Abstract
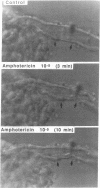
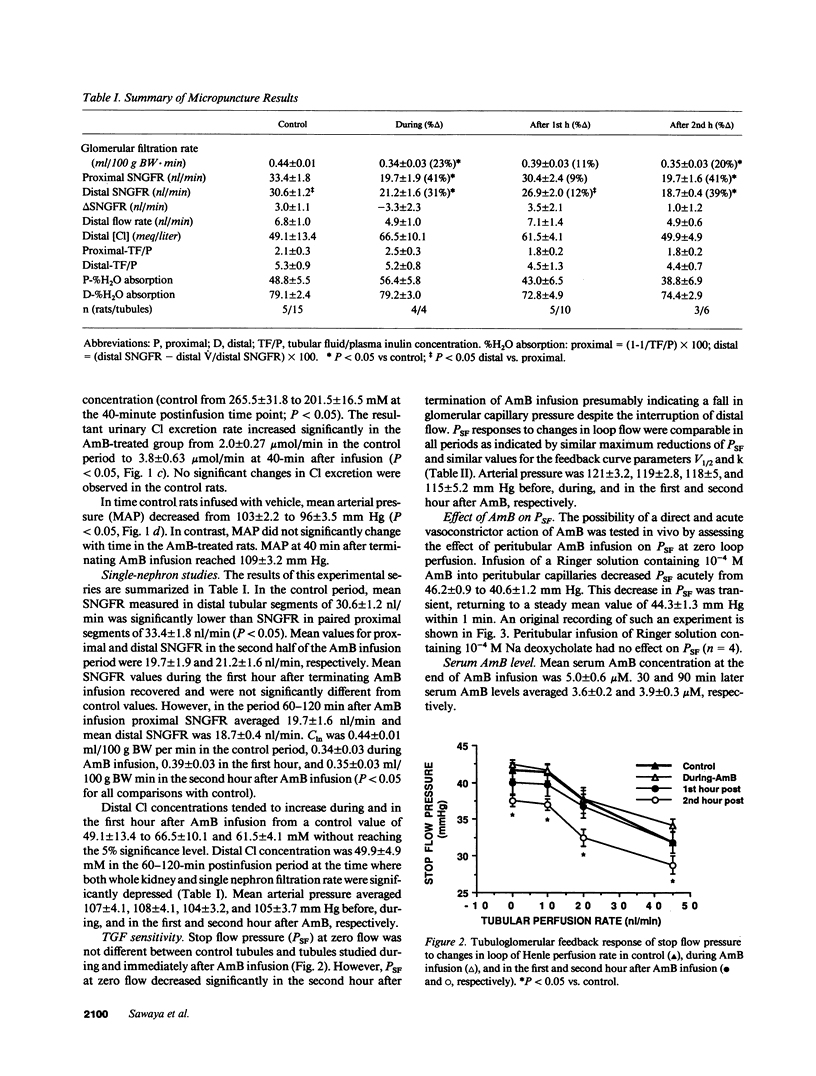
In anesthetized rats we tested the hypothesis that amphotericin B (AmB) reduces glomerular filtration rate (GFR) by activating the tubuloglomerular feedback (TGF) mechanism. Infusion of 1 mg/kg AmB over 50 min was followed by a reduction in kidney GFR (from 0.47 +/- 0.03 to 0.39 +/- 0.02 ml/min per 100 g body wt during the second hour after infusion; P less than 0.05) and by an increase in urine flow and urinary chloride excretion. Single-nephron GFR (SNGFR) measured in proximal (TGF interrupted) or distal tubules (TGF intact) decreased to a similar degree from 33.4 +/- 1.8 and 30.6 +/- 1.2 nl/min in the control period to 19.7 +/- 1.9 and 21.2 +/- 1.6 nl/min during the second hour after AmB infusion (P less than 0.05). Distal chloride concentrations and TGF responses to changes in loop of Henle flow rate were not significantly altered by AmB. AmB at 10(-5) M reduced the diameter of isolated perfused afferent arterioles from rabbit kidneys. In isometrically contracting rings of rabbit aorta and renal artery in vitro AmB produced endothelium-independent constriction, with half-maximal contraction (EC50) being achieved by 1.8 x 10(-6) and 2.6 x 10(-6) M in intact vessels and 1.3 x 10(-6) and 1.7 x 10(-6) M in endothelium-denuded vessels respectively. Tension development did not occur in Ca-free media or in the presence of Ca channel blockers. Pretreatment with ouabain or Bay K 8644 potentiated the effect of AmB. The vasoconstrictive effect of AmB was counteracted by aminophylline and atrial natriuretic peptide. We conclude that the AmB-induced reduction in GFR is not caused by TGF activation and that AmB has a direct vasoconstrictor effect that is probably initiated by depolarization-induced opening of Ca channels. This effect may be an important component of the nephrotoxic actions of AmB.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Monahan M. The interaction of polyene antibiotics with thin lipid membranes. J Gen Physiol. 1968 Aug;52(2):300–325. doi: 10.1085/jgp.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL N. H., ANDRIOLE V. T., SABESIN S. M., UTZ J. P. On the nephrotoxicity of amphotericin B in man. Am J Med. 1962 Jul;33:64–69. doi: 10.1016/0002-9343(62)90277-2. [DOI] [PubMed] [Google Scholar]
- BUTLER W. T., BENNETT J. E., ALLING D. W., WERTLAKE P. T., UTZ J. P., HILL G. J., 2nd NEPHROTOXICITY OF AMPHOTERICIN B; EARLY AND LATE EFFECTS IN 81 PATIENTS. Ann Intern Med. 1964 Aug;61:175–187. doi: 10.7326/0003-4819-61-2-175. [DOI] [PubMed] [Google Scholar]
- BUTLER W. T., HILL G. J., 2nd, SZWED C. F., KNIGHT V. AMPHOTERICIN B RENAL TOXICITY IN THE DOG. J Pharmacol Exp Ther. 1964 Jan;143:47–56. [PubMed] [Google Scholar]
- Bullock W. E., Luke R. G., Nuttall C. E., Bhathena D. Can mannitol reduce amphotericin B nephrotoxicity? Double-blind study and description of a new vascular lesion in kidneys. Antimicrob Agents Chemother. 1976 Sep;10(3):555–563. doi: 10.1128/aac.10.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G., Schuetz H., Vickermann B., Kinne R. Amphotericin B and amphotericin B methylester: effect on brush border membrane permeability. Kidney Int. 1986 Sep;30(3):311–317. doi: 10.1038/ki.1986.186. [DOI] [PubMed] [Google Scholar]
- Cheng J. T., Witty R. T., Robinson R. R., Yarger W. E. Amphotericin B nephrotoxicity: increased renal resistance and tubule permeability. Kidney Int. 1982 Dec;22(6):626–633. doi: 10.1038/ki.1982.221. [DOI] [PubMed] [Google Scholar]
- Coulombe A., Schanne O. F., Reisin I., Ruiz-Ceretti E. Effects of amphotericin B on the electrical properties and electrolyte content of frog sartorius muscle. Can J Physiol Pharmacol. 1980 Sep;58(9):1138–1141. doi: 10.1139/y80-172. [DOI] [PubMed] [Google Scholar]
- Deth R. C., Payne R. A., Peecher D. M. Influence of furosemide on rubidium-86 uptake and alpha-adrenergic responsiveness of arterial smooth muscle. Blood Vessels. 1987;24(6):321–333. doi: 10.1159/000158709. [DOI] [PubMed] [Google Scholar]
- Fields B. T., Jr, Bates J. H., Abernathy R. S. Amphotericin B serum concentrations during therapy. Appl Microbiol. 1970 Jun;19(6):955–959. doi: 10.1128/am.19.6.955-959.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkens J. F., Branch R. A. The influence of sodium status and furosemide on canine acute amphotericin B nephrotoxicity. J Pharmacol Exp Ther. 1980 Aug;214(2):306–311. [PubMed] [Google Scholar]
- Gerkens J. F., Heidemann H. T., Jackson E. K., Branch R. A. Effect of aminophylline on amphotericin B nephrotoxicity in the dog. J Pharmacol Exp Ther. 1983 Mar;224(3):609–613. [PubMed] [Google Scholar]
- Gouge T. H., Andriole V. T. An experimental model of amphotericin B nephrotoxicity with renal tubular acidosis. J Lab Clin Med. 1971 Nov;78(5):713–724. [PubMed] [Google Scholar]
- Greger R., Hampel W. A modified system for in vitro perfusion of isolated renal tubules. Pflugers Arch. 1981 Jan;389(2):175–176. doi: 10.1007/BF00582110. [DOI] [PubMed] [Google Scholar]
- Heidemann H. T., Gerkens J. F., Jackson E. K., Branch R. A. Effect of aminophylline on renal vasoconstriction produced by amphotericin B in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1983 Sep;324(2):148–152. doi: 10.1007/BF00497021. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Nagel W., Fisher R. S. Ouabain on active transepithelial sodium transport in frog skin: studies with microelectrodes. J Gen Physiol. 1979 Jul;74(1):105–127. doi: 10.1085/jgp.74.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Loutzenhiser R., Rüegg U. T., Hof A., Hof R. P. Studies on the mechanism of action of the vasoconstrictive dihydropyridine, CGP 28392. Eur J Pharmacol. 1984 Oct 15;105(3-4):229–237. doi: 10.1016/0014-2999(84)90614-9. [DOI] [PubMed] [Google Scholar]
- McCurdy D. K., Frederic M., Elkinton J. R. Renal tubular acidosis due to amphotericin B. N Engl J Med. 1968 Jan 18;278(3):124–130. doi: 10.1056/NEJM196801182780302. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. K., Katz M. A., Michael U. F., Ogden D. A. Mechanisms of hemodynamic actions of furosemide: differentiation of vascular and renal effects on blood pressure in functionally anephric hypertensive patients. Am Heart J. 1981 Mar;101(3):313–318. doi: 10.1016/0002-8703(81)90196-4. [DOI] [PubMed] [Google Scholar]
- Müller-Suur R., Gutsche H. U., Schurek H. J. Acute and reversible inhibition of tubuloglomerular feedback mediated afferent vasoconstriction by the calcium-antagonist verapamil. Curr Probl Clin Biochem. 1976;6:291–298. [PubMed] [Google Scholar]
- Osswald H., Nabakowski G., Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem. 1980;12(1-2):263–267. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Persson A. E., Schnermann J., Wright F. S. Modification of feedback influence on glomerular filtration rate by acute isotonic extracellular volume expansion. Pflugers Arch. 1979 Aug;381(2):99–105. doi: 10.1007/BF00582339. [DOI] [PubMed] [Google Scholar]
- Ramos H., Attias de Murciano A., Cohen B. E., Bolard J. The polyene antibiotic amphotericin B acts as a Ca2+ ionophore in sterol-containing liposomes. Biochim Biophys Acta. 1989 Jul 10;982(2):303–306. doi: 10.1016/0005-2736(89)90069-2. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Thompson W. L. Dopamine and saralasin antagonism of renal vasoconstriction and oliguria caused by amphotericin B in dogs. J Infect Dis. 1979 Oct;140(4):564–575. doi: 10.1093/infdis/140.4.564. [DOI] [PubMed] [Google Scholar]
- Reuss L. Effects of amphotericin b on the electrical properties of Necturus gallbladder: intracellular microelectrode studies. J Membr Biol. 1978 Jun 22;41(1):65–86. doi: 10.1007/BF01873340. [DOI] [PubMed] [Google Scholar]
- Sabra R., Takahashi K., Branch R. A., Badr K. F. Mechanisms of amphotericin B-induced reduction of the glomerular filtration rate: a micropuncture study. J Pharmacol Exp Ther. 1990 Apr;253(1):34–37. [PubMed] [Google Scholar]
- Schnermann J., Briggs J. P. Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol. 1989 Mar;256(3 Pt 2):F421–F429. doi: 10.1152/ajprenal.1989.256.3.F421. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R., Lawson L. R. Effect of luminal pH on ion permeability and flows of Na+and H+ in turtle bladder. Am J Physiol. 1971 Jun;220(6):1573–1580. doi: 10.1152/ajplegacy.1971.220.6.1573. [DOI] [PubMed] [Google Scholar]
- Thomas G., Chung M., Cohen C. J. A dihydropyridine (Bay k 8644) that enhances calcium currents in guinea pig and calf myocardial cells. A new type of positive inotropic agent. Circ Res. 1985 Jan;56(1):87–96. doi: 10.1161/01.res.56.1.87. [DOI] [PubMed] [Google Scholar]
- Tolins J. P., Raij L. Adverse effect of amphotericin B administration on renal hemodynamics in the rat. Neurohumoral mechanisms and influence of calcium channel blockade. J Pharmacol Exp Ther. 1988 May;245(2):594–599. [PubMed] [Google Scholar]
- Tolins J. P., Raij L. Chronic amphotericin B nephrotoxicity in the rat, protective effect of prophylactic salt loading. Am J Kidney Dis. 1988 Apr;11(4):313–317. doi: 10.1016/s0272-6386(88)80136-7. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974 Jun;53(6):1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]