Abstract
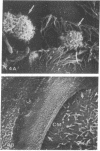
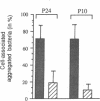
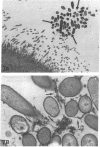
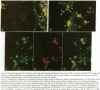
Human nasal polyps in outgrowth culture were used to study the Pseudomonas aeruginosa adhesion to respiratory cells. By scanning electron microscopy, P. aeruginosa were seen associated with ciliated cells, but by transmission electron microscopy, bacteria were never seen at the interciliary spaces or attached along cilia, but were identified trapped at the extremities of cilia, usually as bacterial aggregates. A fibronectin-containing fibrillar material was seen associated with aggregated bacteria. By time-lapse video microscopy, bacteria were seen to aggregate in the culture medium following their addition to the culture wells. Progressively, these aggregates were trapped by cilia or attached to migrating cells of a lower cell layer that protruded beneath the upper layer cells, at the outgrowth periphery. P. aeruginosa adhesion to these lower cell layer migrating cells was significantly higher than to ciliated or nonciliated cells of the upper cell layer. Migrating cells were intensely labeled by the complexes Con A and arachis hypogea agglutinin (PNA)-FITC, in contrast to the other cells. The percentage of PNA-labeled cells with attached bacteria was significantly higher than that without bacteria. These results suggest that changes of cell surface glycoconjugates related with cell migration may favor P. aeruginosa adhesion to respiratory cells.
Full text
PDF


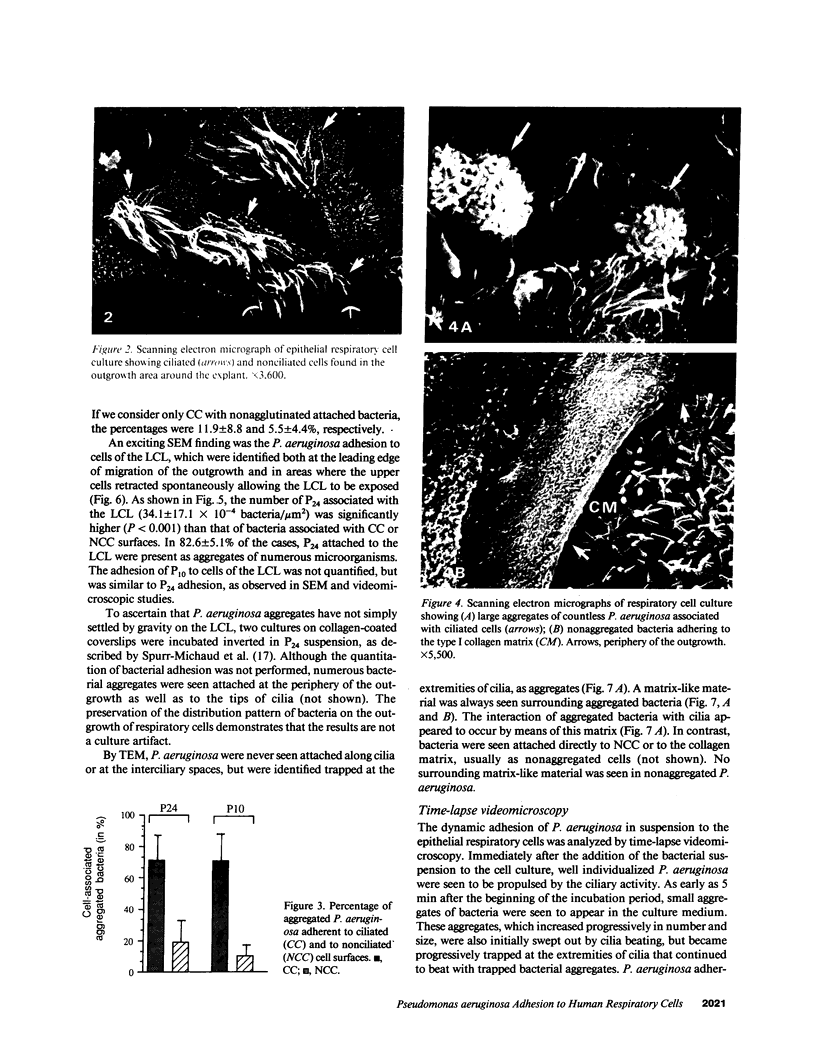
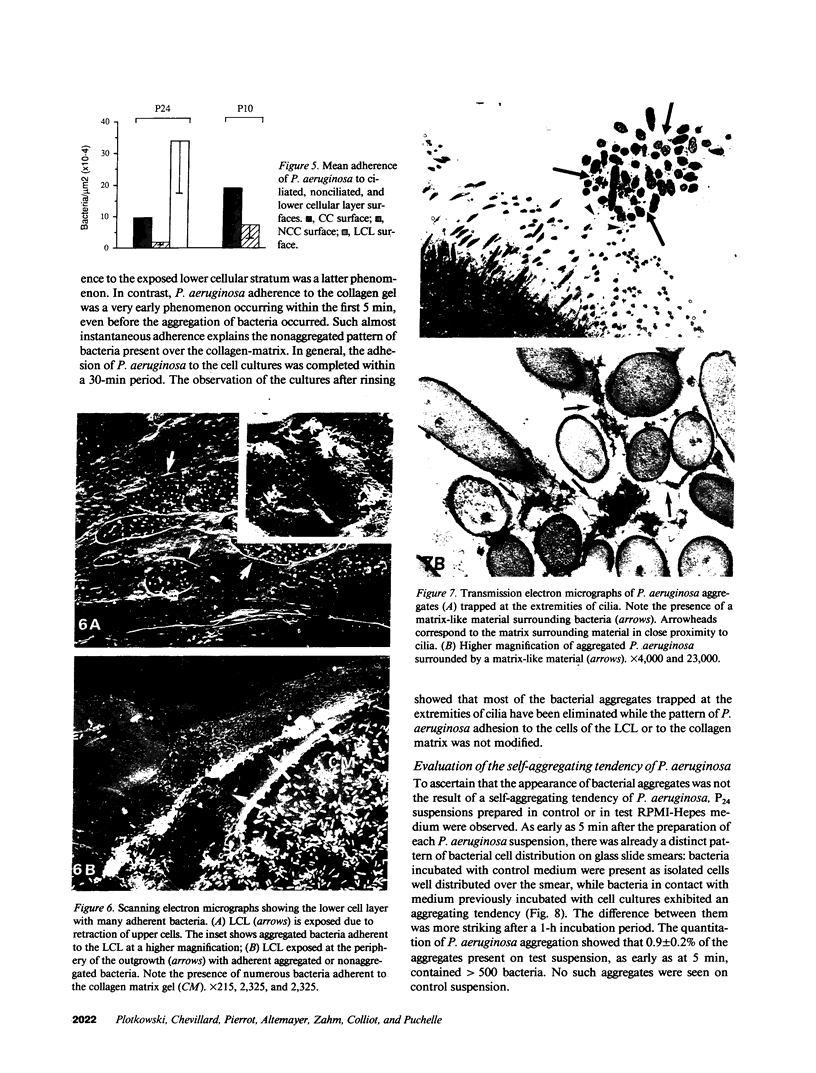


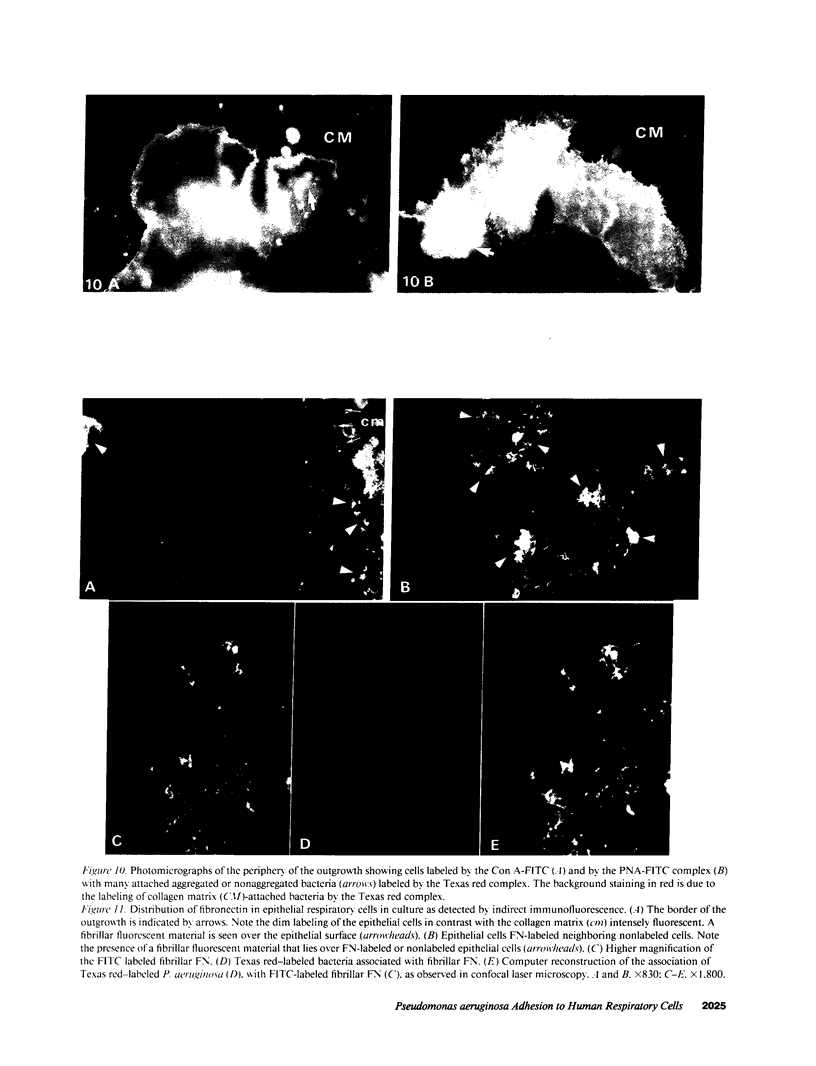



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Svanborg-Edén C. Role of alginate in the adherence of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1989;42:72–79. doi: 10.1159/000417607. [DOI] [PubMed] [Google Scholar]
- Ball R. Y., Stoddart R. W., Jones C. J., Mitchinson M. J. Saccharide expression on wounded endothelial cell monolayers in vitro. J Cell Sci. 1989 May;93(Pt 1):163–172. doi: 10.1242/jcs.93.1.163. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Christie C. D., Smith G. J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989 Dec;140(6):1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Kaufman B., Khorrami A., Enriquez J. I., Manna B. Fibronectin: source of mannose in a highly purified respiratory mucin. Inflammation. 1988 Oct;12(5):433–446. doi: 10.1007/BF00919437. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Franklin A. L., Todd T., Gurman G., Black D., Mankinen-Irvin P. M., Irvin R. T. Adherence of Pseudomonas aeruginosa to cilia of human tracheal epithelial cells. Infect Immun. 1987 Jun;55(6):1523–1525. doi: 10.1128/iai.55.6.1523-1525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D. A., Lallement A. V., Petit A. F., Puchelle E. S. In vivo ciliogenesis in human fetal tracheal epithelium. Am J Anat. 1989 Aug;185(4):415–428. doi: 10.1002/aja.1001850405. [DOI] [PubMed] [Google Scholar]
- Gipson I. K., Riddle C. V., Kiorpes T. C., Spurr S. J. Lectin binding to cell surfaces: comparisons between normal and migrating corneal epithelium. Dev Biol. 1983 Apr;96(2):337–345. doi: 10.1016/0012-1606(83)90171-9. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Gordon S. R. Changes in distribution of extracellular matrix proteins during wound repair in corneal endothelium. J Histochem Cytochem. 1988 Apr;36(4):409–416. doi: 10.1177/36.4.3279112. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Ruoslahti E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J Cell Biol. 1979 Oct;83(1):255–259. doi: 10.1083/jcb.83.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Mathieu P. Glycoconjugates on corneal epithelial surface: effect of neuraminidase treatment. J Histochem Cytochem. 1989 Aug;37(8):1215–1224. doi: 10.1177/37.8.2754252. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M., Berk R. S. In vivo identification of sialic acid as the ocular receptor for Pseudomonas aeruginosa. Infect Immun. 1986 Feb;51(2):687–689. doi: 10.1128/iai.51.2.687-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman C. A., Marchok A. C., Nettesheim P. Respiratory tract epithelium in primary culture: concurrent growth and differentiation during establishment. J Cell Sci. 1978 Aug;32:269–291. doi: 10.1242/jcs.32.1.269. [DOI] [PubMed] [Google Scholar]
- Hergott G. J., Sandig M., Kalnins V. I. Cytoskeletal organization of migrating retinal pigment epithelial cells during wound healing in organ culture. Cell Motil Cytoskeleton. 1989;13(2):83–93. doi: 10.1002/cm.970130203. [DOI] [PubMed] [Google Scholar]
- Kim K. C., Rearick J. I., Nettesheim P., Jetten A. M. Biochemical characterization of mucous glycoproteins synthesized and secreted by hamster tracheal epithelial cells in primary culture. J Biol Chem. 1985 Apr 10;260(7):4021–4027. [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R., Fishman P. H. Ganglioside inhibition of fibronectin-mediated cell adhesion to collagen. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3367–3371. doi: 10.1073/pnas.76.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama K., Habbick B. F., Tumber S. K. Whole, submandibular, and parotid saliva-mediated aggregation of Pseudomonas aeruginosa in cystic fibrosis. Infect Immun. 1989 Apr;57(4):1299–1304. doi: 10.1128/iai.57.4.1299-1304.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Carlson D. M., St George J. A., Plopper C. G., Wu R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am J Respir Cell Mol Biol. 1989 Jul;1(1):41–48. doi: 10.1165/ajrcmb/1.1.41. [DOI] [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann P. L., Lopez-Colberg I., Kelley R. O. Cell surface oligosaccharide modulation during differentiation. I. Modulation of lectin binding. Mech Ageing Dev. 1987 May;38(3):207–217. doi: 10.1016/0047-6374(87)90090-x. [DOI] [PubMed] [Google Scholar]
- Marcus H., Austria A., Baker N. R. Adherence of Pseudomonas aeruginosa to tracheal epithelium. Infect Immun. 1989 Apr;57(4):1050–1053. doi: 10.1128/iai.57.4.1050-1053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Becci P. J., Schürch W., Trump B. F. The respiratory epithelium. VII. Epidermoid metaplasia of hamster tracheal epithelium during regeneration following mechanical injury. J Natl Cancer Inst. 1979 Apr;62(4):995–1008. [PubMed] [Google Scholar]
- McDowell E. M., Ben T., Newkirk C., Chang S., De Luca L. M. Differentiation of tracheal mucociliary epithelium in primary cell culture recapitulates normal fetal development and regeneration following injury in hamsters. Am J Pathol. 1987 Dec;129(3):511–522. [PMC free article] [PubMed] [Google Scholar]
- McEachran D. W., Irvin R. T. Adhesion of Pseudomonas aeruginosa to human buccal epithelial cells: evidence for two classes of receptors. Can J Microbiol. 1985 Jun;31(6):563–569. doi: 10.1139/m85-105. [DOI] [PubMed] [Google Scholar]
- Merchant D. J. Terminally differentiating epithelial tissues in primary explant culture: a model of growth and development. In Vitro Cell Dev Biol. 1990 Jun;26(6):543–553. doi: 10.1007/BF02624202. [DOI] [PubMed] [Google Scholar]
- Niederman M. S., Rafferty T. D., Sasaki C. T., Merrill W. W., Matthay R. A., Reynolds H. Y. Comparison of bacterial adherence to ciliated and squamous epithelial cells obtained from the human respiratory tract. Am Rev Respir Dis. 1983 Jan;127(1):85–90. doi: 10.1164/arrd.1983.127.1.85. [DOI] [PubMed] [Google Scholar]
- Panjwani N., Zaidi T. S., Gigstad J. E., Jungalwala F. B., Barza M., Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990 Jan;58(1):114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkowski M. C., Beck G., Tournier J. M., Bernardo-Filho M., Marques E. A., Puchelle E. Adherence of Pseudomonas aeruginosa to respiratory epithelium and the effect of leucocyte elastase. J Med Microbiol. 1989 Dec;30(4):285–293. doi: 10.1099/00222615-30-4-285. [DOI] [PubMed] [Google Scholar]
- Plotkowski M. C., Puchelle E., Beck G., Jacquot J., Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986 Nov;134(5):1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sabet M. D., Gordon S. R. Ultrastructural immunocytochemical localization of fibronectin deposition during corneal endothelial wound repair. Evidence for cytoskeletal involvement. Biol Cell. 1989;65(2):171–179. [PubMed] [Google Scholar]
- Sawada H., Furthmayr H., Konomi H., Nagai Y. Immunoelectronmicroscopic localization of extracellular matrix components produced by bovine corneal endothelial cells in vitro. Exp Cell Res. 1987 Jul;171(1):94–109. doi: 10.1016/0014-4827(87)90254-0. [DOI] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Shoji S., Rickard K. A., Ertl R. F., Robbins R. A., Linder J., Rennard S. I. Bronchial epithelial cells produce lung fibroblast chemotactic factor: fibronectin. Am J Respir Cell Mol Biol. 1989 Jul;1(1):13–20. doi: 10.1165/ajrcmb/1.1.13. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Singh A., Hazlett L. D., Berk R. S. Characterization of Pseudomonas aeruginosa adherence to mouse corneas in organ culture. Infect Immun. 1990 May;58(5):1301–1307. doi: 10.1128/iai.58.5.1301-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma-Moreira R. E., Caffarena R. M., Somma S., Pérez G., Monteiro M. Analysis of Q fever in Uruguay. Rev Infect Dis. 1987 Mar-Apr;9(2):386–387. doi: 10.1093/clinids/9.2.386. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S. J., Barza M., Gipson I. K. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Invest Ophthalmol Vis Sci. 1988 Mar;29(3):379–386. [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. K., Horowitz P. M., Bentley K. L., Thomas D. D., Alderete J. F., Klebe R. J. Localization of the ganglioside-binding site of fibronectin. J Biol Chem. 1986 Apr 15;261(11):5209–5214. [PubMed] [Google Scholar]
- Varsano S., Basbaum C. B., Forsberg L. S., Borson D. B., Caughey G., Nadel J. A. Dog tracheal epithelial cells in culture synthesize sulfated macromolecular glycoconjugates and release them from the cell surface upon exposure to extracellular proteinases. Exp Lung Res. 1987;13(2):157–184. doi: 10.3109/01902148709064316. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. The reorganization of microfilaments, centrosomes, and microtubules during in vitro small wound reendothelialization. J Cell Biol. 1988 Nov;107(5):1777–1783. doi: 10.1083/jcb.107.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981 Jun;143(6):784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]