Abstract
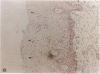
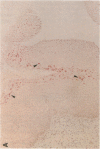
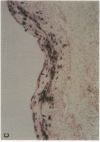
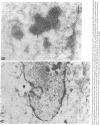
The role of human papillomavirus (HPV) proteins in the pathogenesis of cervical intra-epithelial neoplasia (CIN) and invasive cervical cancer is poorly understood. To characterize E4 protein expression in 49 paraffin-embedded cervical biopsies representing different histopathologic grades of disease, antibodies were elicited to a synthetic peptide corresponding to amino acids 20-34 of a protein predicted to be encoded by the HPV 16 E4 open reading frame. The E4 protein was detected throughout the spectrum of CIN, from CIN1 to CIN3. Expression was localized to the cell nucleus, primarily in the superficial layers of the squamous cervical epithelium. Ultrastructural studies showed that the E4 protein was organized into compact, intranuclear arrays 25-35 nm in diameter. E4 protein expression was also demonstrated in some histologically normal tissues containing HPV 16 DNA, but not in any of five cervical cancers containing HPV 16 DNA. These results suggest that E4 protein expression may precede development of light microscopic tissue abnormalities, that it may continue through the spectrum of CIN, and that expression of this protein may be reduced or terminated in invasive cancer. The function of this protein remains unknown, but its nuclear localization may be consistent with a role in viral maturation.
Full text
PDF


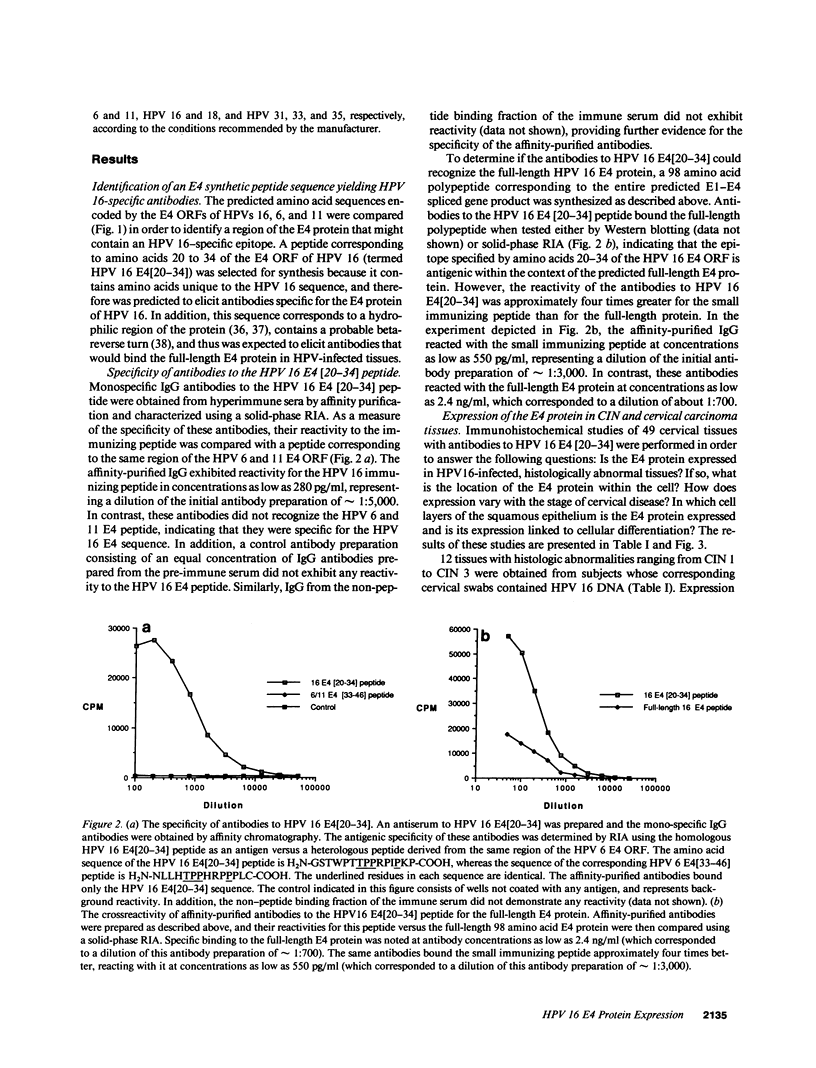
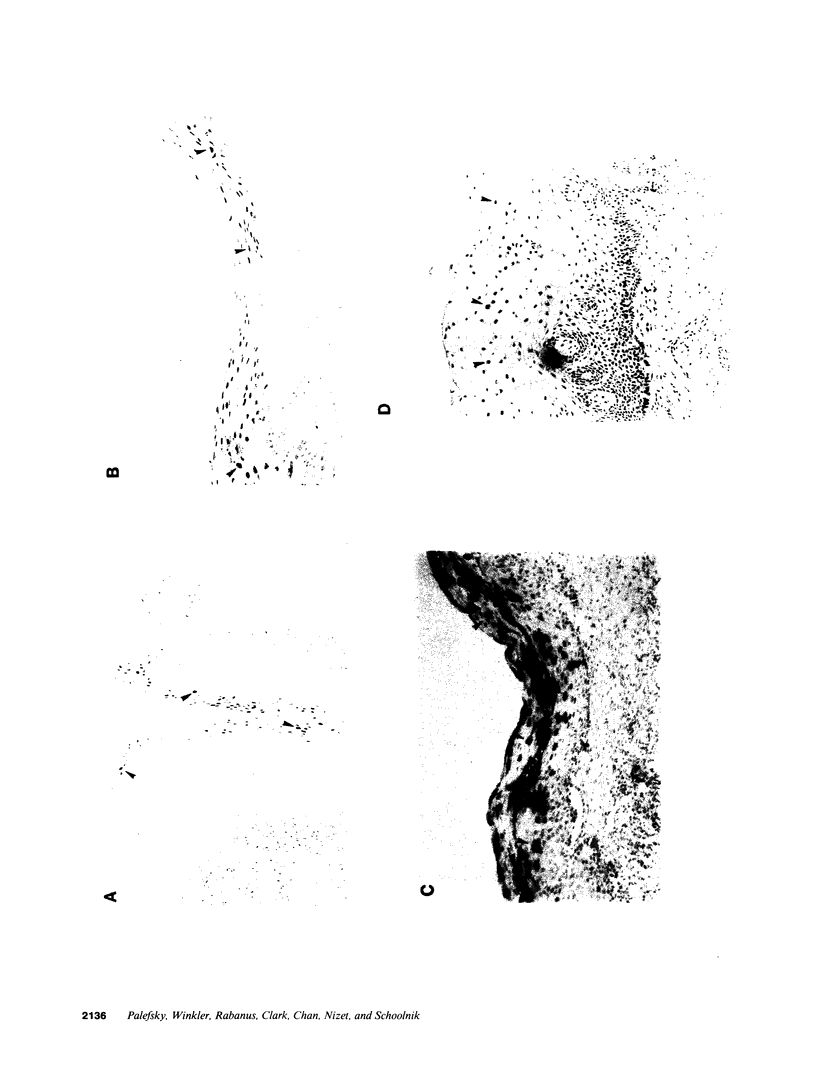
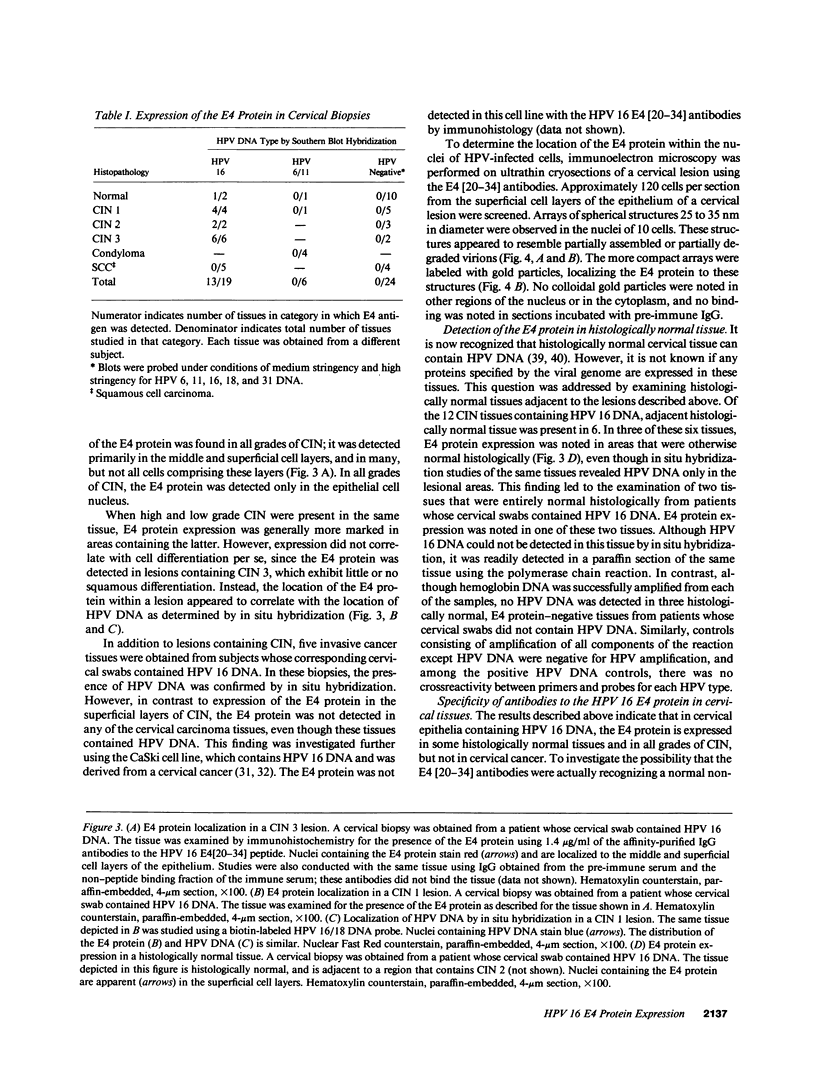
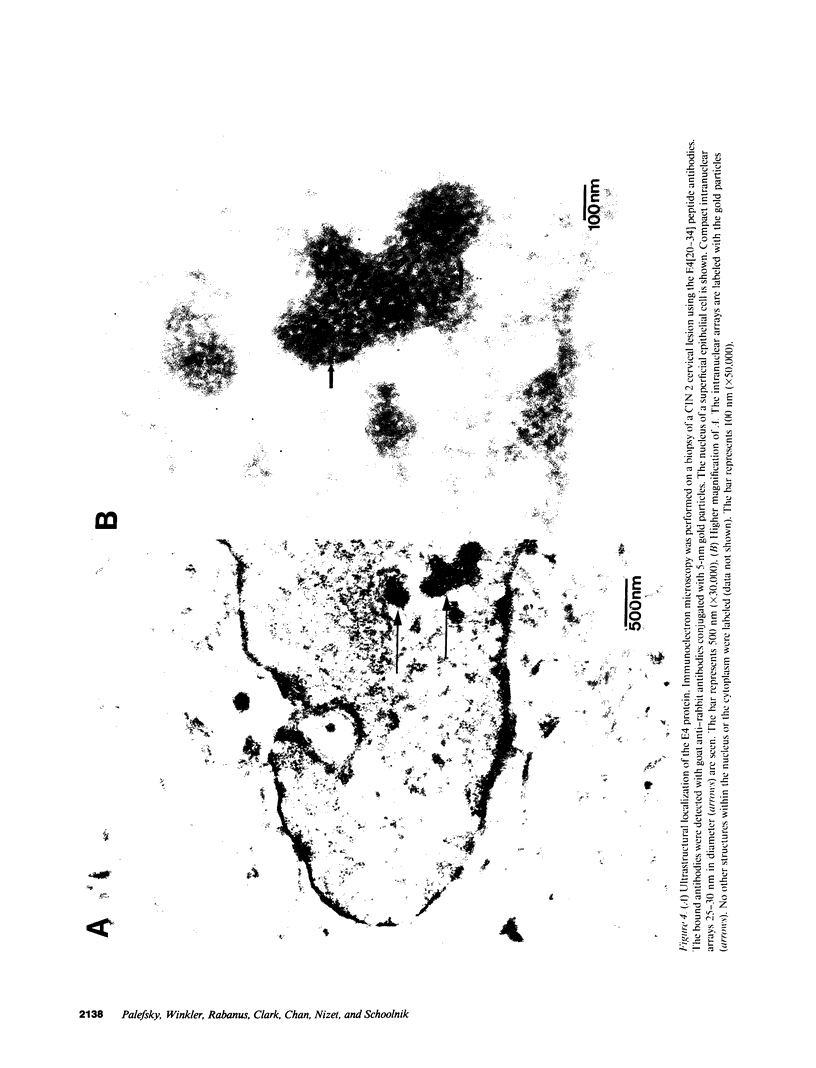



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudenon S., Kremsdorf D., Croissant O., Jablonska S., Wain-Hobson S., Orth G. A novel type of human papillomavirus associated with genital neoplasias. Nature. 1986 May 15;321(6067):246–249. doi: 10.1038/321246a0. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion M. J., McCance D. J., Cuzick J., Singer A. Progressive potential of mild cervical atypia: prospective cytological, colposcopic, and virological study. Lancet. 1986 Aug 2;2(8501):237–240. doi: 10.1016/s0140-6736(86)92067-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Condyloma acuminatum - United States, 1966-1981. MMWR Morb Mortal Wkly Rep. 1983 Jun 17;32(23):306–308. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Nasseri M., Wolinsky S. M., Broker T. R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987 Aug;61(8):2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Barber S., Symbula M., Snyder K., Saleh A. M., Roche J. K. Coexpression of the human papillomavirus type 16 E4 and L1 open reading frames in early cervical neoplasia. Virology. 1990 Sep;178(1):238–246. doi: 10.1016/0042-6822(90)90399-c. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Nuovo G., Friedman D., Silverstein S. J. Accumulation of RNA homologous to human papillomavirus type 16 open reading frames in genital precancers. J Virol. 1988 Jan;62(1):84–90. doi: 10.1128/jvi.62.1.84-90.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartmann K., Schwarz E., Gissmann L., zur Hausen H. The nucleotide sequence and genome organization of human papilloma virus type 11. Virology. 1986 May;151(1):124–130. doi: 10.1016/0042-6822(86)90110-8. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Campbell D., Grand R. J., Gallimore P. H. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 1986 Feb;5(2):355–362. doi: 10.1002/j.1460-2075.1986.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Croce C. M., Gissmann L., Schwarz E., Huebner K. Papillomavirus sequences integrate near cellular oncogenes in some cervical carcinomas. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1070–1074. doi: 10.1073/pnas.84.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczy A., Mitao M., Nagai N., Silverstein S. J., Crum C. P. Latent papillomavirus and recurring genital warts. N Engl J Med. 1985 Sep 26;313(13):784–788. doi: 10.1056/NEJM198509263131304. [DOI] [PubMed] [Google Scholar]
- Firzlaff J. M., Kiviat N. B., Beckmann A. M., Jenison S. A., Galloway D. A. Detection of human papillomavirus capsid antigens in various squamous epithelial lesions using antibodies directed against the L1 and L2 open reading frames. Virology. 1988 Jun;164(2):467–477. doi: 10.1016/0042-6822(88)90561-2. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Girardi F., Pfister H. Human papillomavirus DNA in normal, metaplastic, preneoplastic and neoplastic epithelia of the cervix uteri. Int J Cancer. 1988 Jan 15;41(1):41–45. doi: 10.1002/ijc.2910410109. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Kocks C., L'Age-Stehr J., Reupke H. Comparative immunoelectron microscopy with monoclonal antibodies on yellow fever virus-infected cells: pre-embedding labelling versus immunocryoultramicrotomy. J Virol Methods. 1985 Mar;10(3):225–239. doi: 10.1016/0166-0934(85)90063-1. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Boshart M., Dürst M., Ikenberg H., Wagner D., zur Hausen H. Presence of human papillomavirus in genital tumors. J Invest Dermatol. 1984 Jul;83(1 Suppl):26s–28s. doi: 10.1111/1523-1747.ep12281143. [DOI] [PubMed] [Google Scholar]
- Griffiths G., McDowall A., Back R., Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984 Oct;89(1):65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd A. K., Schoolnik G. K. Peptides: chemistry, biology, and pharmacology. Adv Pharmacol. 1990;21:221–285. doi: 10.1016/s1054-3589(08)60344-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Howley P. M. Bovine papillomavirus type 1 E1 replication-defective mutants are altered in their transcriptional regulation. J Virol. 1988 Nov;62(11):4009–4015. doi: 10.1128/jvi.62.11.4009-4015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. A simplified representation of protein conformations for rapid simulation of protein folding. J Mol Biol. 1976 Jun 14;104(1):59–107. doi: 10.1016/0022-2836(76)90004-8. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Lancaster W. D., Temple G. F. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J Virol. 1986 Apr;58(1):225–229. doi: 10.1128/jvi.58.1.225-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A. T., Quinn A. P., Lancaster W. D., Temple G. F. A new type of papillomavirus associated with cancer of the uterine cervix. Virology. 1987 Jul;159(1):187–190. doi: 10.1016/0042-6822(87)90366-7. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Genetic analysis of bovine papillomavirus type 1 trans-acting replication factors. J Virol. 1985 Mar;53(3):955–965. doi: 10.1128/jvi.53.3.955-965.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab J. C., Walkinshaw S. A., Cordiner J. W., Clements J. B. Human papillomavirus in clinically and histologically normal tissue of patients with genital cancer. N Engl J Med. 1986 Oct 23;315(17):1052–1058. doi: 10.1056/NEJM198610233151703. [DOI] [PubMed] [Google Scholar]
- Moskaluk C., Bastia D. The E2 "gene" of bovine papillomavirus encodes an enhancer-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1215–1218. doi: 10.1073/pnas.84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash J. D., Burke T. W., Hoskins W. J. Biologic course of cervical human papillomavirus infection. Obstet Gynecol. 1987 Feb;69(2):160–162. [PubMed] [Google Scholar]
- Neary K., DiMaio D. Open reading frames E6 and E7 of bovine papillomavirus type 1 are both required for full transformation of mouse C127 cells. J Virol. 1989 Jan;63(1):259–266. doi: 10.1128/jvi.63.1.259-266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary K., Horwitz B. H., DiMaio D. Mutational analysis of open reading frame E4 of bovine papillomavirus type 1. J Virol. 1987 Apr;61(4):1248–1252. doi: 10.1128/jvi.61.4.1248-1252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Reid R., Greenberg M., Jenson A. B., Husain M., Willett J., Daoud Y., Temple G., Stanhope C. R., Sherman A. I., Phibbs G. D. Sexually transmitted papillomaviral infections. I. The anatomic distribution and pathologic grade of neoplastic lesions associated with different viral types. Am J Obstet Gynecol. 1987 Jan;156(1):212–222. doi: 10.1016/0002-9378(87)90241-9. [DOI] [PubMed] [Google Scholar]
- Rosenbert M. J., Schulz K. F., Burton N. Sexually transmitted diseases in sub-Saharan Africa. A priority list based on Family Health International's Meeting. Lancet. 1986 Jul 19;2(8499):152–153. doi: 10.1016/s0140-6736(86)91958-6. [DOI] [PubMed] [Google Scholar]
- Sadeghi S. B., Hsieh E. W., Gunn S. W. Prevalence of cervical intraepithelial neoplasia in sexually active teenagers and young adults. Results of data analysis of mass Papanicolaou screening of 796,337 women in the United States in 1981. Am J Obstet Gynecol. 1984 Mar 15;148(6):726–729. doi: 10.1016/0002-9378(84)90555-6. [DOI] [PubMed] [Google Scholar]
- Schmidt M. A., O'Hanley P., Schoolnik G. K. Gal-Gal pyelonephritis Escherichia coli pili linear immunogenic and antigenic epitopes. J Exp Med. 1985 Apr 1;161(4):705–717. doi: 10.1084/jem.161.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg E. Cancer statistics. 1986. CA Cancer J Clin. 1986 Jan-Feb;36(1):9–25. doi: 10.3322/canjclin.36.1.9. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Wolinsky S. M., Whitbeck A., Broker T. R., Chow L. T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989 Sep;172(1):331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988 Jun;7(6):1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]