Abstract
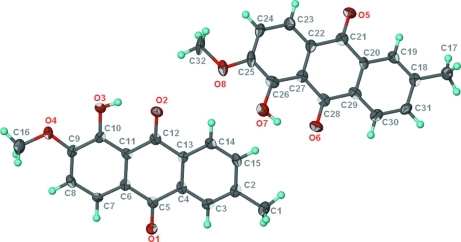
The title compound, C16H12O4, exists as planar molecules in the solid state (r.m.s. deviation of 0.02 Å in one molecule and 0.07 Å in the second independent molecule comprising the asymmetric unit). In each molecule, the 1-hydroxy group forms an intramolecular hydrogen bond to the adjacent carbonyl O atom.
Related literature
The existence of the title natural product has only been reported for Crucianella maritima L. (El-Lakany et al., 2004 ▶). For another anthraquinone isolated from Rennellia elliptica Korth., see: Ismail et al. (2009 ▶).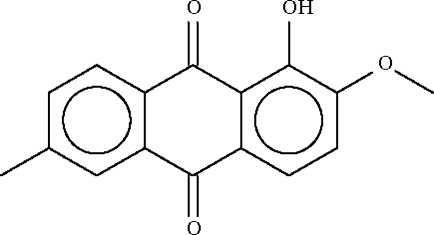
Experimental
Crystal data
C16H12O4
M r = 268.26
Triclinic,
a = 7.1755 (3) Å
b = 11.9082 (5) Å
c = 14.9683 (7) Å
α = 91.409 (3)°
β = 100.603 (3)°
γ = 105.666 (3)°
V = 1206.73 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 100 K
0.25 × 0.20 × 0.01 mm
Data collection
Bruker SMART APEX diffractometer
Absorption correction: none
6750 measured reflections
4142 independent reflections
2248 reflections with I > 2σ(I)
R int = 0.051
Refinement
R[F 2 > 2σ(F 2)] = 0.082
wR(F 2) = 0.267
S = 1.08
4142 reflections
367 parameters
H-atom parameters constrained
Δρmax = 0.59 e Å−3
Δρmin = −0.35 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809017619/tk2448sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809017619/tk2448Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H3⋯O2 | 0.84 | 1.80 | 2.538 (4) | 147 |
| O7—H7⋯O6 | 0.84 | 1.81 | 2.551 (5) | 146 |
Acknowledgments
We thank Universiti Teknologi MARA and the University of Malaya for supporting this study.
supplementary crystallographic information
Experimental
About 1 kg of the root of Rennelia elliptica Korth., which was collected from the Kuala Keniam National Park, Malaysia, was extracted with dichloromethane. The solvent was removed to give a crude material (approx. 10 g) that was fractionated on a chromatography column (60 x 5 cm) packed with silica. The silica had been previously immersed in 4% oxalic acid and then activated by heating to 363 K. The fractions were eluted with hexane–dichloromethane (3:7 v/v), and those fractions having an identical TLC pattern were combined and then subjected to column chromatography (330 x 15 mm), with dichloromethane as eluent. The compound was further purified on a short glass column (50 x 5 mm). The solvent was removed and the product recrystallized from chloroform to furnish yellow crystals (about 10 mg). The formulation was established by 1H- and 13C-NMR spectroscopy.
Refinement
Hydrogen atoms were placed at calculated positions (C–H 0.95–0.98 Å) and were treated as riding on their parent carbon atoms, with U(H) set to 1.2–1.5 times Ueq(C). The hydroxy H-atoms were similarly generated (O–H 0.84 Å).
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of the two independent molecules of C16H12O4 at the 70% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C16H12O4 | Z = 4 |
| Mr = 268.26 | F(000) = 560 |
| Triclinic, P1 | Dx = 1.477 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.1755 (3) Å | Cell parameters from 1159 reflections |
| b = 11.9082 (5) Å | θ = 2.8–26.8° |
| c = 14.9683 (7) Å | µ = 0.11 mm−1 |
| α = 91.409 (3)° | T = 100 K |
| β = 100.603 (3)° | Plate, yellow |
| γ = 105.666 (3)° | 0.25 × 0.20 × 0.01 mm |
| V = 1206.73 (9) Å3 |
Data collection
| Bruker SMART APEX diffractometer | 2248 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.051 |
| graphite | θmax = 25.0°, θmin = 1.8° |
| ω scans | h = −8→8 |
| 6750 measured reflections | k = −14→13 |
| 4142 independent reflections | l = −17→17 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.082 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.267 | H-atom parameters constrained |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.1397P)2 + 0.3435P] where P = (Fo2 + 2Fc2)/3 |
| 4142 reflections | (Δ/σ)max = 0.001 |
| 367 parameters | Δρmax = 0.59 e Å−3 |
| 0 restraints | Δρmin = −0.35 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.2331 (5) | 0.2601 (3) | 0.5314 (2) | 0.0207 (8) | |
| O2 | 0.2924 (5) | 0.7172 (3) | 0.4917 (2) | 0.0219 (9) | |
| O3 | 0.3202 (5) | 0.6932 (3) | 0.3261 (2) | 0.0205 (8) | |
| H3 | 0.3084 | 0.7272 | 0.3737 | 0.031* | |
| O4 | 0.3344 (5) | 0.5601 (3) | 0.1875 (2) | 0.0207 (8) | |
| O5 | 0.9299 (6) | 1.2275 (3) | 1.0962 (2) | 0.0327 (10) | |
| O6 | 0.5739 (6) | 0.7665 (3) | 1.0100 (2) | 0.0281 (9) | |
| O7 | 0.5636 (6) | 0.7944 (3) | 0.8409 (2) | 0.0297 (10) | |
| H7 | 0.5365 | 0.7587 | 0.8866 | 0.045* | |
| O8 | 0.6524 (5) | 0.9285 (3) | 0.7122 (2) | 0.0279 (9) | |
| C1 | 0.1303 (8) | 0.4169 (5) | 0.8389 (3) | 0.0247 (13) | |
| H1A | 0.2063 | 0.3608 | 0.8555 | 0.037* | |
| H1B | −0.0110 | 0.3773 | 0.8316 | 0.037* | |
| H1C | 0.1682 | 0.4806 | 0.8872 | 0.037* | |
| C2 | 0.1725 (7) | 0.4662 (4) | 0.7505 (3) | 0.0188 (11) | |
| C3 | 0.1867 (7) | 0.3958 (4) | 0.6786 (3) | 0.0186 (11) | |
| H3A | 0.1746 | 0.3153 | 0.6858 | 0.022* | |
| C4 | 0.2183 (7) | 0.4406 (4) | 0.5959 (3) | 0.0163 (11) | |
| C5 | 0.2389 (7) | 0.3634 (4) | 0.5212 (3) | 0.0158 (11) | |
| C6 | 0.2613 (7) | 0.4126 (4) | 0.4328 (3) | 0.0155 (11) | |
| C7 | 0.2703 (7) | 0.3443 (4) | 0.3595 (3) | 0.0146 (11) | |
| H7A | 0.2615 | 0.2640 | 0.3662 | 0.017* | |
| C8 | 0.2921 (7) | 0.3884 (4) | 0.2752 (3) | 0.0187 (12) | |
| H8 | 0.2951 | 0.3385 | 0.2253 | 0.022* | |
| C9 | 0.3092 (7) | 0.5067 (4) | 0.2654 (3) | 0.0179 (11) | |
| C10 | 0.3034 (7) | 0.5792 (4) | 0.3390 (3) | 0.0160 (11) | |
| C11 | 0.2798 (7) | 0.5340 (4) | 0.4234 (3) | 0.0153 (11) | |
| C12 | 0.2727 (7) | 0.6111 (4) | 0.4991 (3) | 0.0176 (11) | |
| C13 | 0.2400 (7) | 0.5601 (4) | 0.5865 (3) | 0.0143 (11) | |
| C14 | 0.2299 (7) | 0.6314 (4) | 0.6590 (3) | 0.0184 (11) | |
| H14 | 0.2462 | 0.7125 | 0.6527 | 0.022* | |
| C15 | 0.1964 (7) | 0.5858 (4) | 0.7401 (3) | 0.0195 (12) | |
| H15 | 0.1894 | 0.6355 | 0.7893 | 0.023* | |
| C16 | 0.3257 (8) | 0.4888 (5) | 0.1080 (4) | 0.0295 (14) | |
| H16A | 0.3329 | 0.5367 | 0.0558 | 0.044* | |
| H16B | 0.2014 | 0.4261 | 0.0959 | 0.044* | |
| H16C | 0.4370 | 0.4546 | 0.1179 | 0.044* | |
| C17 | 0.8428 (9) | 1.0596 (5) | 1.4057 (3) | 0.0262 (13) | |
| H17A | 0.9719 | 1.1182 | 1.4193 | 0.039* | |
| H17B | 0.8450 | 0.9950 | 1.4449 | 0.039* | |
| H17C | 0.7409 | 1.0957 | 1.4171 | 0.039* | |
| C18 | 0.7980 (7) | 1.0137 (5) | 1.3075 (3) | 0.0204 (12) | |
| C19 | 0.8440 (7) | 1.0856 (5) | 1.2383 (3) | 0.0204 (12) | |
| H19 | 0.9084 | 1.1664 | 1.2537 | 0.024* | |
| C20 | 0.7990 (7) | 1.0432 (4) | 1.1479 (3) | 0.0167 (11) | |
| C21 | 0.8479 (8) | 1.1216 (5) | 1.0761 (3) | 0.0221 (12) | |
| C22 | 0.7990 (7) | 1.0732 (4) | 0.9807 (3) | 0.0206 (12) | |
| C23 | 0.8403 (8) | 1.1438 (5) | 0.9115 (3) | 0.0230 (12) | |
| H23 | 0.9019 | 1.2251 | 0.9259 | 0.028* | |
| C24 | 0.7944 (8) | 1.0992 (5) | 0.8208 (3) | 0.0236 (12) | |
| H24 | 0.8261 | 1.1500 | 0.7745 | 0.028* | |
| C25 | 0.7032 (8) | 0.9821 (5) | 0.7979 (3) | 0.0236 (13) | |
| C26 | 0.6581 (7) | 0.9084 (4) | 0.8672 (4) | 0.0218 (12) | |
| C27 | 0.7028 (7) | 0.9509 (4) | 0.9582 (3) | 0.0193 (12) | |
| C28 | 0.6543 (7) | 0.8737 (4) | 1.0285 (3) | 0.0196 (12) | |
| C29 | 0.7021 (7) | 0.9217 (4) | 1.1244 (3) | 0.0196 (12) | |
| C30 | 0.6537 (7) | 0.8511 (5) | 1.1930 (3) | 0.0221 (12) | |
| H30 | 0.5869 | 0.7705 | 1.1781 | 0.027* | |
| C31 | 0.7009 (8) | 0.8955 (4) | 1.2837 (3) | 0.0224 (12) | |
| H31 | 0.6666 | 0.8449 | 1.3299 | 0.027* | |
| C32 | 0.6916 (9) | 0.9996 (6) | 0.6386 (4) | 0.0317 (14) | |
| H32A | 0.6461 | 0.9508 | 0.5809 | 0.047* | |
| H32B | 0.8338 | 1.0370 | 0.6471 | 0.047* | |
| H32C | 0.6216 | 1.0600 | 0.6374 | 0.047* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.025 (2) | 0.0202 (19) | 0.0184 (19) | 0.0103 (16) | 0.0018 (15) | 0.0029 (15) |
| O2 | 0.027 (2) | 0.0162 (19) | 0.023 (2) | 0.0089 (16) | 0.0025 (16) | 0.0009 (15) |
| O3 | 0.026 (2) | 0.0153 (18) | 0.020 (2) | 0.0053 (16) | 0.0048 (16) | 0.0001 (15) |
| O4 | 0.023 (2) | 0.025 (2) | 0.0140 (19) | 0.0080 (16) | 0.0030 (15) | −0.0005 (15) |
| O5 | 0.048 (3) | 0.018 (2) | 0.025 (2) | −0.0013 (19) | 0.0036 (19) | −0.0023 (16) |
| O6 | 0.035 (2) | 0.018 (2) | 0.028 (2) | 0.0044 (18) | 0.0015 (17) | −0.0022 (16) |
| O7 | 0.037 (2) | 0.026 (2) | 0.024 (2) | 0.0076 (19) | 0.0049 (18) | −0.0012 (17) |
| O8 | 0.033 (2) | 0.038 (2) | 0.014 (2) | 0.0150 (19) | −0.0003 (16) | −0.0025 (17) |
| C1 | 0.021 (3) | 0.033 (3) | 0.024 (3) | 0.010 (3) | 0.011 (2) | 0.007 (2) |
| C2 | 0.011 (3) | 0.028 (3) | 0.017 (3) | 0.007 (2) | −0.001 (2) | 0.003 (2) |
| C3 | 0.012 (3) | 0.020 (3) | 0.023 (3) | 0.005 (2) | 0.000 (2) | 0.005 (2) |
| C4 | 0.006 (2) | 0.025 (3) | 0.017 (3) | 0.005 (2) | −0.002 (2) | −0.004 (2) |
| C5 | 0.008 (3) | 0.020 (3) | 0.019 (3) | 0.005 (2) | 0.000 (2) | 0.000 (2) |
| C6 | 0.008 (3) | 0.022 (3) | 0.015 (3) | 0.005 (2) | −0.003 (2) | 0.000 (2) |
| C7 | 0.010 (3) | 0.013 (2) | 0.020 (3) | 0.002 (2) | 0.004 (2) | 0.001 (2) |
| C8 | 0.016 (3) | 0.023 (3) | 0.016 (3) | 0.008 (2) | −0.001 (2) | −0.005 (2) |
| C9 | 0.013 (3) | 0.025 (3) | 0.016 (3) | 0.005 (2) | 0.002 (2) | 0.004 (2) |
| C10 | 0.010 (3) | 0.022 (3) | 0.015 (3) | 0.004 (2) | −0.001 (2) | 0.004 (2) |
| C11 | 0.011 (3) | 0.018 (3) | 0.015 (3) | 0.007 (2) | −0.004 (2) | −0.001 (2) |
| C12 | 0.010 (3) | 0.022 (3) | 0.019 (3) | 0.004 (2) | −0.002 (2) | 0.000 (2) |
| C13 | 0.007 (2) | 0.021 (3) | 0.016 (3) | 0.007 (2) | 0.0013 (19) | 0.002 (2) |
| C14 | 0.017 (3) | 0.019 (3) | 0.018 (3) | 0.007 (2) | −0.002 (2) | −0.001 (2) |
| C15 | 0.015 (3) | 0.027 (3) | 0.014 (3) | 0.006 (2) | −0.003 (2) | −0.005 (2) |
| C16 | 0.031 (3) | 0.034 (3) | 0.020 (3) | 0.003 (3) | 0.005 (2) | 0.000 (2) |
| C17 | 0.034 (3) | 0.027 (3) | 0.018 (3) | 0.011 (3) | −0.001 (2) | 0.002 (2) |
| C18 | 0.017 (3) | 0.028 (3) | 0.018 (3) | 0.013 (2) | 0.000 (2) | 0.002 (2) |
| C19 | 0.020 (3) | 0.022 (3) | 0.019 (3) | 0.009 (2) | −0.003 (2) | −0.002 (2) |
| C20 | 0.017 (3) | 0.023 (3) | 0.014 (3) | 0.013 (2) | 0.003 (2) | 0.000 (2) |
| C21 | 0.019 (3) | 0.025 (3) | 0.024 (3) | 0.011 (3) | 0.001 (2) | 0.004 (2) |
| C22 | 0.022 (3) | 0.025 (3) | 0.017 (3) | 0.012 (2) | 0.003 (2) | 0.004 (2) |
| C23 | 0.021 (3) | 0.026 (3) | 0.021 (3) | 0.005 (2) | 0.005 (2) | 0.000 (2) |
| C24 | 0.028 (3) | 0.031 (3) | 0.013 (3) | 0.010 (3) | 0.003 (2) | 0.005 (2) |
| C25 | 0.019 (3) | 0.042 (3) | 0.015 (3) | 0.015 (3) | 0.006 (2) | −0.002 (2) |
| C26 | 0.015 (3) | 0.022 (3) | 0.030 (3) | 0.010 (2) | 0.004 (2) | 0.000 (2) |
| C27 | 0.013 (3) | 0.025 (3) | 0.022 (3) | 0.010 (2) | 0.003 (2) | 0.002 (2) |
| C28 | 0.009 (3) | 0.026 (3) | 0.025 (3) | 0.008 (2) | 0.000 (2) | 0.000 (2) |
| C29 | 0.014 (3) | 0.020 (3) | 0.026 (3) | 0.010 (2) | −0.001 (2) | −0.002 (2) |
| C30 | 0.020 (3) | 0.025 (3) | 0.027 (3) | 0.012 (2) | 0.007 (2) | 0.007 (2) |
| C31 | 0.027 (3) | 0.022 (3) | 0.022 (3) | 0.012 (2) | 0.008 (2) | 0.010 (2) |
| C32 | 0.030 (3) | 0.056 (4) | 0.013 (3) | 0.017 (3) | 0.008 (2) | 0.005 (3) |
Geometric parameters (Å, °)
| O1—C5 | 1.235 (6) | C14—C15 | 1.377 (7) |
| O2—C12 | 1.243 (6) | C14—H14 | 0.9500 |
| O3—C10 | 1.351 (6) | C15—H15 | 0.9500 |
| O3—H3 | 0.8400 | C16—H16A | 0.9800 |
| O4—C9 | 1.360 (6) | C16—H16B | 0.9800 |
| O4—C16 | 1.426 (6) | C16—H16C | 0.9800 |
| O5—C21 | 1.242 (6) | C17—C18 | 1.502 (7) |
| O6—C28 | 1.251 (6) | C17—H17A | 0.9800 |
| O7—C26 | 1.356 (6) | C17—H17B | 0.9800 |
| O7—H7 | 0.8400 | C17—H17C | 0.9800 |
| O8—C25 | 1.359 (6) | C18—C19 | 1.390 (7) |
| O8—C32 | 1.429 (6) | C18—C31 | 1.396 (7) |
| C1—C2 | 1.509 (7) | C19—C20 | 1.382 (7) |
| C1—H1A | 0.9800 | C19—H19 | 0.9500 |
| C1—H1B | 0.9800 | C20—C29 | 1.429 (7) |
| C1—H1C | 0.9800 | C20—C21 | 1.469 (7) |
| C2—C3 | 1.383 (7) | C21—C22 | 1.470 (7) |
| C2—C15 | 1.404 (7) | C22—C23 | 1.375 (7) |
| C3—C4 | 1.394 (7) | C22—C27 | 1.434 (7) |
| C3—H3A | 0.9500 | C23—C24 | 1.392 (7) |
| C4—C13 | 1.402 (7) | C23—H23 | 0.9500 |
| C4—C5 | 1.483 (7) | C24—C25 | 1.376 (8) |
| C5—C6 | 1.478 (7) | C24—H24 | 0.9500 |
| C6—C7 | 1.372 (7) | C25—C26 | 1.402 (7) |
| C6—C11 | 1.428 (7) | C26—C27 | 1.390 (7) |
| C7—C8 | 1.397 (7) | C27—C28 | 1.443 (7) |
| C7—H7A | 0.9500 | C28—C29 | 1.476 (7) |
| C8—C9 | 1.395 (7) | C29—C30 | 1.377 (7) |
| C8—H8 | 0.9500 | C30—C31 | 1.392 (7) |
| C9—C10 | 1.397 (7) | C30—H30 | 0.9500 |
| C10—C11 | 1.406 (7) | C31—H31 | 0.9500 |
| C11—C12 | 1.459 (7) | C32—H32A | 0.9800 |
| C12—C13 | 1.487 (7) | C32—H32B | 0.9800 |
| C13—C14 | 1.386 (7) | C32—H32C | 0.9800 |
| C10—O3—H3 | 109.5 | H16A—C16—H16C | 109.5 |
| C9—O4—C16 | 117.9 (4) | H16B—C16—H16C | 109.5 |
| C26—O7—H7 | 109.5 | C18—C17—H17A | 109.5 |
| C25—O8—C32 | 117.7 (4) | C18—C17—H17B | 109.5 |
| C2—C1—H1A | 109.5 | H17A—C17—H17B | 109.5 |
| C2—C1—H1B | 109.5 | C18—C17—H17C | 109.5 |
| H1A—C1—H1B | 109.5 | H17A—C17—H17C | 109.5 |
| C2—C1—H1C | 109.5 | H17B—C17—H17C | 109.5 |
| H1A—C1—H1C | 109.5 | C19—C18—C31 | 118.3 (5) |
| H1B—C1—H1C | 109.5 | C19—C18—C17 | 122.3 (5) |
| C3—C2—C15 | 118.9 (5) | C31—C18—C17 | 119.3 (5) |
| C3—C2—C1 | 121.5 (5) | C20—C19—C18 | 122.1 (5) |
| C15—C2—C1 | 119.7 (5) | C20—C19—H19 | 119.0 |
| C2—C3—C4 | 121.4 (5) | C18—C19—H19 | 119.0 |
| C2—C3—H3A | 119.3 | C19—C20—C29 | 119.1 (5) |
| C4—C3—H3A | 119.3 | C19—C20—C21 | 120.8 (5) |
| C3—C4—C13 | 118.9 (5) | C29—C20—C21 | 120.0 (4) |
| C3—C4—C5 | 119.9 (4) | O5—C21—C22 | 120.6 (5) |
| C13—C4—C5 | 121.1 (4) | O5—C21—C20 | 120.1 (5) |
| O1—C5—C6 | 120.5 (4) | C22—C21—C20 | 119.2 (5) |
| O1—C5—C4 | 121.3 (4) | C23—C22—C27 | 118.8 (5) |
| C6—C5—C4 | 118.2 (4) | C23—C22—C21 | 121.1 (5) |
| C7—C6—C11 | 118.8 (4) | C27—C22—C21 | 120.1 (4) |
| C7—C6—C5 | 121.2 (4) | C22—C23—C24 | 121.7 (5) |
| C11—C6—C5 | 120.0 (4) | C22—C23—H23 | 119.1 |
| C6—C7—C8 | 122.5 (4) | C24—C23—H23 | 119.1 |
| C6—C7—H7A | 118.8 | C25—C24—C23 | 120.4 (5) |
| C8—C7—H7A | 118.8 | C25—C24—H24 | 119.8 |
| C9—C8—C7 | 119.0 (5) | C23—C24—H24 | 119.8 |
| C9—C8—H8 | 120.5 | O8—C25—C24 | 125.8 (5) |
| C7—C8—H8 | 120.5 | O8—C25—C26 | 115.3 (5) |
| O4—C9—C10 | 115.4 (4) | C24—C25—C26 | 119.0 (5) |
| O4—C9—C8 | 124.5 (5) | O7—C26—C27 | 121.6 (5) |
| C10—C9—C8 | 120.1 (4) | O7—C26—C25 | 116.7 (5) |
| O3—C10—C9 | 117.9 (4) | C27—C26—C25 | 121.7 (5) |
| O3—C10—C11 | 121.6 (4) | C26—C27—C22 | 118.5 (5) |
| C9—C10—C11 | 120.5 (4) | C26—C27—C28 | 120.8 (5) |
| C10—C11—C6 | 119.1 (4) | C22—C27—C28 | 120.7 (5) |
| C10—C11—C12 | 119.6 (4) | O6—C28—C27 | 121.4 (5) |
| C6—C11—C12 | 121.3 (4) | O6—C28—C29 | 119.1 (5) |
| O2—C12—C11 | 121.5 (4) | C27—C28—C29 | 119.5 (5) |
| O2—C12—C13 | 120.0 (4) | C30—C29—C20 | 118.6 (5) |
| C11—C12—C13 | 118.5 (4) | C30—C29—C28 | 121.0 (5) |
| C14—C13—C4 | 119.9 (4) | C20—C29—C28 | 120.3 (5) |
| C14—C13—C12 | 119.5 (4) | C29—C30—C31 | 121.3 (5) |
| C4—C13—C12 | 120.6 (4) | C29—C30—H30 | 119.3 |
| C15—C14—C13 | 120.6 (5) | C31—C30—H30 | 119.3 |
| C15—C14—H14 | 119.7 | C30—C31—C18 | 120.5 (5) |
| C13—C14—H14 | 119.7 | C30—C31—H31 | 119.7 |
| C14—C15—C2 | 120.3 (5) | C18—C31—H31 | 119.7 |
| C14—C15—H15 | 119.8 | O8—C32—H32A | 109.5 |
| C2—C15—H15 | 119.8 | O8—C32—H32B | 109.5 |
| O4—C16—H16A | 109.5 | H32A—C32—H32B | 109.5 |
| O4—C16—H16B | 109.5 | O8—C32—H32C | 109.5 |
| H16A—C16—H16B | 109.5 | H32A—C32—H32C | 109.5 |
| O4—C16—H16C | 109.5 | H32B—C32—H32C | 109.5 |
| C15—C2—C3—C4 | −1.8 (7) | C31—C18—C19—C20 | −0.9 (7) |
| C1—C2—C3—C4 | 177.5 (4) | C17—C18—C19—C20 | −178.7 (5) |
| C2—C3—C4—C13 | 1.3 (7) | C18—C19—C20—C29 | 0.0 (7) |
| C2—C3—C4—C5 | 178.1 (4) | C18—C19—C20—C21 | 179.4 (4) |
| C3—C4—C5—O1 | −1.6 (7) | C19—C20—C21—O5 | −0.3 (7) |
| C13—C4—C5—O1 | 175.1 (5) | C29—C20—C21—O5 | 179.1 (5) |
| C3—C4—C5—C6 | 176.7 (4) | C19—C20—C21—C22 | 179.6 (4) |
| C13—C4—C5—C6 | −6.6 (6) | C29—C20—C21—C22 | −1.0 (7) |
| O1—C5—C6—C7 | 1.9 (7) | O5—C21—C22—C23 | −0.8 (8) |
| C4—C5—C6—C7 | −176.5 (4) | C20—C21—C22—C23 | 179.3 (4) |
| O1—C5—C6—C11 | −176.2 (4) | O5—C21—C22—C27 | −179.5 (5) |
| C4—C5—C6—C11 | 5.4 (6) | C20—C21—C22—C27 | 0.6 (7) |
| C11—C6—C7—C8 | −1.7 (7) | C27—C22—C23—C24 | −1.1 (7) |
| C5—C6—C7—C8 | −179.8 (4) | C21—C22—C23—C24 | −179.8 (5) |
| C6—C7—C8—C9 | 1.3 (7) | C22—C23—C24—C25 | 0.7 (8) |
| C16—O4—C9—C10 | −175.1 (4) | C32—O8—C25—C24 | −1.8 (7) |
| C16—O4—C9—C8 | 5.6 (7) | C32—O8—C25—C26 | 178.9 (4) |
| C7—C8—C9—O4 | 178.8 (4) | C23—C24—C25—O8 | −179.5 (5) |
| C7—C8—C9—C10 | −0.4 (7) | C23—C24—C25—C26 | −0.2 (7) |
| O4—C9—C10—O3 | 0.8 (6) | O8—C25—C26—O7 | −2.4 (7) |
| C8—C9—C10—O3 | −179.9 (4) | C24—C25—C26—O7 | 178.3 (4) |
| O4—C9—C10—C11 | −179.4 (4) | O8—C25—C26—C27 | 179.5 (4) |
| C8—C9—C10—C11 | −0.1 (7) | C24—C25—C26—C27 | 0.2 (7) |
| O3—C10—C11—C6 | 179.5 (4) | O7—C26—C27—C22 | −178.6 (4) |
| C9—C10—C11—C6 | −0.2 (7) | C25—C26—C27—C22 | −0.5 (7) |
| O3—C10—C11—C12 | 0.1 (7) | O7—C26—C27—C28 | 1.7 (7) |
| C9—C10—C11—C12 | −179.6 (4) | C25—C26—C27—C28 | 179.7 (4) |
| C7—C6—C11—C10 | 1.1 (7) | C23—C22—C27—C26 | 1.0 (7) |
| C5—C6—C11—C10 | 179.2 (4) | C21—C22—C27—C26 | 179.7 (4) |
| C7—C6—C11—C12 | −179.6 (4) | C23—C22—C27—C28 | −179.2 (4) |
| C5—C6—C11—C12 | −1.4 (7) | C21—C22—C27—C28 | −0.5 (7) |
| C10—C11—C12—O2 | −2.0 (7) | C26—C27—C28—O6 | 1.1 (7) |
| C6—C11—C12—O2 | 178.7 (4) | C22—C27—C28—O6 | −178.6 (5) |
| C10—C11—C12—C13 | 177.7 (4) | C26—C27—C28—C29 | −179.5 (4) |
| C6—C11—C12—C13 | −1.7 (7) | C22—C27—C28—C29 | 0.8 (7) |
| C3—C4—C13—C14 | 0.0 (7) | C19—C20—C29—C30 | 1.1 (7) |
| C5—C4—C13—C14 | −176.7 (4) | C21—C20—C29—C30 | −178.3 (4) |
| C3—C4—C13—C12 | −179.6 (4) | C19—C20—C29—C28 | −179.3 (4) |
| C5—C4—C13—C12 | 3.6 (7) | C21—C20—C29—C28 | 1.3 (7) |
| O2—C12—C13—C14 | 0.6 (7) | O6—C28—C29—C30 | −2.1 (7) |
| C11—C12—C13—C14 | −179.1 (4) | C27—C28—C29—C30 | 178.4 (4) |
| O2—C12—C13—C4 | −179.7 (4) | O6—C28—C29—C20 | 178.3 (4) |
| C11—C12—C13—C4 | 0.6 (6) | C27—C28—C29—C20 | −1.2 (7) |
| C4—C13—C14—C15 | −0.8 (7) | C20—C29—C30—C31 | −1.3 (7) |
| C12—C13—C14—C15 | 178.9 (4) | C28—C29—C30—C31 | 179.1 (4) |
| C13—C14—C15—C2 | 0.2 (7) | C29—C30—C31—C18 | 0.4 (7) |
| C3—C2—C15—C14 | 1.1 (7) | C19—C18—C31—C30 | 0.7 (7) |
| C1—C2—C15—C14 | −178.3 (4) | C17—C18—C31—C30 | 178.6 (5) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3···O2 | 0.84 | 1.80 | 2.538 (4) | 147 |
| O7—H7···O6 | 0.84 | 1.81 | 2.551 (5) | 146 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2448).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- El-Lakany, A. M., Aboul-Ela, M. A., Abdel-Kader, M. S., Badr, J. M., Sabri, N. N. & Goher, Y. (2004). Nat. Prod. Sci.10, 63–68.
- Ismail, N. H., Osman, C. P., Ahmad, R., Awang, K. & Ng, S. W. (2009). Acta Cryst. E65, o1433–o1434. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2009). publCIF In preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809017619/tk2448sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809017619/tk2448Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report