Abstract
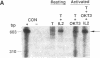
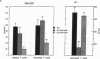
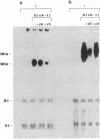
Most biologic responses to IL-2 have been attributed to interaction of IL-2 with a high affinity receptor which consists of a heterodimer composed of two distinct IL-2-binding proteins (IL-2R alpha/IL-2R beta). However, both low affinity IL-2R alpha (55 kD) and intermediate affinity IL-2R beta (70-75 kD) also appear to be expressed independently on the cell surface. We investigated the receptor-specific regulatory effects of IL-2 on cytokine production in unstimulated and activated T cells. T cells were activated by stimulation of the antigen receptor complex with anti-CD3 mAb. IL-2 (10(2) U/ml, 1 nM) stimulation of resting cells resulted in a fivefold increase in GM-CSF release but in only minimal IFN-gamma release. IL-2 markedly augmented mRNA expression of GM-CSF but not IFN-gamma in unstimulated T cells. IL-2R beta mAb but not IL-2R alpha mAb decreased IL-2-induced GM-CSF release and mRNA expression from unstimulated T cells. IL-2 concentrations required for GM-CSF release from resting cells suggested ligand binding to an intermediate affinity receptor. GM-CSF and IFN-gamma release from activated T cells increased four- to fivefold in response to 1 nM IL-2 and IL-2 augmented both GM-CSF and IFN-gamma mRNA. IL-2R beta mAb but not IL-2R alpha mAb reduced GM-CSF release and mRNA expression in activated T cells stimulated with 1 nM IL-2. IL-2R alpha blockade markedly decreased IL-2-induced IFN-gamma release and mRNA expression from activated cells, while IL-2R beta blockade had little effect on IFN-gamma production in activated cells. IL-2R alpha blockade altered the affinity of the receptor mediating activated cell GM-CSF release from a high affinity to an intermediate affinity state. These studies indicate an independent role for IL-2R beta in mediating GM-CSF production from T cells. They also suggest that unstimulated and activated T cells, which express distinct IL-2 receptor moieties, mediate release of separate lymphokines and that different subunits of the IL-2 receptor may play an important role in the regulation of cytokine production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allouche M., Sahraoui Y., Augery-Bourget Y., Ohashi Y., Sugamura K., Jasmin C., Georgoulias V. Presence of a p70 IL-2-binding peptide on leukemic cells from various hemopoietic lineages. J Immunol. 1989 Oct 1;143(7):2223–2229. [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Berger S. L. Isolation of cytoplasmic RNA: ribonucleoside-vanadyl complexes. Methods Enzymol. 1987;152:227–234. doi: 10.1016/0076-6879(87)52024-9. [DOI] [PubMed] [Google Scholar]
- Bich-Thuy L. T., Dukovich M., Peffer N. J., Fauci A. S., Kehrl J. H., Greene W. C. Direct activation of human resting T cells by IL 2: the role of an IL 2 receptor distinct from the Tac protein. J Immunol. 1987 Sep 1;139(5):1550–1556. [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Burdach S. E., Levitt L. J. Receptor-specific inhibition of bone marrow erythropoiesis by recombinant DNA-derived interleukin-2. Blood. 1987 May;69(5):1368–1375. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M. Growth of human T lymphocytes: an analysis of interleukin 2 and its cellular receptor. Prog Hematol. 1986;14:283–301. [PubMed] [Google Scholar]
- Greene W. C. The human interleukin-2 receptor: a molecular and biochemical analysis of structure and function. Clin Res. 1987 Sep;35(5):439–450. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Gemmell L., Larson N., Luh J., Arai K., Rennick D. Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4360–4364. doi: 10.1073/pnas.82.13.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt L., Kipps T. J., Engleman E. G., Greenberg P. L. Human bone marrow and peripheral blood T lymphocyte depletion: efficacy and effects of both T cells and monocytes on growth of hematopoietic progenitors. Blood. 1985 Mar;65(3):663–679. [PubMed] [Google Scholar]
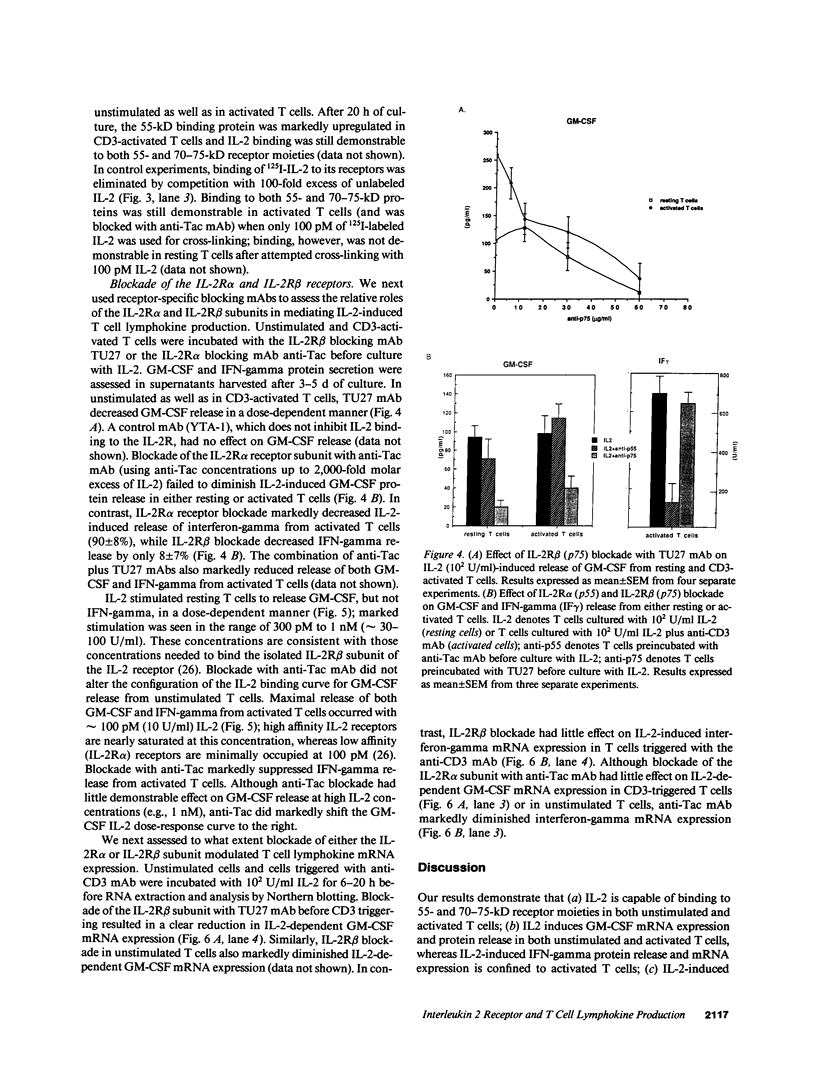
- Lowenthal J. W., Greene W. C. Contrasting interleukin 2 binding properties of the alpha (p55) and beta (p70) protein subunits of the human high-affinity interleukin 2 receptor. J Exp Med. 1987 Oct 1;166(4):1156–1161. doi: 10.1084/jem.166.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G. B., May C. Binding of interleukin 2 to its 75-kDa intermediate affinity receptor is sufficient to activate Na+/H+ exchange. J Immunol. 1987 Dec 15;139(12):4083–4087. [PubMed] [Google Scholar]
- Mills G. B., May C., McGill M., Fung M., Baker M., Sutherland R., Greene W. C. Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated. J Biol Chem. 1990 Feb 25;265(6):3561–3567. [PubMed] [Google Scholar]
- Nakamura Y., Inamoto T., Sugie K., Masutani H., Shindo T., Tagaya Y., Yamauchi A., Ozawa K., Yodoi J. Mitogenicity and down-regulation of high-affinity interleukin 2 receptor by YTA-1 and YTA-2, monoclonal antibodies that recognize 75-kDa molecules on human large granular lymphocytes. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1318–1322. doi: 10.1073/pnas.86.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Otsuka T., Miyajima A., Brown N., Otsu K., Abrams J., Saeland S., Caux C., de Waal Malefijt R., de Vries J., Meyerson P. Isolation and characterization of an expressible cDNA encoding human IL-3. Induction of IL-3 mRNA in human T cell clones. J Immunol. 1988 Apr 1;140(7):2288–2295. [PubMed] [Google Scholar]
- Owen C. S. Magnetic cell sorting using colloidal protein-magnetite. J Immunogenet. 1989 Apr;16(2):117–123. doi: 10.1111/j.1744-313x.1989.tb00453.x. [DOI] [PubMed] [Google Scholar]
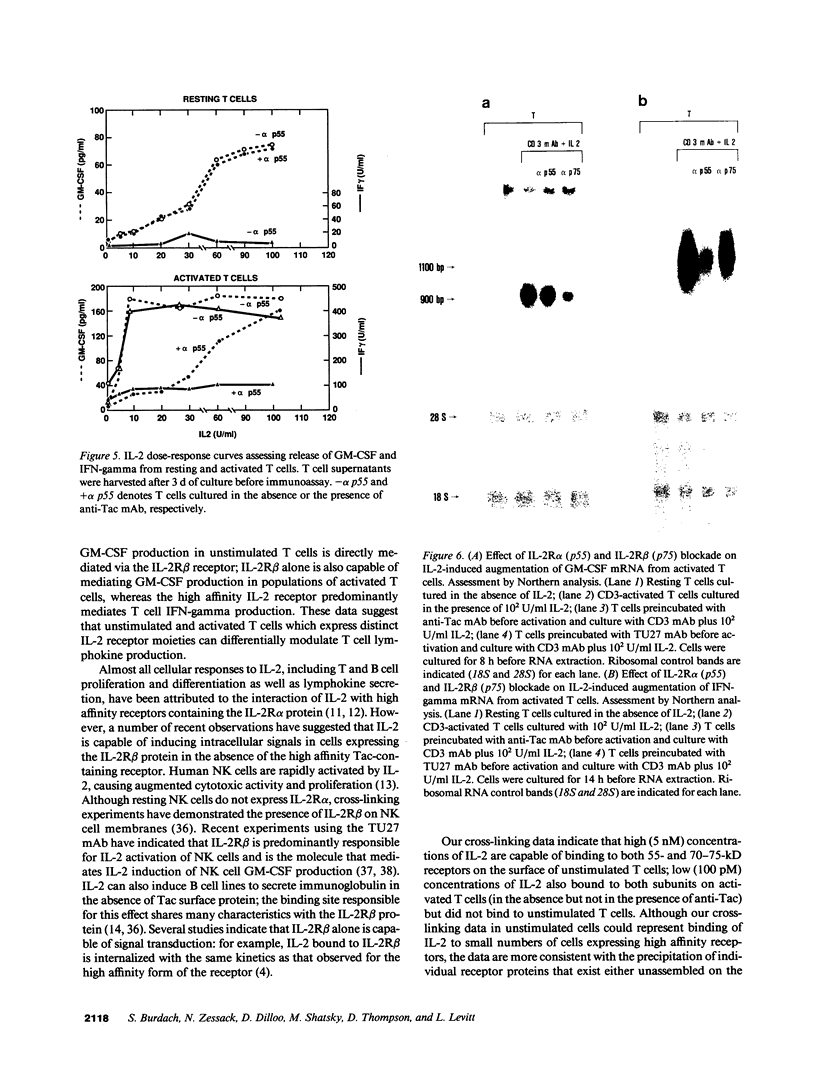
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Jeong G., Welte K., Mertelsmann R., Rabin H., Henderson L. E., Souza L. M., Boone T. C., Robb R. J. Stimulation of immunoglobulin secretion in human B lymphocytes as a direct effect of high concentrations of IL 2. J Immunol. 1984 Nov;133(5):2442–2445. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain P. L., Schlossman S. F. Human T lymphocyte subsets. Functional heterogeneity and surface recognition structures. J Clin Invest. 1984 Nov;74(5):1559–1565. doi: 10.1172/JCI111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Hoekzema G. S., Nelson D. L., Greene W. C., Jay G. Reconstitution of a functional interleukin 2 receptor in a nonlymphoid cell. J Immunol. 1987 Oct 1;139(7):2355–2360. [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sharon M., Siegel J. P., Tosato G., Yodoi J., Gerrard T. L., Leonard W. J. The human interleukin 2 receptor beta chain (p70). Direct identification, partial purification, and patterns of expression on peripheral blood mononuclear cells. J Exp Med. 1988 Mar 1;167(3):1265–1270. doi: 10.1084/jem.167.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kitamura F., Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1982–1986. doi: 10.1073/pnas.86.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita H., Nakata M., Azuma A., Nitta T., Takeshita T., Sugamura K., Okumura K. Activation of peripheral blood T cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Oct 1;170(4):1445–1450. doi: 10.1084/jem.170.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]