4) | 0.009 (3) | −0.007 (3) |
| C19 | 0.077 (3) | 0.072 (3) | 0.062 (3) | −0.001 (3) | 0.006 (3) | 0.003 (3) |
Geometric parameters (Å, °)
| S1—C2 | 1.695 (5) | C10—C11 | 1.352 (6) |
| S1—C5 | 1.703 (5) | C10—C19 | 1.432 (6) |
| C2—C3 | 1.332 (7) | C11—C12 | 1.407 (7) |
| C2—H2A | 0.9300 | C11—H11A | 0.9300 |
| C3—C4 | 1.404 (7) | C12—C13 | 1.341 (7) |
| C3—H3A | 0.9300 | C12—H12A | 0.9300 |
| C4—C5 | 1.373 (6) | C13—C14 | 1.405 (7) |
| C4—H4A | 0.9300 | C13—H13A | 0.9300 |
| C5—C6 | 1.455 (6) | C14—C15 | 1.401 (7) |
| C6—N7 | 1.261 (5) | C14—C19 | 1.425 (7) |
| C6—H6A | 0.9300 | C15—C16 | 1.349 (7) |
| N7—C8 | 1.479 (6) | C15—H15A | 0.9300 |
| C8—C9 | 1.509 (7) | C16—C17 | 1.378 (9) |
| C8—C10 | 1.519 (7) | C16—H16A | 0.9300 |
| C8—H8A | 0.9800 | C17—C18 | 1.361 (8) |
| C9—H9A | 0.9600 | C17—H17A | 0.9300 |
| C9—H9B | 0.9600 | C18—C19 | 1.414 (6) |
| C9—H9C | 0.9600 | C18—H18A | 0.9300 |
| C2—S1—C5 | 91.0 (3) | C11—C10—C8 | 121.5 (5) |
| C3—C2—S1 | 112.7 (5) | C19—C10—C8 | 119.9 (4) |
| C3—C2—H2A | 123.7 | C10—C11—C12 | 122.5 (5) |
| S1—C2—H2A | 123.7 | C10—C11—H11A | 118.8 |
| C2—C3—C4 | 113.4 (5) | C12—C11—H11A | 118.8 |
| C2—C3—H3A | 123.3 | C13—C12—C11 | 120.2 (5) |
| C4—C3—H3A | 123.3 | C13—C12—H12A | 119.9 |
| C5—C4—C3 | 110.8 (5) | C11—C12—H12A | 119.9 |
| C5—C4—H4A | 124.6 | C12—C13—C14 | 120.4 (6) |
| C3—C4—H4A | 124.6 | C12—C13—H13A | 119.8 |
| C4—C5—C6 | 127.2 (4) | C14—C13—H13A | 119.8 |
| C4—C5—S1 | 112.1 (4) | C15—C14—C13 | 122.0 (5) |
| C6—C5—S1 | 120.6 (4) | C15—C14—C19 | 118.1 (5) |
| N7—C6—C5 | 121.9 (5) | C13—C14—C19 | 119.8 (5) |
| N7—C6—H6A | 119.0 | C16—C15—C14 | 122.4 (6) |
| C5—C6—H6A | 119.0 | C16—C15—H15A | 118.8 |
| C6—N7—C8 | 117.9 (4) | C14—C15—H15A | 118.8 |
| N7—C8—C9 | 108.2 (4) | C15—C16—C17 | 120.1 (6) |
| N7—C8—C10 | 107.0 (4) | C15—C16—H16A | 119.9 |
| C9—C8—C10 | 114.2 (4) | C17—C16—H16A | 119.9 |
| N7—C8—H8A | 109.1 | C18—C17—C16 | 119.9 (6) |
| C9—C8—H8A | 109.1 | C18—C17—H17A | 120.0 |
| C10—C8—H8A | 109.1 | C16—C17—H17A | 120.0 |
| C8—C9—H9A | 109.5 | C17—C18—C19 | 122.0 (6) |
| C8—C9—H9B | 109.5 | C17—C18—H18A | 119.0 |
| H9A—C9—H9B | 109.5 | C19—C18—H18A | 119.0 |
| C8—C9—H9C | 109.5 | C18—C19—C14 | 117.3 (5) |
| H9A—C9—H9C | 109.5 | C18—C19—C10 | 124.1 (5) |
| H9B—C9—H9C | 109.5 | C14—C19—C10 | 118.5 (5) |
| C11—C10—C19 | 118.5 (5) | ||
| C5—S1—C2—C3 | 0.2 (4) | C11—C12—C13—C14 | 0.6 (9) |
| S1—C2—C3—C4 | −0.4 (6) | C12—C13—C14—C15 | 176.8 (5) |
| C2—C3—C4—C5 | 0.5 (7) | C12—C13—C14—C19 | 0.2 (9) |
| C3—C4—C5—C6 | 179.1 (5) | C13—C14—C15—C16 | −177.9 (6) |
| C3—C4—C5—S1 | −0.4 (6) | C19—C14—C15—C16 | −1.3 (9) |
| C2—S1—C5—C4 | 0.1 (4) | C14—C15—C16—C17 | 0.5 (11) |
| C2—S1—C5—C6 | −179.4 (4) | C15—C16—C17—C18 | −0.1 (10) |
| C4—C5—C6—N7 | 174.2 (5) | C16—C17—C18—C19 | 0.6 (9) |
| S1—C5—C6—N7 | −6.4 (6) | C17—C18—C19—C14 | −1.4 (8) |
| C5—C6—N7—C8 | −177.8 (4) | C17—C18—C19—C10 | 178.7 (5) |
| C6—N7—C8—C9 | 101.4 (5) | C15—C14—C19—C18 | 1.7 (7) |
| C6—N7—C8—C10 | −135.0 (5) | C13—C14—C19—C18 | 178.4 (5) |
| N7—C8—C10—C11 | −94.5 (6) | C15—C14—C19—C10 | −178.5 (5) |
| C9—C8—C10—C11 | 25.3 (7) | C13—C14—C19—C10 | −1.7 (7) |
| N7—C8—C10—C19 | 83.5 (6) | C11—C10—C19—C18 | −177.6 (5) |
| C9—C8—C10—C19 | −156.7 (5) | C8—C10—C19—C18 | 4.3 (8) |
| C19—C10—C11—C12 | −1.9 (8) | C11—C10—C19—C14 | 2.5 (7) |
| C8—C10—C11—C12 | 176.1 (5) | C8—C10—C19—C14 | −175.5 (5) |
| C10—C11—C12—C13 | 0.3 (8) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9B···CgAi | 0.96 | 2.85 | 3.682 (6) | 145 |
| C6—H6A···CgBii | 0.93 | 3.03 | 3.891 (5) | 155 |
| C13—H13A···CgCiii | 0.93 | 3.54 | 4.399 (6) | 155 |
| C15—H15A···CgAiii | 0.93 | 3.22 | 4.030 (6) | 147 |
Symmetry codes: (i) x+1, y, z; (ii) −x+1, y+1/2, −z+1/2; (iii) x−1/2, −y+5/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2331).
References
- Arjona, O., Carreiro, C., Perez Ossorio, R., Plumet, J., Cativiela, C., Mayoral, J. A. & Melendez, E. (1986). An. Quim.82, 115–118.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Jeon, S.-J., Li, H. & Walsh, P. J. (2005). J. Am. Chem. Soc.127, 16416–16425. [DOI] [PubMed]
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). XSCANS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Tanaka, K. & Toda, F. (2000). Chem. Rev.100, 1025–1074. [DOI] [PubMed]
- Tovar, A., Peña, U., Hernández, G., Portillo, R. & Gutiérrez, R. (2007). Synthesis, pp. 22–24.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809022375/wn2331sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809022375/wn2331Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report