Abstract
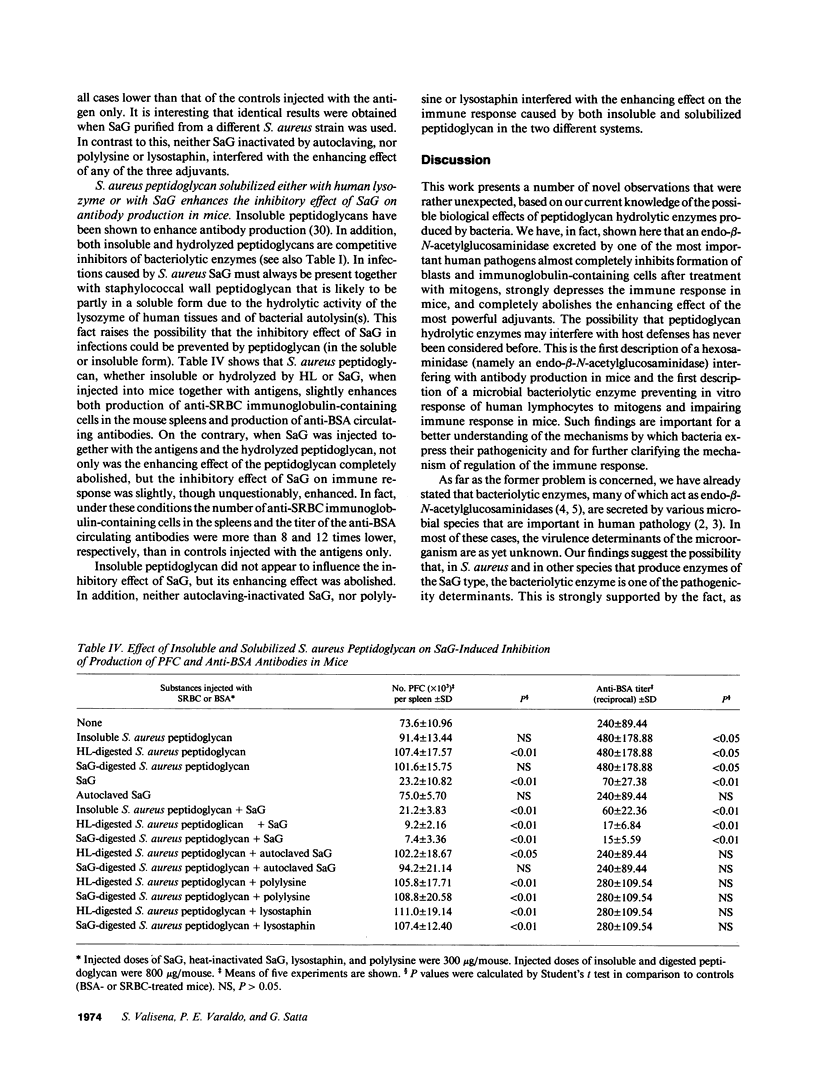
The effect of a bacteriolytic enzyme, the endo-beta-N-acetylglucosaminidase excreted by Staphylococcus aureus (SaG) on the response of human lymphocytes to mitogens and on the immune response in mice has been studied. SaG inhibited incorporation of [3H]thymidine into TCA-precipitable material by human peripheral lymphocytes stimulated either by phytohemagglutinin or by concanavalin A, as well as formation of cytoplasmic immunoglobulin-containing cells by B lymphocytes treated with pokeweed mitogen. In all cases the level of inhibition first increased with the SaG concentrations reaching values of over 80% at an enzyme concentration of 100 micrograms/ml, and then decreased. Heat-inactivated SaG as well as SaG treated with both polyclonal and monoclonal specific antibodies or enzyme inhibitors such as chitotriose or hydrolyzed peptidoglycan had no effect on lymphocyte response to mitogens. In mice, SaG at a dose of 300 micrograms per mouse was found to cause a fourfold decrease in the anti-BSA antibody titer and an approximately 70-75% reduction in the immunoglobulin-containing cells in the spleens of mice injected with sheep red blood cells. SaG also completely abolished the enhancing effect of adjuvants such as muramyldipeptide, Freund's complete adjuvant, and Escherichia coli lipopolysaccharide. When SaG was injected into mice together with S. aureus peptidoglycan hydrolyzed either by SaG or by human lysozyme, the inhibitory effect on both production of anti-BSA circulating antibodies and appearance of Igc cells in the spleens of mice injected with sheep red blood cells was enhanced. As we know that (a) human tissues contain endo-beta-N-acetylglucosaminidases; (b) other human hexosaminidases (lysozymes) have previously been shown to interfere with the functions of immunocompetent cells; and (c) products of hexosaminidase hydrolysis of peptidoglycan (muropeptides) known to modulate immune response are ordinarily found in the urine of healthy persons, the possibility that hexosaminidases play a major role in the regulation of the immune response is raised and discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- Berry A. M., Lock R. A., Hansman D., Paton J. C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989 Aug;57(8):2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C. Methods of quantitative microbiological analyses that support the diagnosis, treatment, and prognosis of human infection. Crit Rev Microbiol. 1981;8(4):339–397. doi: 10.3109/10408418109085083. [DOI] [PubMed] [Google Scholar]
- Gordon L. I., Douglas S. D., Kay N. E., Yamada O., Osserman E. F., Jacob H. S. Modulation of neutrophil function by lysozyme. Potential negative feedback system of inflammation. J Clin Invest. 1979 Jul;64(1):226–232. doi: 10.1172/JCI109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A. A., Brandriss M. W. Passive hemagglutination procedures for protein and polysaccharide antigens using erythrocytes stabilized by aldehydes. J Immunol. 1968 Mar;100(3):641–646. [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982 Feb 25;257(4):1664–1669. [PubMed] [Google Scholar]
- Lachica R. V., Deibel R. H. Detection of nuclease activity in semisolid and broth cultures. Appl Microbiol. 1969 Aug;18(2):174–176. doi: 10.1128/am.18.2.174-176.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahuran D. J., Tsui F., Gravel R. A., Lowden J. A. Evidence for two dissimilar polypeptide chains in the beta 2 subunit of hexosaminidase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1602–1605. doi: 10.1073/pnas.79.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Moretta A., Cooper M. D. Human T lymphocyte subpopulations: studies of the mechanism by which T cells bearing Fc receptors for IgG suppress T-dependent B cell differentiation induced by pokeweed mitogen. J Immunol. 1979 Mar;122(3):984–990. [PubMed] [Google Scholar]
- Musson R. A., Shafran H., Henson P. M. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothel Soc. 1980 Sep;28(3):249–264. [PubMed] [Google Scholar]
- Nishigaki M., Muramatsu T., Kobata A. Endoglycosidases acting on carbohydrate moieties of glycoproteins: demonstration in mammalian tissue. Biochem Biophys Res Commun. 1974 Jul 24;59(2):638–645. doi: 10.1016/s0006-291x(74)80027-6. [DOI] [PubMed] [Google Scholar]
- Noda M., Kato I., Hirayama T., Matsuda F. Fixation and inactivation of staphylococcal leukocidin by phosphatidylcholine and ganglioside GM1 in rabbit polymorphonuclear leukocytes. Infect Immun. 1980 Aug;29(2):678–684. doi: 10.1128/iai.29.2.678-684.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdijk B., van der Kroef W. M., Lisman J. J., Pierce R. J., Montreuil J., Spik G. Demonstration and partial characterization of endo-N-acetyl-beta-D-glucosaminidase in human tissues. FEBS Lett. 1981 Jun 15;128(2):364–366. doi: 10.1016/0014-5793(81)80118-4. [DOI] [PubMed] [Google Scholar]
- Owens J. J. The egg yolk reaction produced by several species of bacteria. J Appl Bacteriol. 1974 Mar;37(1):137–148. doi: 10.1111/j.1365-2672.1974.tb00424.x. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. The rate of formation of hyaluronidase, coagulase and total extracellular protein by strains of Staphylococcus aureus. J Gen Microbiol. 1954 Apr;10(2):209–220. doi: 10.1099/00221287-10-2-209. [DOI] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Cerilli J. G., Jacob H. S., Osserman E. F. Lysozyme stimulates lymphocyte proliferation in monocyte-depleted mixed lymphocyte cultures. J Lab Clin Med. 1982 Mar;99(3):370–381. [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. PURIFICATION AND PROPERTIES OF LYSOSTAPHIN--A LYTIC AGENT FOR STAPHYLOCOCCUS AUREUS. Biochim Biophys Acta. 1965 Feb 15;97:242–250. doi: 10.1016/0304-4165(65)90088-7. [DOI] [PubMed] [Google Scholar]
- Satta G., Azzarone B., Varaldo P. E., Fontana R., Valisena S. Stimulation of spreading of trypsinized human fibroblasts by lysozymes from Staphylococcus aureus, hen egg white, and human urine. In Vitro. 1980 Sep;16(9):738–750. doi: 10.1007/BF02619307. [DOI] [PubMed] [Google Scholar]
- Satta G., Grazi G., Varaldo P. E., Fontana R. Detection of bacterial phosphatase activity by means of an original and simple test. J Clin Pathol. 1979 Apr;32(4):391–395. doi: 10.1136/jcp.32.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Varaldo P. E., Azzarone B., Romanzi C. A. Effects of Staphylococcus aureus lysozyme on human fibroblasts. Cell Biol Int Rep. 1979 Sep;3(6):525–533. doi: 10.1016/0309-1651(79)90088-2. [DOI] [PubMed] [Google Scholar]
- Satta G., Varaldo P. E., Grazi G., Fontana R. Bacteriolytic activity in staphylococci. Infect Immun. 1977 Apr;16(1):37–42. doi: 10.1128/iai.16.1.37-42.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker R., Kline M. E., Haak R. A., Rest R. F., Rosenthal R. S. Degradation of gonococcal peptidoglycan by granule extract from human neutrophils: demonstration of N-acetylglucosaminidase activity that utilizes peptidoglycan substrates. Infect Immun. 1987 Nov;55(11):2579–2584. doi: 10.1128/iai.55.11.2579-2584.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from hen oviduct. J Biol Chem. 1976 Nov 10;251(21):6537–6543. [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Valisena S., Varaldo P. E., Satta G. Biochemical and physical properties of the endo-beta-N-acetylglucosaminidases from Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus saprophyticus. Microbiologica. 1983 Oct;6(4):277–291. [PubMed] [Google Scholar]
- Valisena S., Varaldo P. E., Satta G. Purification and characterization of three separate bacteriolytic enzymes excreted by Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus saprophyticus. J Bacteriol. 1982 Aug;151(2):636–647. doi: 10.1128/jb.151.2.636-647.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaldo P. E., Valisena S., Mingari M. C., Satta G. Lysozyme-induced inhibition of the lymphocyte response to mitogenic lectins. Proc Soc Exp Biol Med. 1989 Jan;190(1):54–62. doi: 10.3181/00379727-190-42829. [DOI] [PubMed] [Google Scholar]