Abstract
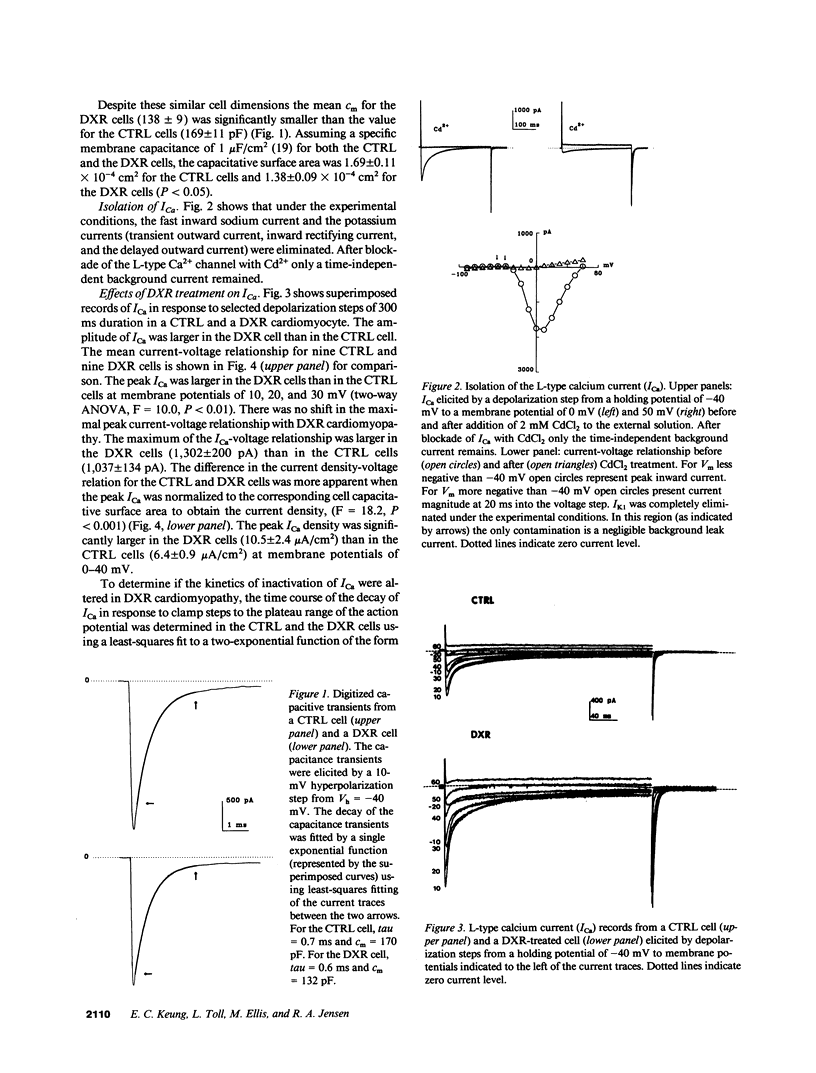
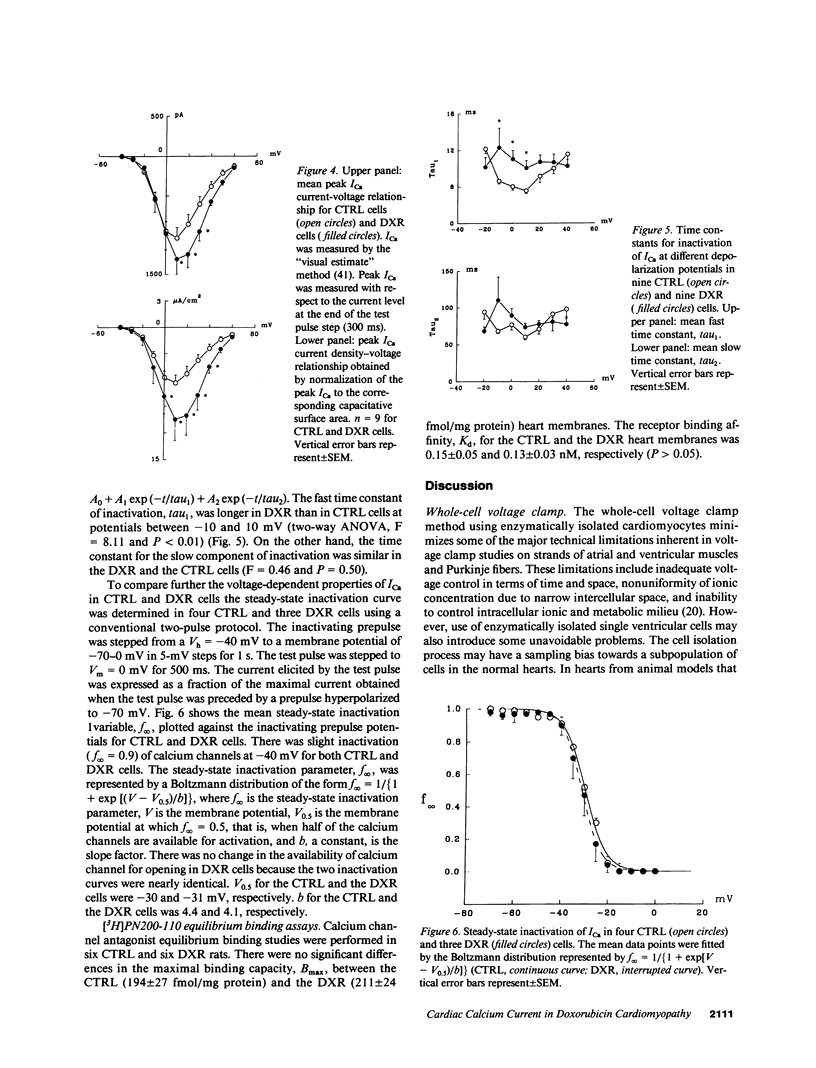
Doxorubicin (DXR) is an effective antitumor agent in a wide spectrum of neoplasms. Chronic treatment is associated with cardiomyopathy and characteristic myocardial ultrastructural changes, which include swelling of the t tubules. Accordingly, we investigated excitation-contraction coupling in cardiomyopathic rat heart resulting from chronic DXR treatment. Using the whole-cell patch clamp technique, we studied the L-type calcium channel in single cells enzymatically isolated from normal (CTRL) and DXR rat hearts. Despite similar cell dimensions, the total membrane capacitance was significantly smaller in the DXR cells (138 +/- 9 pF) than in the CTRL cells (169 +/- 11 pF) (mean +/- SEM, n = 9, P less than 0.05). The mean current and the current density-voltage relationships of the CTRL and the DXR cells were significantly different (n = 9, P less than 0.001) with the maximal peak L-type calcium current (ICa) density increased from 6.4 +/- 0.9 in CTRL cells to 10.5 +/- 2.4 microA/cm2 in the DXR cells (P less than 0.05). There was no shift either in the current-voltage relationship or the steady-state inactivation curve in the two cell groups. However, the fast time constant of inactivation was increased at a membrane voltage of -10 to 10 mV. Calcium channel antagonist equilibrium binding assays using [3H]-PN200-110 revealed no difference in the maximal receptor binding capacity (CTRL, 194 +/- 27 and DXR 211 +/- 24 fmol/mg protein; P greater than 0.05, n = 6) and in receptor affinity (CTRL, 0.15 +/- 0.05 and DXR 0.13 +/- 0.03 nM; P less than 0.05). These data suggest that a decrease in effective capacitance might be associated with t-tubular damage. Despite this decrease, ICa was increased in the DXR cells. Such an increase may result from an alteration in the properties of the calcium channels and/or recruitment of "hibernating" channels in the remaining surface and t-tubular membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. J., Buck E., Salama G., Casida J. E., Pessah I. N. Mechanism of anthraquinone-induced calcium release from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1988 Dec 15;263(35):18750–18758. [PubMed] [Google Scholar]
- Andrawis N. S., Kuo T. H., Giacomelli F., Wiener J. Altered calcium regulation in the cardiac plasma membrane in experimental renal hypertension. J Mol Cell Cardiol. 1988 Jul;20(7):625–634. doi: 10.1016/s0022-2828(88)80120-2. [DOI] [PubMed] [Google Scholar]
- Cameron J. S., Kimura S., Jackson-Burns D. A., Smith D. B., Bassett A. L. ATP-sensitive K+ channels are altered in hypertrophied ventricular myocytes. Am J Physiol. 1988 Nov;255(5 Pt 2):H1254–H1258. doi: 10.1152/ajpheart.1988.255.5.H1254. [DOI] [PubMed] [Google Scholar]
- Chatelain P., Demol D., Roba J. Comparison of [3H]nitrendipine binding to heart membranes of normotensive and spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1984 Mar-Apr;6(2):220–223. doi: 10.1097/00005344-198403000-00002. [DOI] [PubMed] [Google Scholar]
- Dillon J. S., Gu X. H., Nayler W. G. Effect of age and of hypertrophy on cardiac Ca2+ antagonist binding sites. J Cardiovasc Pharmacol. 1989 Aug;14(2):233–240. doi: 10.1097/00005344-198908000-00008. [DOI] [PubMed] [Google Scholar]
- Ehlert F. J., Roeske W. R., Itoga E., Yamamura H. I. The binding of [3H]nitrendipine to receptors for calcium channel antagonists in the heart, cerebral cortex, and ileum of rats. Life Sci. 1982 Jun 21;30(25):2191–2202. doi: 10.1016/0024-3205(82)90293-4. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Marks E. S., Patterson R. E., Speir E. H., Steadman K. A., Keiser H. R. Correlation of changes in cardiac calcium channels with hemodynamics in Syrian hamster cardiomyopathy and heart failure. Life Sci. 1987 Jul 13;41(2):153–159. doi: 10.1016/0024-3205(87)90488-7. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials, afterpotentials, and excitation-contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol. 1969 Mar;53(3):298–310. doi: 10.1085/jgp.53.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green F. J., Farmer B. B., Wiseman G. L., Jose M. J., Watanabe A. M. Effect of membrane depolarization on binding of [3H]nitrendipine to rat cardiac myocytes. Circ Res. 1985 Apr;56(4):576–585. doi: 10.1161/01.res.56.4.576. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Howell J. N. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969 May;201(3):515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett S. E., Rafuse V. F., Gordon T. [3H]-nitrendipine binding sites in normal and cardiomyopathic hamsters: absence of a selective increase in putative calcium channels in cardiomyopathic hearts. Cardiovasc Res. 1988 Nov;22(11):840–846. doi: 10.1093/cvr/22.11.840. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Acton E. M., Peters J. H. Doxorubicin cardiotoxicity in the rat: comparison of electrocardiogram, transmembrane potential, and structural effects. J Cardiovasc Pharmacol. 1984 Jan-Feb;6(1):186–200. [PubMed] [Google Scholar]
- Jensen R. A., Acton E. M., Peters J. H. Electrocardiographic and transmembrane potential effects of 5-iminodaunorubicin in the rat. Cancer Res. 1984 Sep;44(9):4030–4039. [PubMed] [Google Scholar]
- Jensen R. A. Doxorubicin cardiotoxicity: contractile changes after long-term treatment in the rat. J Pharmacol Exp Ther. 1986 Jan;236(1):197–203. [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Keung E. C. Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circ Res. 1989 Apr;64(4):753–763. doi: 10.1161/01.res.64.4.753. [DOI] [PubMed] [Google Scholar]
- Kleiman R. B., Houser S. R. Outward currents in normal and hypertrophied feline ventricular myocytes. Am J Physiol. 1989 May;256(5 Pt 2):H1450–H1461. doi: 10.1152/ajpheart.1989.256.5.H1450. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. S., Hume J. R., Giles W., Brown A. M. Sodium current depression by lidocaine and quinidine in isolated ventricular cells. Nature. 1981 May 28;291(5813):325–327. doi: 10.1038/291325a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoux E., Callens F., Swynghedauw B., Charlemagne D. Adaptational process of the cardiac Ca2+ channels to pressure overload: biochemical and physiological properties of the dihydropyridine receptors in normal and hypertrophied rat hearts. J Cardiovasc Pharmacol. 1988 Oct;12(4):390–396. doi: 10.1097/00005344-198810000-00003. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Cavalié A., Trautwein W., Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986 May;406(5):437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Dillon J. S., Elz J. S., McKelvie M. An effect of ischemia on myocardial dihydropyridine binding sites. Eur J Pharmacol. 1985 Sep 10;115(1):81–89. doi: 10.1016/0014-2999(85)90587-4. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., deBruyn Kops A., Dzau V. J. Identification of two calcium channel receptor sites for [3H]nitrendipine in mammalian cardiac and smooth muscle membrane. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7452–7456. doi: 10.1073/pnas.83.19.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. H., Hemwall E. L., Marino T. A., Houser S. R. Isolation and morphology of calcium-tolerant feline ventricular myocytes. Am J Physiol. 1983 Nov;245(5 Pt 1):H891–H896. doi: 10.1152/ajpheart.1983.245.5.H891. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Deally C. M., Weinberg L. E. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987 Aug;19(8):817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Reynolds I. J., Weisman H. F., Dudeck P., Weisfeldt M. L., Snyder S. H. Calcium antagonist receptors in cardiomyopathic hamster: selective increases in heart, muscle, brain. Science. 1986 Apr 25;232(4749):515–518. doi: 10.1126/science.3008330. [DOI] [PubMed] [Google Scholar]



