Abstract
In the title compound, [PdCl2(C10H8N2)]·CH2Cl2, the Pd2+ ion is four-coordinated in a slightly distorted square-planar environment by two N atoms of the 2,2′-bipyridine (bipy) ligand and two chloride ions. The compound displays intramolecular C—H⋯Cl hydrogen bonds and pairs of complex molecules are connected by intermolecular C—H⋯Cl hydrogen bonds. Intermolecular π–π interactions are present between the pyridine rings of the ligand, the shortest centroid–centroid distance being 4.096 (3) Å. As a result of the electronic nature of the chelate ring, it is possible to create π–π interactions to its symmetry-related counterpart [3.720 (2) Å] and also with a pyridine ring [3.570 (3) Å] of the bipy unit. The present structure is a redetermination of a previous structure [Vicente et al. (1997 ▶). Private communication (refcode PYCXMN02). CCDC, Cambridge, England]. In the new structure refinement all H atoms were located in a difference Fourier synthesis. Their coordinates were refined freely, together with isotropic displacement parameters.
Related literature
For crystal structures of [PdX
2(bipy)] (X = Cl or Br), see: Maekawa et al. (1991 ▶); Vicente et al. (1997 ▶); Smeets et al. (1997 ▶). For related Pt(II, IV)–bipyridine complexes, see: Osborn & Rogers (1974 ▶); Hambley (1986 ▶); Sartori et al. (2005 ▶); Momeni et al. (2007 ▶); Kim et al. (2009 ▶).
Experimental
Crystal data
[PdCl2(C10H8N2)]·CH2Cl2
M r = 418.41
Triclinic,

a = 8.7913 (10) Å
b = 9.1115 (11) Å
c = 10.1846 (12) Å
α = 72.481 (2)°
β = 66.983 (2)°
γ = 81.429 (2)°
V = 715.58 (15) Å3
Z = 2
Mo Kα radiation
μ = 2.03 mm−1
T = 293 K
0.20 × 0.08 × 0.08 mm
Data collection
Bruker SMART 1000 CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.623, T max = 0.850
4221 measured reflections
2862 independent reflections
2298 reflections with I > 2σ(I)
R int = 0.023
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.097
S = 1.05
2862 reflections
203 parameters
All H-atom parameters refined
Δρmax = 0.56 e Å−3
Δρmin = −0.69 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809016262/kp2220sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809016262/kp2220Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Pd1—N2 | 2.025 (4) |
| Pd1—N1 | 2.029 (4) |
| Pd1—Cl2 | 2.2853 (14) |
| Pd1—Cl1 | 2.2964 (14) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1⋯Cl2 | 0.93 (5) | 2.59 (5) | 3.230 (6) | 126 (4) |
| C2—H2⋯Cl2i | 0.93 (5) | 2.79 (5) | 3.595 (7) | 145 (4) |
| C10—H10⋯Cl1 | 0.86 (5) | 2.68 (5) | 3.248 (6) | 125 (4) |
| C11—H11B⋯Cl1 | 0.99 (6) | 2.63 (6) | 3.578 (8) | 161 (5) |
Symmetry code: (i)  .
.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007–412-J02001).
supplementary crystallographic information
Comment
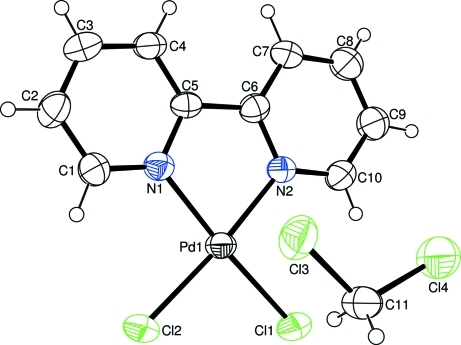
The asymmetric unit of the title compound, [PdCl2(C10H8N2)].CH2Cl2, contains a neutral PdII complex and a solvent molecule (Fig. 1). The compound crystallized in the triclinic space group P1, whereas the previously reported complex [PdCl2(C10H8N2)] crystallized in the orthorhombic space group C2221 (Maekawa et al., 1991). The X-ray structure analysis of the title compound was previously carried out (Vicente et al., 1997) and the structure is stored in CCDC code: RAGVUQ. The stored and our crystal structures are in agreement, however, the new structure determination provides data on hydrogen bonding based on H atoms positions located from a difference Fourier synthesis and refined isotropically.
In the complex, the Pd2+ ion is four-coordinated in a distorted square-planar environment by two N atoms of the 2,2'-bipyridine (bipy) ligand and two Cl ions. The main contribution to the distortion is the tight N1—Pd1—N2 chelate angle (81.04 (16)°), which results in non-linear trans arrangement (<N1—Pd1—Cl1 = 175.78 (12)° and <N2—Pd1—Cl2 = 174.97 (12)°). The Pd1—N and Pd1—Cl bond lengths are almost equal (Pd1—N: 2.029 (4) and 2.025 (4) Å; Pd1—Cl 2.2964 (14) and 2.2853 (14) Å), respectively (Table 1), and close to those reported for [PdCl2(C10H8N2)] (Maekawa et al., 1991). The best least-squares plane of the atoms of the planar complex, except Cl2, reveals mean deviation of 0.026 (5) Å; deviation of Cl2 from this plane is 0.184 (2) Å. The compound displays the inter- and intramolecular C—H···Cl hydrogen bonds (Table 2) and molecules are connected by intermolecular hydrogen bonds and stacked in layres along the c axis (Fig. 2). Intermolecular π-π interactions (defined by separation distance between the ring centroids (the symmetry operation for second plane 1-x,1-y,-z) involve the planes: Pd1,N1→ N2 and ity symmetry related counterpart, 3.720 (2) Å, planes are parallel and shifted for 1.506 Å; Pd1,N1→ N2 and N1→ C5, 3.570 (3) Å, the dihedral angle between planes is 1.54 °, no shift; N1→ C5 and N2→ C10, 4.096 (3) Å the dihedral angle between planes is 2.72 °, no shift.
Experimental
To a solution of Na2PdCl4 (0.300 g, 1.020 mmol) in EtOH (30 ml) was added 2,2'-bipyridine (0.159 g, 1.018 mmol) and stirred for 5 h at room temperature. The precipitate obtained was separated by filtration and washed with EtOH and water and dried under vacuum, to give a yellow powder (0.302 g). Crystals suitable for X-ray analysis were obtained by slow evaporation from a CH2Cl2 solution.
Refinement
All H atoms were located from Fourier difference maps and refined isotropically.
Figures
Fig. 1.
The structure of the title compound, with displacement ellipsoids drawn at the 40% probability level for non-H atoms.
Fig. 2.
Crystal packing of I. Hydrogen-bond interactions are drawn with dashed lines.
Crystal data
| [PdCl2(C10H8N2)]·CH2Cl2 | Z = 2 |
| Mr = 418.41 | F(000) = 408 |
| Triclinic, P1 | Dx = 1.942 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.7913 (10) Å | Cell parameters from 983 reflections |
| b = 9.1115 (11) Å | θ = 2.3–21.7° |
| c = 10.1846 (12) Å | µ = 2.03 mm−1 |
| α = 72.481 (2)° | T = 293 K |
| β = 66.983 (2)° | Stick, yellow |
| γ = 81.429 (2)° | 0.20 × 0.08 × 0.08 mm |
| V = 715.58 (15) Å3 |
Data collection
| Bruker SMART 1000 CCD diffractometer | 2862 independent reflections |
| Radiation source: fine-focus sealed tube | 2298 reflections with I > 2σ(I) |
| graphite | Rint = 0.023 |
| φ and ω scans | θmax = 26.4°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −10→10 |
| Tmin = 0.623, Tmax = 0.850 | k = −7→11 |
| 4221 measured reflections | l = −12→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.097 | All H-atom parameters refined |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0352P)2 + 0.5604P] where P = (Fo2 + 2Fc2)/3 |
| 2862 reflections | (Δ/σ)max < 0.001 |
| 203 parameters | Δρmax = 0.56 e Å−3 |
| 0 restraints | Δρmin = −0.69 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Pd1 | 0.32112 (5) | 0.28572 (4) | 0.21130 (4) | 0.04479 (15) | |
| Cl1 | 0.36850 (18) | 0.02764 (15) | 0.30403 (16) | 0.0612 (4) | |
| Cl2 | 0.1458 (2) | 0.22805 (17) | 0.11857 (19) | 0.0720 (5) | |
| N1 | 0.2916 (5) | 0.5169 (5) | 0.1348 (4) | 0.0454 (10) | |
| N2 | 0.4786 (5) | 0.3551 (5) | 0.2803 (4) | 0.0446 (10) | |
| C1 | 0.1884 (7) | 0.5895 (7) | 0.0665 (6) | 0.0545 (14) | |
| H1 | 0.126 (6) | 0.526 (6) | 0.052 (5) | 0.058 (16)* | |
| C2 | 0.1803 (8) | 0.7475 (7) | 0.0175 (7) | 0.0610 (15) | |
| H2 | 0.101 (7) | 0.800 (6) | −0.020 (6) | 0.063 (17)* | |
| C3 | 0.2768 (8) | 0.8325 (7) | 0.0413 (7) | 0.0620 (16) | |
| H3 | 0.273 (6) | 0.930 (6) | 0.018 (5) | 0.043 (14)* | |
| C4 | 0.3831 (7) | 0.7585 (6) | 0.1120 (6) | 0.0515 (13) | |
| H4 | 0.451 (6) | 0.809 (6) | 0.128 (5) | 0.046 (14)* | |
| C5 | 0.3887 (6) | 0.6010 (6) | 0.1590 (5) | 0.0417 (11) | |
| C6 | 0.4959 (6) | 0.5099 (6) | 0.2384 (5) | 0.0466 (12) | |
| C7 | 0.6069 (8) | 0.5739 (7) | 0.2675 (7) | 0.0644 (16) | |
| H7 | 0.623 (7) | 0.683 (7) | 0.236 (6) | 0.081 (19)* | |
| C8 | 0.7003 (9) | 0.4784 (8) | 0.3427 (8) | 0.077 (2) | |
| H8 | 0.775 (8) | 0.512 (8) | 0.355 (7) | 0.09 (2)* | |
| C9 | 0.6833 (8) | 0.3223 (8) | 0.3858 (8) | 0.0717 (18) | |
| H9 | 0.734 (6) | 0.255 (6) | 0.442 (5) | 0.049 (15)* | |
| C10 | 0.5692 (7) | 0.2645 (7) | 0.3546 (7) | 0.0579 (15) | |
| H10 | 0.562 (6) | 0.166 (6) | 0.376 (5) | 0.043 (14)* | |
| C11 | 0.0507 (9) | 0.1175 (7) | 0.6220 (8) | 0.0614 (16) | |
| H11A | −0.043 (7) | 0.071 (6) | 0.685 (6) | 0.062 (17)* | |
| H11B | 0.115 (7) | 0.087 (7) | 0.529 (7) | 0.09 (2)* | |
| Cl3 | −0.0049 (2) | 0.31218 (19) | 0.55636 (18) | 0.0740 (5) | |
| Cl4 | 0.1895 (2) | 0.1016 (2) | 0.7099 (2) | 0.0887 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Pd1 | 0.0465 (2) | 0.0387 (2) | 0.0512 (3) | −0.00670 (17) | −0.01825 (18) | −0.01196 (18) |
| Cl1 | 0.0744 (9) | 0.0386 (7) | 0.0735 (9) | −0.0059 (7) | −0.0309 (8) | −0.0121 (7) |
| Cl2 | 0.0836 (11) | 0.0561 (9) | 0.0965 (12) | −0.0203 (8) | −0.0543 (9) | −0.0117 (8) |
| N1 | 0.045 (2) | 0.044 (2) | 0.048 (2) | −0.005 (2) | −0.017 (2) | −0.0118 (19) |
| N2 | 0.049 (2) | 0.039 (2) | 0.050 (2) | −0.0036 (19) | −0.021 (2) | −0.0121 (19) |
| C1 | 0.052 (3) | 0.054 (3) | 0.062 (3) | −0.002 (3) | −0.028 (3) | −0.012 (3) |
| C2 | 0.063 (4) | 0.050 (4) | 0.068 (4) | 0.008 (3) | −0.029 (3) | −0.009 (3) |
| C3 | 0.072 (4) | 0.039 (3) | 0.074 (4) | −0.002 (3) | −0.025 (3) | −0.016 (3) |
| C4 | 0.057 (3) | 0.039 (3) | 0.063 (3) | −0.005 (3) | −0.025 (3) | −0.016 (3) |
| C5 | 0.041 (3) | 0.039 (3) | 0.044 (3) | −0.004 (2) | −0.010 (2) | −0.016 (2) |
| C6 | 0.046 (3) | 0.044 (3) | 0.045 (3) | −0.004 (2) | −0.012 (2) | −0.011 (2) |
| C7 | 0.080 (4) | 0.052 (4) | 0.078 (4) | −0.015 (3) | −0.045 (4) | −0.013 (3) |
| C8 | 0.084 (5) | 0.070 (5) | 0.097 (5) | −0.016 (4) | −0.058 (4) | −0.009 (4) |
| C9 | 0.074 (4) | 0.063 (4) | 0.092 (5) | −0.004 (3) | −0.054 (4) | −0.009 (4) |
| C10 | 0.065 (4) | 0.045 (3) | 0.067 (4) | −0.002 (3) | −0.032 (3) | −0.008 (3) |
| C11 | 0.065 (4) | 0.052 (4) | 0.072 (4) | −0.009 (3) | −0.026 (3) | −0.019 (3) |
| Cl3 | 0.0761 (10) | 0.0652 (10) | 0.0759 (10) | 0.0022 (8) | −0.0354 (9) | −0.0044 (8) |
| Cl4 | 0.0906 (12) | 0.0701 (11) | 0.1211 (15) | 0.0040 (9) | −0.0663 (12) | −0.0139 (10) |
Geometric parameters (Å, °)
| Pd1—N2 | 2.025 (4) | C4—H4 | 0.90 (5) |
| Pd1—N1 | 2.029 (4) | C5—C6 | 1.480 (7) |
| Pd1—Cl2 | 2.2853 (14) | C6—C7 | 1.371 (7) |
| Pd1—Cl1 | 2.2964 (14) | C7—C8 | 1.377 (9) |
| N1—C1 | 1.335 (6) | C7—H7 | 0.96 (6) |
| N1—C5 | 1.360 (6) | C8—C9 | 1.369 (9) |
| N2—C10 | 1.338 (7) | C8—H8 | 0.83 (6) |
| N2—C6 | 1.359 (6) | C9—C10 | 1.375 (8) |
| C1—C2 | 1.375 (8) | C9—H9 | 0.90 (5) |
| C1—H1 | 0.93 (5) | C10—H10 | 0.86 (5) |
| C2—C3 | 1.360 (8) | C11—Cl4 | 1.743 (6) |
| C2—H2 | 0.93 (5) | C11—Cl3 | 1.765 (6) |
| C3—C4 | 1.376 (8) | C11—H11A | 0.90 (5) |
| C3—H3 | 0.85 (5) | C11—H11B | 0.99 (6) |
| C4—C5 | 1.369 (7) | ||
| N2—Pd1—N1 | 81.04 (16) | N1—C5—C4 | 120.6 (5) |
| N2—Pd1—Cl2 | 174.97 (12) | N1—C5—C6 | 115.2 (4) |
| N1—Pd1—Cl2 | 94.27 (12) | C4—C5—C6 | 124.2 (5) |
| N2—Pd1—Cl1 | 94.74 (12) | N2—C6—C7 | 121.4 (5) |
| N1—Pd1—Cl1 | 175.78 (12) | N2—C6—C5 | 115.0 (4) |
| Cl2—Pd1—Cl1 | 89.94 (5) | C7—C6—C5 | 123.6 (5) |
| C1—N1—C5 | 119.3 (5) | C6—C7—C8 | 118.8 (6) |
| C1—N1—Pd1 | 126.5 (4) | C6—C7—H7 | 122 (4) |
| C5—N1—Pd1 | 114.2 (3) | C8—C7—H7 | 119 (4) |
| C10—N2—C6 | 118.9 (4) | C9—C8—C7 | 120.2 (6) |
| C10—N2—Pd1 | 126.6 (4) | C9—C8—H8 | 118 (5) |
| C6—N2—Pd1 | 114.4 (3) | C7—C8—H8 | 121 (5) |
| N1—C1—C2 | 121.4 (6) | C8—C9—C10 | 118.5 (6) |
| N1—C1—H1 | 116 (3) | C8—C9—H9 | 124 (3) |
| C2—C1—H1 | 123 (3) | C10—C9—H9 | 117 (3) |
| C3—C2—C1 | 119.7 (6) | N2—C10—C9 | 122.2 (6) |
| C3—C2—H2 | 118 (4) | N2—C10—H10 | 118 (3) |
| C1—C2—H2 | 122 (4) | C9—C10—H10 | 119 (3) |
| C2—C3—C4 | 119.2 (6) | Cl4—C11—Cl3 | 111.0 (3) |
| C2—C3—H3 | 124 (3) | Cl4—C11—H11A | 111 (4) |
| C4—C3—H3 | 117 (3) | Cl3—C11—H11A | 106 (4) |
| C5—C4—C3 | 119.8 (5) | Cl4—C11—H11B | 106 (3) |
| C5—C4—H4 | 118 (3) | Cl3—C11—H11B | 103 (4) |
| C3—C4—H4 | 123 (3) | H11A—C11—H11B | 120 (5) |
| N2—Pd1—N1—C1 | −177.3 (4) | C3—C4—C5—N1 | 0.8 (8) |
| Cl2—Pd1—N1—C1 | 4.6 (4) | C3—C4—C5—C6 | −178.8 (5) |
| N2—Pd1—N1—C5 | 2.4 (3) | C10—N2—C6—C7 | 1.7 (8) |
| Cl2—Pd1—N1—C5 | −175.7 (3) | Pd1—N2—C6—C7 | −175.3 (4) |
| N1—Pd1—N2—C10 | 179.8 (5) | C10—N2—C6—C5 | −179.1 (4) |
| Cl1—Pd1—N2—C10 | −0.3 (5) | Pd1—N2—C6—C5 | 4.0 (5) |
| N1—Pd1—N2—C6 | −3.5 (3) | N1—C5—C6—N2 | −2.0 (6) |
| Cl1—Pd1—N2—C6 | 176.4 (3) | C4—C5—C6—N2 | 177.6 (5) |
| C5—N1—C1—C2 | 1.2 (8) | N1—C5—C6—C7 | 177.3 (5) |
| Pd1—N1—C1—C2 | −179.2 (4) | C4—C5—C6—C7 | −3.1 (8) |
| N1—C1—C2—C3 | −1.3 (9) | N2—C6—C7—C8 | −1.0 (9) |
| C1—C2—C3—C4 | 1.1 (9) | C5—C6—C7—C8 | 179.8 (6) |
| C2—C3—C4—C5 | −0.8 (9) | C6—C7—C8—C9 | 0.7 (11) |
| C1—N1—C5—C4 | −0.9 (7) | C7—C8—C9—C10 | −1.2 (11) |
| Pd1—N1—C5—C4 | 179.4 (4) | C6—N2—C10—C9 | −2.2 (9) |
| C1—N1—C5—C6 | 178.7 (4) | Pd1—N2—C10—C9 | 174.4 (5) |
| Pd1—N1—C5—C6 | −1.0 (5) | C8—C9—C10—N2 | 1.9 (10) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1···Cl2 | 0.93 (5) | 2.59 (5) | 3.230 (6) | 126 (4) |
| C2—H2···Cl2i | 0.93 (5) | 2.79 (5) | 3.595 (7) | 145 (4) |
| C10—H10···Cl1 | 0.86 (5) | 2.68 (5) | 3.248 (6) | 125 (4) |
| C11—H11B···Cl1 | 0.99 (6) | 2.63 (6) | 3.578 (8) | 161 (5) |
Symmetry codes: (i) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KP2220).
References
- Bruker (2000). SADABS, SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Hambley, T. W. (1986). Acta Cryst. C42, 49–51.
- Kim, N.-H., Hwang, I.-C. & Ha, K. (2009). Acta Cryst. E65, m180. [DOI] [PMC free article] [PubMed]
- Maekawa, M., Munakata, M., Kitagawa, S. & Nakamura, M. (1991). Anal. Sci.7, 521–522.
- Momeni, B. Z., Hamzeh, S., Hosseini, S. S. & Rominger, F. (2007). Inorg. Chim. Acta, 360, 2661–2668.
- Osborn, R. S. & Rogers, D. (1974). J. Chem. Soc. Daton Trans. pp. 1002–1004.
- Sartori, D. A., Hurst, S. K., Wood, N., Larsen, R. D. & Abbott, E. H. (2005). J. Chem. Crystallogr.35, 995–998.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Smeets, W. J. J., Spek, A. L., Hoare, J. L., Canty, A. J., Hovestad, N. & van Koten, G. (1997). Acta Cryst. C53, 1045–1047.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Vicente, J., Abad, J. A., Rink, B. & Arellano, M. C. R. (1997). Private communication (refcode PYCXMN02). CCDC, Cambridge, England.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809016262/kp2220sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809016262/kp2220Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report