Abstract
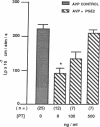
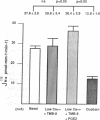
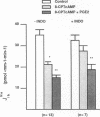
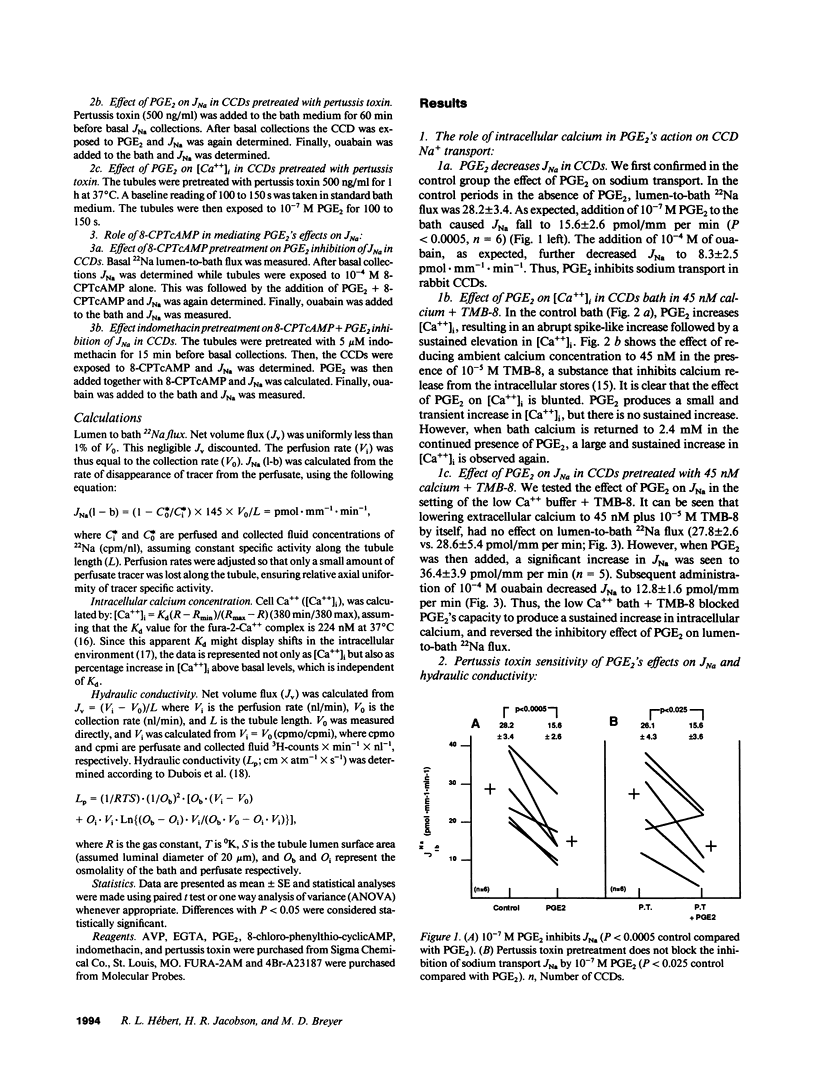
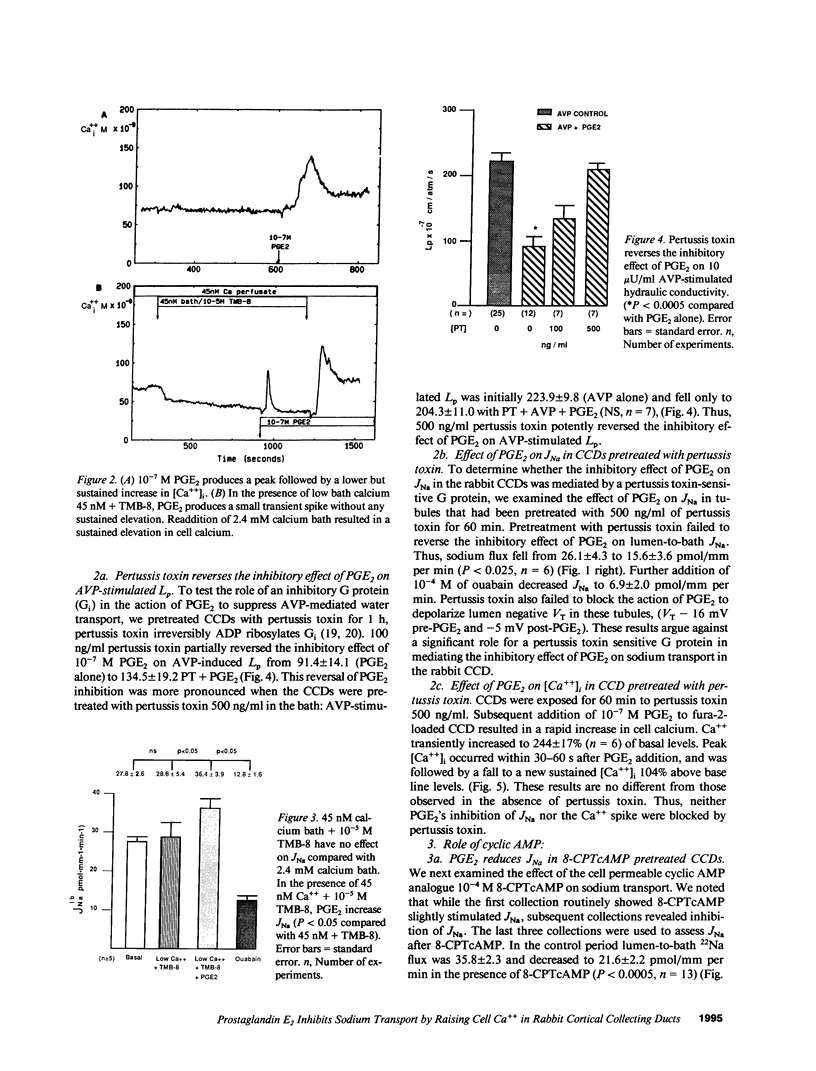
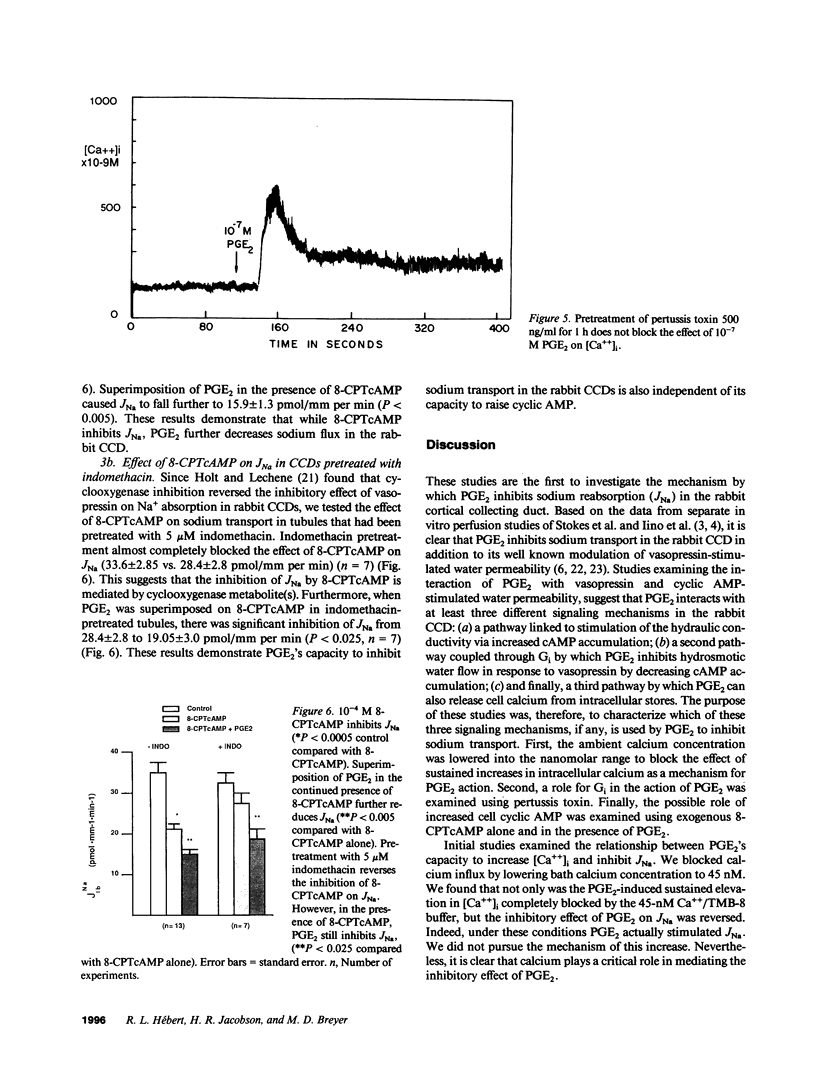
The mechanism by which prostaglandin E2 (PGE2) inhibits sodium absorption (JNa) in the rabbit cortical collecting duct (CCD) was explored. PGE2 activates at least three signaling mechanisms in the CCD: (a) by itself PGE2 increases cAMP generation (b) PGE2 also inhibits vasopressin-stimulated cAMP accumulation, and (c) PGE2 raises intracellular calcium([Ca++]i). We tested the contribution of these signaling pathways to PGE2's effect on Na+ absorption, measuring 22Na flux (JNa) and [Ca++]i (using fura-2) in microperfused rabbit CCDs. In control studies PGE2 reduced JNa from 28.2 +/- 3.4 to 15.6 +/- 2.6 pmol.mm-1.min-1. Lowering bath calcium from 2.4 to 45 nM did not by itself alter JNa but in this setting PGE2 failed to inhibit JNa (28.6 +/- 5.4 to 38.5 +/- 4.0). In separate tubules, PGE2 raised [Ca++]i in a spike-like fashion followed by a sustained elevation. However, in 45 nM bath Ca++, PGE2 failed to produce a sustained [Ca++]i elevation. While pretreatment of CCDs with pertussis toxin blocked PGE2 inhibition of vasopressin-stimulated water permeability, it did not block the effect of PGE2 on JNa. To see if cAMP generation contributes to the effect of PGE2 on JNa, we tested the effect of exogenous cAMP, (8-chlorophenylthio(CPT)cAMP) on JNa. 0.1 mM 8-CPTcAMP reduced JNa from 35.75 +/- 2.3 to 21.6 +/- 2.2. However, the addition of PGE2 further blunted JNa to 15.9 +/- 1.3. In CCDs pretreated with indomethacin, 8-CPTcAMP did not significantly decrease JNa 33.6 +/- 2.8 vs. 28.4 +/- 2. However, superimposed PGE2 reduced JNa to 19.0 +/- 3.0. We conclude that PGE2 inhibits sodium transport predominantly by increasing intracellular calcium. This action is not mediated by a pertussis toxin-sensitive G protein. Finally, cAMP, through a cyclooxygenase-dependent mechanism, also inhibits CCD JNa and may contribute to the effects of PGE2 on JNa in the rabbit CCD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando Y., Jacobson H. R., Breyer M. D. Phorbol myristate acetate, dioctanoylglycerol, and phosphatidic acid inhibit the hydroosmotic effect of vasopressin on rabbit cortical collecting tubule. J Clin Invest. 1987 Aug;80(2):590–593. doi: 10.1172/JCI113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer M. D., Kokko J. P., Jacobson H. R. Regulation of net bicarbonate transport in rabbit cortical collecting tubule by peritubular pH, carbon dioxide tension, and bicarbonate concentration. J Clin Invest. 1986 May;77(5):1650–1660. doi: 10.1172/JCI112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H. S., Jr, Al-Awqati Q. Regulation of the sodium permeability of the luminal border of toad bladder by intracellular sodium and calcium: role of sodium-calcium exchange in the basolateral membrane. J Gen Physiol. 1981 Jun;77(6):693–712. doi: 10.1085/jgp.77.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Ca2+.Calmodulin-dependent release of arachidonic acid for renal medullary prostaglandin synthesis. Evidence for involvement of phospholipases A2 and C. J Biol Chem. 1983 Apr 25;258(8):4814–4823. [PubMed] [Google Scholar]
- Du Bois R., Vernoiry A., Abramow M. Computation of the osmotic water permeability of perfused tubule segments. Kidney Int. 1976 Dec;10(6):478–479. doi: 10.1038/ki.1976.135. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E. Ca2(+)-dependent inhibition of sodium transport in rabbit cortical collecting tubules. Am J Physiol. 1990 Mar;258(3 Pt 2):F568–F582. doi: 10.1152/ajprenal.1990.258.3.F568. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. I. Depression of sodium transport. Am J Physiol. 1981 Oct;241(4):F452–F460. doi: 10.1152/ajprenal.1981.241.4.F452. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Jacobson H. R., Breyer M. D. PGE2 inhibits AVP-induced water flow in cortical collecting ducts by protein kinase C activation. Am J Physiol. 1990 Aug;259(2 Pt 2):F318–F325. doi: 10.1152/ajprenal.1990.259.2.F318. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Lamoureux C., Sirois P., Braquet P., Plante G. E. Interaction between prostaglandin E2 and leukotriene D4 on the excretion of electrolytes by the dog kidney in vivo. Prostaglandins. 1987 Feb;33(2):301–313. doi: 10.1016/0090-6980(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Lamoureux C., Sirois P., Braquet P., Plante G. E. Potentiating effects of leukotriene B4 and prostaglandin E2 on urinary sodium excretion by the dog kidney. Prostaglandins Leukot Med. 1985 Apr;18(1):69–80. doi: 10.1016/0262-1746(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Iino Y., Imai M. Effects of prostaglandins on Na transport in isolated collecting tubules. Pflugers Arch. 1978 Feb 22;373(2):125–132. doi: 10.1007/BF00584850. [DOI] [PubMed] [Google Scholar]
- Kimmel P. L., Goldfarb S. Effects of isoproterenol on potassium secretion by the cortical collecting tubule. Am J Physiol. 1984 Jun;246(6 Pt 2):F804–F810. doi: 10.1152/ajprenal.1984.246.6.F804. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Eaton D. C. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol. 1989 Jun;256(6 Pt 2):F1094–F1103. doi: 10.1152/ajprenal.1989.256.6.F1094. [DOI] [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca++ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in skeletal muscles. Pharmacology. 1974;12(1):20–31. doi: 10.1159/000136517. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Schneider J. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. II. Changes in Na+ and Ca2+ fluxes, Na+/K+ pump activity, and intracellular pH. J Cell Biol. 1986 Jun;102(6):2223–2233. doi: 10.1083/jcb.102.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Padrell E., Carty D. J., Omri G., Landau E. M., Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990 Jan 4;343(6253):79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Nadler S. P., Hebert S. C., Brenner B. M. PGE2, forskolin, and cholera toxin interactions in rabbit cortical collecting tubule. Am J Physiol. 1986 Jan;250(1 Pt 2):F127–F135. doi: 10.1152/ajprenal.1986.250.1.F127. [DOI] [PubMed] [Google Scholar]
- Reif M. C., Troutman S. L., Schafer J. A. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest. 1986 Apr;77(4):1291–1298. doi: 10.1172/JCI112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Ribeiro C. P., Ribeiro-Neto F., Field J. B., Suki W. N. Prevention of alpha 2-adrenergic inhibition on ADH action by pertussis toxin in rabbit CCT. Am J Physiol. 1987 Jul;253(1 Pt 1):C105–C112. doi: 10.1152/ajpcell.1987.253.1.C105. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Schuster V. L. Mechanism of bradykinin, ADH, and cAMP interaction in rabbit cortical collecting duct. Am J Physiol. 1985 Nov;249(5 Pt 2):F645–F653. doi: 10.1152/ajprenal.1985.249.5.F645. [DOI] [PubMed] [Google Scholar]
- Sonnenburg W. K., Smith W. L. Regulation of cyclic AMP metabolism in rabbit cortical collecting tubule cells by prostaglandins. J Biol Chem. 1988 May 5;263(13):6155–6160. [PubMed] [Google Scholar]
- Stokes J. B., Kokko J. P. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977 Jun;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Bollag W. E., Rasmussen H. The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem. 1987 Jan 5;262(1):182–188. [PubMed] [Google Scholar]
- Taylor A., Eich E., Pearl M., Brem A. S., Peeper E. Q. Cytosolic calcium and the action of vasopressin in toad urinary bladder. Am J Physiol. 1987 Jun;252(6 Pt 2):F1028–F1041. doi: 10.1152/ajprenal.1987.252.6.F1028. [DOI] [PubMed] [Google Scholar]
- Taylor A., Windhager E. E. Possible role of cytosolic calcium and Na-Ca exchange in regulation of transepithelial sodium transport. Am J Physiol. 1979 Jun;236(6):F505–F512. doi: 10.1152/ajprenal.1979.236.6.F505. [DOI] [PubMed] [Google Scholar]
- Tomita K., Pisano J. J., Knepper M. A. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest. 1985 Jul;76(1):132–136. doi: 10.1172/JCI111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Umegaki K., Smith W. L. Association of a solubilized prostaglandin E2 receptor from renal medulla with a pertussis toxin-reactive guanine nucleotide regulatory protein. J Biol Chem. 1986 Oct 15;261(29):13430–13437. [PubMed] [Google Scholar]
- Yamaguchi D. T., Hahn T. J., Beeker T. G., Kleeman C. R., Muallem S. Relationship of cAMP and calcium messenger systems in prostaglandin-stimulated UMR-106 cells. J Biol Chem. 1988 Aug 5;263(22):10745–10753. [PubMed] [Google Scholar]
- Yokohama H., Negishi M., Sugama K., Hayashi H., Ito S., Hayaishi O. Inhibition of prostaglandin E2-induced phosphoinositide metabolism by phorbol ester in bovine adrenal chromaffin cells. Biochem J. 1988 Nov 1;255(3):957–962. doi: 10.1042/bj2550957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokohama H., Tanaka T., Ito S., Negishi M., Hayashi H., Hayaishi O. Prostaglandin E receptor enhancement of catecholamine release may be mediated by phosphoinositide metabolism in bovine adrenal chromaffin cells. J Biol Chem. 1988 Jan 25;263(3):1119–1122. [PubMed] [Google Scholar]