Abstract
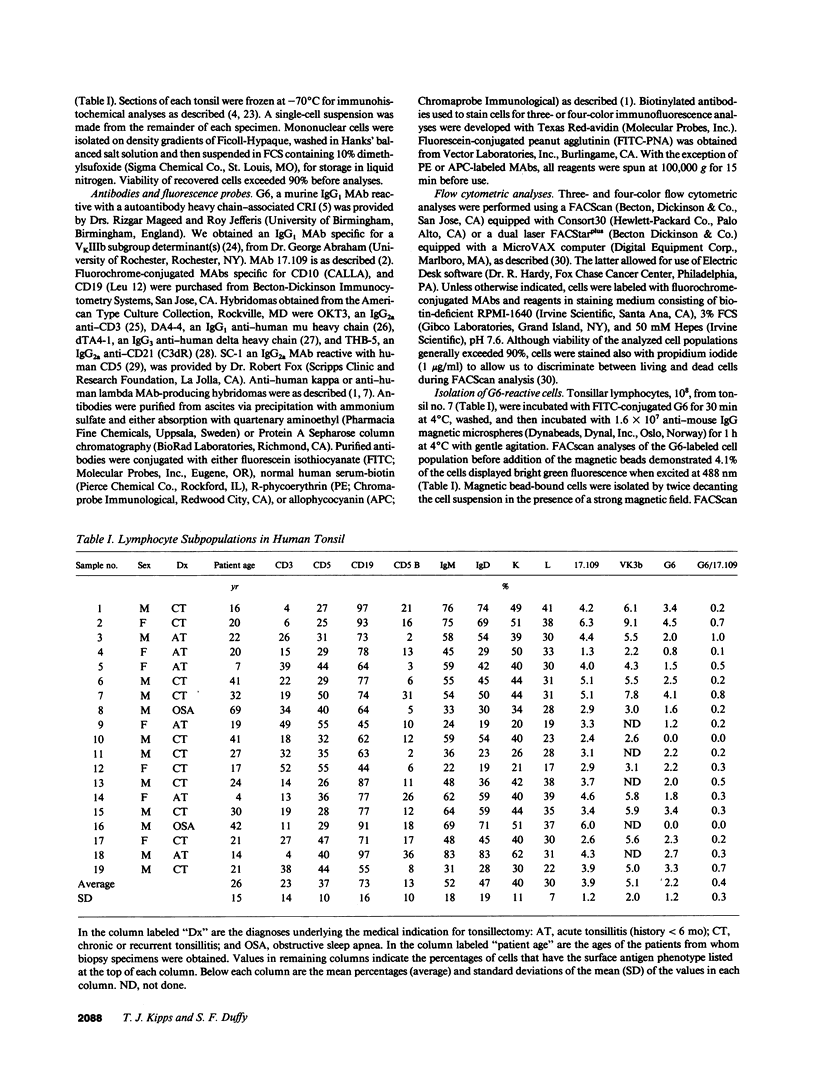
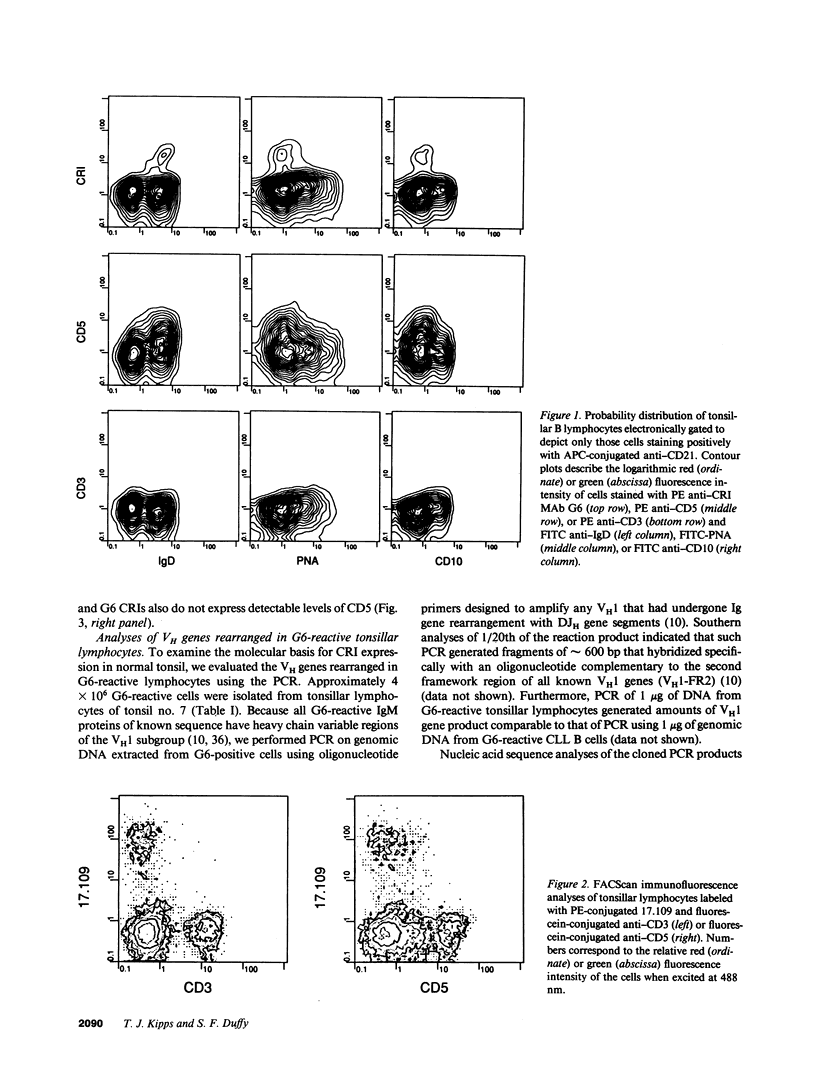
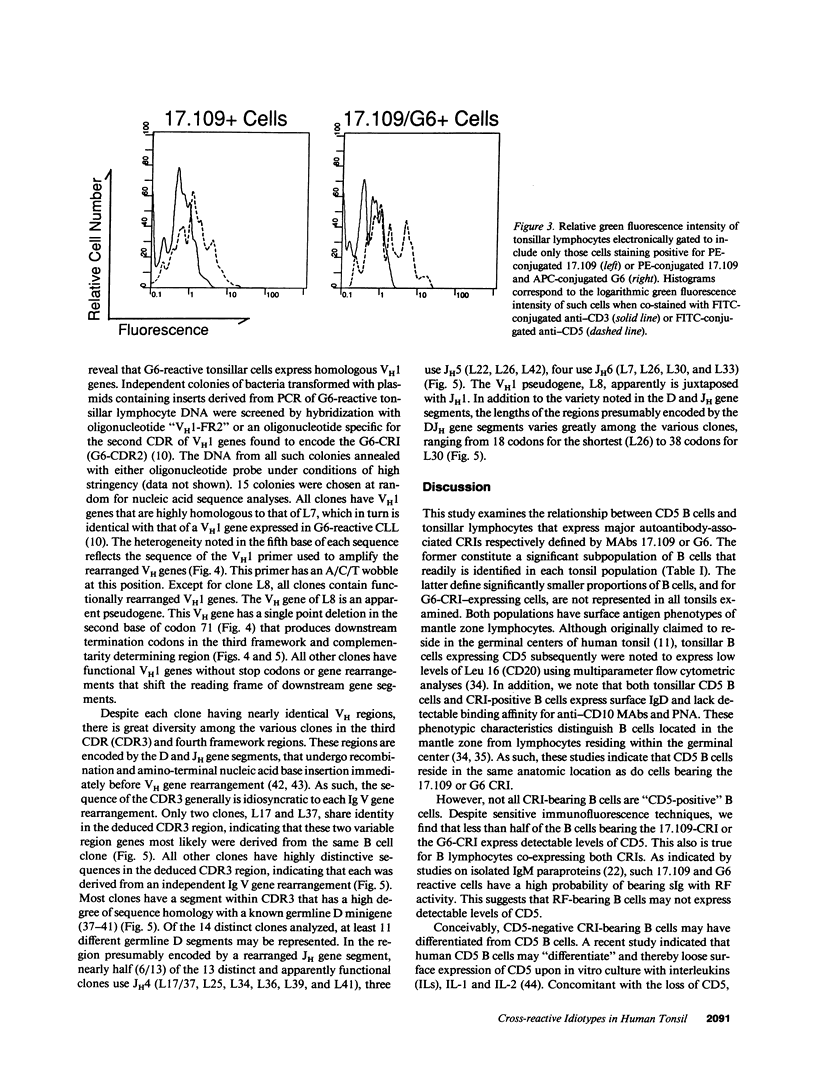
We examined human tonsillar B cells for expression of autoantibody heavy-chain or kappa light-chain cross-reactive idiotypes (CRIs), respectively defined by murine MAbs G6 or 17.109. We find 17.109 or G6 each specifically binds a subpopulation of B cells, respectively reacting with 3.8 +/- 3% (mean +/- SD) or 2.0 +/- 1.2% of all tonsillar lymphocytes. Cells reactive with both 17.109 and G6 comprise only 0.4 +/- 0.3% of tonsillar lymphocytes. Although each tested specimen had 17.109-positive cells, 2 of 19 tonsils (11%) did not have any G6-reactive cells. We find that CRI-positive cells and CD5 B cells both co-express slgD but fail to bind peanut agglutinin or MAbs specific for CD10, indicating that both cell types reside in the mantle zones of secondary B cell follicles. However, less than half of the B cells bearing one or both of these CRIs express detectable levels of CD5. Nevertheless, we find that G6-reactive lymphocytes constitute a multiclonal population of cells that express homologous heavy chain variable region genes, each rearranged to one of several distinct and apparently nonmutated D and JH gene segments. Collectively, these studies indicate that expression of nondiversified autoantibody-encoding variable region genes may not be an exclusive property of B cells that bear detectable levels of the CD5 surface antigen.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antin J. H., Emerson S. G., Martin P., Gadol N., Ault K. A. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986 Jan;136(2):505–510. [PubMed] [Google Scholar]
- Baccala R., Quang T. V., Gilbert M., Ternynck T., Avrameas S. Two murine natural polyreactive autoantibodies are encoded by nonmutated germ-line genes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4624–4628. doi: 10.1073/pnas.86.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill M., Janossy G., Janossa M., Burford G. D., Seymour G. J., Wernet P., Kelemen E. Human B cell development. II. Subpopulations in the human fetus. J Immunol. 1985 Mar;134(3):1531–1538. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Riva M., Tesio L., Schena M., Gaidano G., Bergui L. Human normal CD5+ B lymphocytes can be induced to differentiate to CD5- B lymphocytes with germinal center cell features. Blood. 1989 Apr;73(5):1259–1263. [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Kipps T. J., Radoux V., Jirik F. R., Goldfien R. D., Fox R. I., Silverman G. J., Fong S. Idiotypic and genetic studies of human rheumatoid factors. Arthritis Rheum. 1987 Dec;30(12):1321–1325. doi: 10.1002/art.1780301201. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Kipps T. J., Radoux V., Jirik F., Goldfien R. D., Fox R. I., Silverman G. J., Fong S. Molecular basis for the cross-reactive idiotypes on human anti-IgG autoantibodies (rheumatoid factors). Ciba Found Symp. 1987;129:123–134. doi: 10.1002/9780470513484.ch9. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Fong S. A common idiotope on human rheumatoid factors identified by a hybridoma antibody. Mol Immunol. 1983 Oct;20(10):1081–1087. doi: 10.1016/0161-5890(83)90116-5. [DOI] [PubMed] [Google Scholar]
- Casali P., Burastero S. E., Nakamura M., Inghirami G., Notkins A. L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Harlow D., Royston I., Elder J. Structural characterization of the human T cell surface antigen (p67) isolated from normal and neoplastic lymphocytes. J Immunol. 1982 Jul;129(1):401–405. [PubMed] [Google Scholar]
- Förster I., Gu H., Rajewsky K. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 1988 Dec 1;7(12):3693–3703. doi: 10.1002/j.1460-2075.1988.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadol N., Ault K. A. Phenotypic and functional characterization of human Leu1 (CD5) B cells. Immunol Rev. 1986 Oct;93:23–34. doi: 10.1111/j.1600-065x.1986.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Gadol N., Peacock M. A., Ault K. A. Antigenic phenotype and functional characterization of human tonsil B cells. Blood. 1988 Apr;71(4):1048–1055. [PubMed] [Google Scholar]
- Gobbi M., Caligaris-Cappio F., Janossy G. Normal equivalent cells of B cell malignancies: analysis with monoclonal antibodies. Br J Haematol. 1983 Jul;54(3):393–403. doi: 10.1111/j.1365-2141.1983.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Greenstein J. L., Solomon A., Abraham G. N. Monoclonal antibodies reactive with idiotypic and variable-region specific determinants on human immunoglobulins. Immunology. 1984 Jan;51(1):17–25. [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986 Oct;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Shimizu M., Yamasaki K., Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A. Toward a layered immune system. Cell. 1989 Dec 22;59(6):953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M. Conventional and Ly-1 B-cell lineages in normal and mu transgenic mice. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):219–225. doi: 10.1101/sqb.1989.054.01.027. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of B-lymphocytes. 2. Immunoglobulin expression of germinal center cells. Am J Pathol. 1984 Mar;114(3):396–402. [PMC free article] [PubMed] [Google Scholar]
- Ichihara Y., Abe M., Yasui H., Matsuoka H., Kurosawa Y. At least five DH genes of human immunoglobulin heavy chains are encoded in 9-kilobase DNA fragments. Eur J Immunol. 1988 Apr;18(4):649–652. doi: 10.1002/eji.1830180426. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988 Dec 20;7(13):4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Fong S., Tomhave E., Chen P. P., Goldfien R. D., Carson D. A. High-frequency expression of a conserved kappa light-chain variable-region gene in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 May;84(9):2916–2920. doi: 10.1073/pnas.84.9.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Carson D. A. Uniform high frequency expression of autoantibody-associated crossreactive idiotypes in the primary B cell follicles of human fetal spleen. J Exp Med. 1990 Jan 1;171(1):189–196. doi: 10.1084/jem.171.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Kuster P., Carson D. A. Autoantibody-associated cross-reactive idiotypes expressed at high frequency in chronic lymphocytic leukemia relative to B-cell lymphomas of follicular center cell origin. Blood. 1988 Aug;72(2):422–428. [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Tefferi A., Meisenholder G., Banks P. M., Carson D. A. CD5-positive B-cell malignancies frequently express cross-reactive idiotypes associated with IgM autoantibodies. Am J Pathol. 1990 Apr;136(4):809–816. [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J. The CD5 B cell. Adv Immunol. 1989;47:117–185. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- Kipps T. J., Tomhave E., Chen P. P., Carson D. A. Autoantibody-associated kappa light chain variable region gene expressed in chronic lymphocytic leukemia with little or no somatic mutation. Implications for etiology and immunotherapy. J Exp Med. 1988 Mar 1;167(3):840–852. doi: 10.1084/jem.167.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Tomhave E., Pratt L. F., Duffy S., Chen P. P., Carson D. A. Developmentally restricted immunoglobulin heavy chain variable region gene expressed at high frequency in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5913–5917. doi: 10.1073/pnas.86.15.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Vaughan J. H. Genetic influence on the levels of circulating CD5 B lymphocytes. J Immunol. 1987 Aug 15;139(4):1060–1064. [PubMed] [Google Scholar]
- Kubagawa H., Gathings W. E., Levitt D., Kearney J. F., Cooper M. D. Immunoglobulin isotype expression of normal pre-B cells as determined by immunofluorescence. J Clin Immunol. 1982 Oct;2(4):264–269. doi: 10.1007/BF00915065. [DOI] [PubMed] [Google Scholar]
- Mageed R. A., Dearlove M., Goodall D. M., Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins. XVII--Monoclonal antibodies reactive with common and restricted idiotopes to the heavy chain of human rheumatoid factors. Rheumatol Int. 1986;6(4):179–183. doi: 10.1007/BF00541285. [DOI] [PubMed] [Google Scholar]
- Maruyama S., Kubagawa H., Cooper M. D. Activation of human B cells and inhibition of their terminal differentiation by monoclonal anti-mu antibodies. J Immunol. 1985 Jul;135(1):192–199. [PubMed] [Google Scholar]
- Matsuda F., Lee K. H., Nakai S., Sato T., Kodaira M., Zong S. Q., Ohno H., Fukuhara S., Honjo T. Dispersed localization of D segments in the human immunoglobulin heavy-chain locus. EMBO J. 1988 Apr;7(4):1047–1051. doi: 10.1002/j.1460-2075.1988.tb02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Ueki Y., Larrick J. W., Notkins A. L., Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto's disease and systemic lupus erythematosus. J Immunol. 1988 Dec 15;141(12):4165–4172. [PubMed] [Google Scholar]
- Newkirk M. M., Gram H., Heinrich G. F., Ostberg L., Capra J. D., Wasserman R. L. Complete protein sequences of the variable regions of the cloned heavy and light chains of a human anti-cytomegalovirus antibody reveal a striking similarity to human monoclonal rheumatoid factors of the Wa idiotypic family. J Clin Invest. 1988 May;81(5):1511–1518. doi: 10.1172/JCI113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk M. M., Mageed R. A., Jefferis R., Chen P. P., Capra J. D. Complete amino acid sequences of variable regions of two human IgM rheumatoid factors, BOR and KAS of the Wa idiotypic family, reveal restricted use of heavy and light chain variable and joining region gene segments. J Exp Med. 1987 Aug 1;166(2):550–564. doi: 10.1084/jem.166.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. F., Rassenti L., Larrick J., Robbins B., Banks P. M., Kipps T. J. Ig V region gene expression in small lymphocytic lymphoma with little or no somatic hypermutation. J Immunol. 1989 Jul 15;143(2):699–705. [PubMed] [Google Scholar]
- Radoux V., Chen P. P., Sorge J. A., Carson D. A. A conserved human germline V kappa gene directly encodes rheumatoid factor light chains. J Exp Med. 1986 Dec 1;164(6):2119–2124. doi: 10.1084/jem.164.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Schlossman S. F. A monoclonal antibody blocking human T cell function. Eur J Immunol. 1980 Oct;10(10):758–762. doi: 10.1002/eji.1830101006. [DOI] [PubMed] [Google Scholar]
- Reininger L., Ollier P., Poncet P., Kaushik A., Jaton J. C. Novel V genes encode virtually identical variable regions of six murine monoclonal anti-bromelain-treated red blood cell autoantibodies. J Immunol. 1987 Jan 1;138(1):316–323. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanz I., Casali P., Thomas J. W., Notkins A. L., Capra J. D. Nucleotide sequences of eight human natural autoantibody VH regions reveals apparent restricted use of VH families. J Immunol. 1989 Jun 1;142(11):4054–4061. [PubMed] [Google Scholar]
- Sanz I., Dang H., Takei M., Talal N., Capra J. D. VH sequence of a human anti-Sm autoantibody. Evidence that autoantibodies can be unmutated copies of germline genes. J Immunol. 1989 Feb 1;142(3):883–887. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Shokri F., Mageed R. A., Tunn E., Bacon P. A., Jefferis R. Qualitative and quantitative expression of VHI associated cross reactive idiotopes within IgM rheumatoid factor from patients with early synovitis. Ann Rheum Dis. 1990 Mar;49(3):150–154. doi: 10.1136/ard.49.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Silverman G. J., Goldfien R. D., Chen P., Mageed R. A., Jefferis R., Goñi F., Frangione B., Fong S., Carson D. A. Idiotypic and subgroup analysis of human monoclonal rheumatoid factors. Implications for structural and genetic basis of autoantibodies in humans. J Clin Invest. 1988 Aug;82(2):469–475. doi: 10.1172/JCI113620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall A. M., Fariñas M. C., Tarlinton D. M., Lalor P. A., Herzenberg L. A., Strober S., Herzenberg L. A. Ly-1 B-cell clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7312–7316. doi: 10.1073/pnas.85.19.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D., Stall A. M., Herzenberg L. A. Repetitive usage of immunoglobulin VH and D gene segments in CD5+ Ly-1 B clones of (NZB x NZW)F1 mice. EMBO J. 1988 Dec 1;7(12):3705–3710. doi: 10.1002/j.1460-2075.1988.tb03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Clement L. T., Cooper M. D. Expression of C3d receptors during human B cell differentiation: immunofluorescence analysis with the HB-5 monoclonal antibody. J Immunol. 1984 Aug;133(2):678–683. [PubMed] [Google Scholar]
- Weinberg D. S., Ault K. A., Gurley M., Pinkus G. S. The human lymph node germinal center cell: characterization and isolation by using two-color flow cytometry. J Immunol. 1986 Sep 1;137(5):1486–1494. [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]



