Abstract
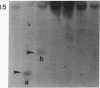
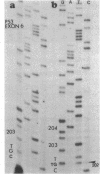
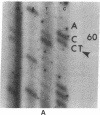
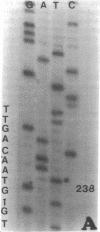
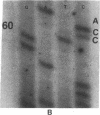
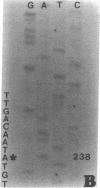
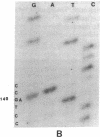
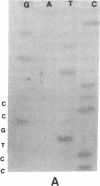
DNA from 135 patients with chronic myelogenous leukemia (CML) at various clinical stages and Philadelphia (Ph1) chromosome positive acute lymphoblastic leukemia was investigated for alterations in a variety of proto-oncogenes which have been implicated in the evolution of CML from its chronic phase to blast crisis. The most common genetic change found in the evolution of typical Ph1 chromosome positive CML to blast crisis was an alteration of the p53 gene involving either a rearrangement, a deletion, or a point mutation in the coding sequence of the gene. Alterations of the p53 gene were found in the myeloid and the rare megakaryocytic variant of blast crisis but were absent in the lymphoid leukemic transformants. Gross structural alterations were seen in 11 of 54 (20%) of myeloid or unknown phenotypes of blast crisis and in only 1 of 44 chronic phase cases. Eight examples of mutations in the open reading frame of the p53 gene at codons 49, 53, 60, 140, 202, 204, 238, and 239 were observed in blast crisis patients. Mutations in the N-RAS gene were rare in typical blast crisis (2 of 27 cases) but were found in megakaryocytic and Ph1 negative myeloid blast crisis. We concluded that heterogeneous alterations in the p53 gene and occasionally in the N-RAS genes accompany the evolution of chronic phase CML to blast crisis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahuja H. G., Foti A., Bar-Eli M., Cline M. J. The pattern of mutational involvement of RAS genes in human hematologic malignancies determined by DNA amplification and direct sequencing. Blood. 1990 Apr 15;75(8):1684–1690. [PubMed] [Google Scholar]
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bar-Eli M., Ahuja H., Gonzalez-Cadavid N., Foti A., Cline M. J. Analysis of N-RAS exon-1 mutations in myelodysplastic syndromes by polymerase chain reaction and direct sequencing. Blood. 1989 Jan;73(1):281–283. [PubMed] [Google Scholar]
- Bartram C. R., de Klein A., Hagemeijer A., van Agthoven T., Geurts van Kessel A., Bootsma D., Grosveld G., Ferguson-Smith M. A., Davies T., Stone M. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y., Daley G. Q., Mes-Masson A. M., Witte O. N., Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., van der Eb A. J., Janssen J. W., Delwel R., Löwenberg B., Colly L. P. Mutations in N-ras predominate in acute myeloid leukemia. Blood. 1987 Apr;69(4):1237–1241. [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Crist W. M., Champlin R., Witte O. N. Unique forms of the abl tyrosine kinase distinguish Ph1-positive CML from Ph1-positive ALL. Science. 1987 Jan 2;235(4784):85–88. doi: 10.1126/science.3541203. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Dreazen O., Canaani E., Gale R. P. Molecular biology of chronic myelogenous leukemia. Semin Hematol. 1988 Jan;25(1):35–48. [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., Beard M. E., Morris C. M., Heaton D. C., Reeve A. E. Ph-negative chronic myeloid leukaemia. Br J Haematol. 1987 Jul;66(3):311–314. doi: 10.1111/j.1365-2141.1987.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Fleischman E. W., Prigogina E. L., Volkova M. A., Frenkel M. A., Zakhartchenko N. A., Konstantinova L. N., Puchkova G. P., Balakirev S. A. Correlations between the clinical course, characteristics of blast cells, and karyotype patterns in chronic myeloid leukemia. Hum Genet. 1981;58(3):285–293. doi: 10.1007/BF00294925. [DOI] [PubMed] [Google Scholar]
- Foti A., Bar-Eli M., Ahuja H. G., Cline M. J. A splicing mutation accounts for the lack of p53 gene expression in a CML blast crisis cell line: a novel mechanism of p53 gene inactivation. Br J Haematol. 1990 Sep;76(1):143–145. doi: 10.1111/j.1365-2141.1990.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Kelman Z., Prokocimer M., Peller S., Kahn Y., Rechavi G., Manor Y., Cohen A., Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989 Nov 15;74(7):2318–2324. [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Hjelle B., Bishop J. M. Transforming genes in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1952–1956. doi: 10.1073/pnas.85.6.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashal R., Shtalrid M., Talpaz M., Kantarjian H., Smith L., Beran M., Cork A., Trujillo J., Gutterman J., Deisseroth A. Rearrangement and expression of p53 in the chronic phase and blast crisis of chronic myelogenous leukemia. Blood. 1990 Jan 1;75(1):180–189. [PubMed] [Google Scholar]
- Masuda H., Battifora H., Yokota J., Meltzer S., Cline M. J. Specificity of proto-oncogene amplification in human malignant diseases. Mol Biol Med. 1987 Aug;4(4):213–227. [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M., Rassool F. V., Goldman J. M., Graham S. V., Birnie G. D. Genomic alterations involving the c-myc proto-oncogene locus during the evolution of a case of chronic granulocytic leukaemia. Lancet. 1984 Dec 15;2(8416):1362–1365. doi: 10.1016/s0140-6736(84)92058-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Muehleck S. D., McKenna R. W., Arthur D. C., Parkin J. L., Brunning R. D. Transformation of chronic myelogenous leukemia: clinical, morphologic, and cytogenetic features. Am J Clin Pathol. 1984 Jul;82(1):1–14. doi: 10.1093/ajcp/82.1.1. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Gale R. P., Dreazen O., Berrebi A., Zaizov R., Kubonishi I., Miyoshi I., Canaani E. bcr-abl RNA in patients with chronic myelogenous leukemia. Blood. 1987 Mar;69(3):971–973. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Inactivation of p53 gene expression by an insertion of Moloney murine leukemia virus-like DNA sequences. Mol Cell Biol. 1984 Jul;4(7):1402–1410. doi: 10.1128/mcb.4.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Tsunetsugu-Yokota Y., Battifora H., Le Fevre C., Cline M. J. Alterations of myc, myb, and rasHa proto-oncogenes in cancers are frequent and show clinical correlation. Science. 1986 Jan 17;231(4735):261–265. doi: 10.1126/science.3941898. [DOI] [PubMed] [Google Scholar]