Abstract
The title compound, C8H12N2O3S2, has been isolated as an intermediate in the synthesis of methylene blue dye, the best known phenothiazine dye, and structurally characterized as a zwitterion. The crystal structure is dominated by intermolecular N—H⋯O hydrogen bonds between the amine and sulfothioate groups, with graph-set motif C(9)R 2 2(8), involving antiparallel chains and a centrosymmetric eight-membered ring. A hydrogen bond with graph-set motif R 2 2(14) between the ammonium and sulfothioate groups completes the two-dimensional network in the ab plane. Intermolecular C—H⋯O hydrogen bonds are also present in the crystal.
Related literature
For methylene blue dye, see: Bernthasen (1889 ▶); Zollinger (1991 ▶); Hunger (2003 ▶). For its preparation, see: Leventis et al. (1997 ▶). For the synthesis of the title compound, see: Bogert & Updike (1927 ▶); Bennett & Bell (1943 ▶). For bond-length data, see: Trinajstić (1968 ▶); Allen et al. (1987 ▶).
Experimental
Crystal data
C8H12N2O3S2
M r = 248.32
Monoclinic,
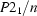
a = 12.0593 (1) Å
b = 7.3651 (1) Å
c = 12.2312 (1) Å
β = 95.0766 (8)°
V = 1082.09 (2) Å3
Z = 4
Cu Kα radiation
μ = 4.41 mm−1
T = 296 K
0.48 × 0.37 × 0.29 mm
Data collection
Xcalibur Nova diffractometer with enhance (Cu) X-ray source and Onyx CCD
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2008 ▶) T min = 0.628, T max = 1.000 (expected range = 0.175–0.279)
5132 measured reflections
2153 independent reflections
2006 reflections with I > 2σ(I)
R int = 0.015
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.092
S = 1.07
2153 reflections
150 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.33 e Å−3
Δρmin = −0.19 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2008 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2008 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXL97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809016158/kp2216sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809016158/kp2216Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H11N⋯O1i | 0.87 (3) | 2.36 (3) | 3.136 (3) | 148 (2) |
| N1—H21N⋯O1ii | 0.82 (2) | 2.28 (2) | 3.010 (2) | 148 (2) |
| N2—H12N⋯O3iii | 0.88 (2) | 1.89 (2) | 2.769 (2) | 175 (2) |
| C5—H5⋯O3i | 0.93 | 2.55 | 3.376 (2) | 148 |
| C8—H8A⋯O2iv | 0.96 | 2.41 | 3.209 (3) | 141 |
Symmetry codes: (i) 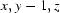 ; (ii)
; (ii) 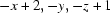 ; (iii)
; (iii) 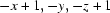 ; (iv)
; (iv) 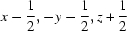 .
.
Acknowledgments
This research was supported by the Ministry of Science and Technology of the Republic of Croatia (grant No. 117–0000000–3283 and 098–1191344–2943).
supplementary crystallographic information
Comment
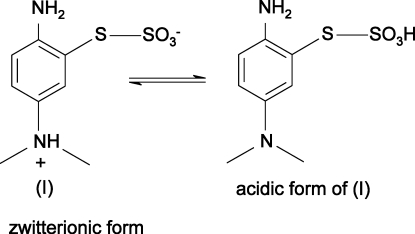
Phenothiazine dyes are class of colorants with application in various fields of which the methylene blue is the most well known (Zollinger, 1991; Hunger, 2003). Commercially, methylene blue is produced by oxidation of 4-N,N-dimethylaminoaniline with Na2Cr2O7 in the presence of Na2S2O3, followed by the further oxidation in the presence of N,N-dimethylaniline, usually without isolation of intermediate 4-N,N-dimethylaminoaniline-2-tiosulfuric acid (Leventis et al., 1997). Namely, this compound was first described in 1889 (Bernthasen, 1889) and in the last hundred years reported by several authors. Moreover, in the literature the compound is described as phenyl O-hydrogen sulfothioate acid but the possibility of zwitterionic form (I) (Scheme 1) was not reported. Following one of the known method for preparation of 4-N,N-dimethylaminoaniline-2-tiosulfuric acid (Bogert & Updike, 1927), we isolated S-2-amino-5-(dimethylammonio)phenyl sulfothioate (I) determined by single-crystal structure analysis (Scheme, Fig. 1).
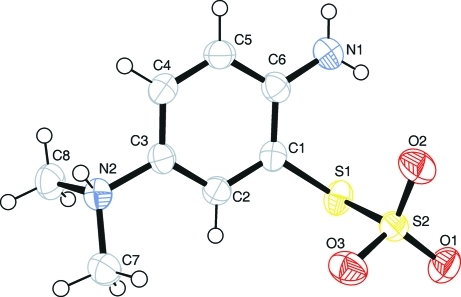
The single S—S bond distance value of sulfothioate group is 2.0985 (5) Å. The S—C bond in (I) is 1.768 (2) Å, reflecting aprox. 20% of π bond character according to N. Trinajstić (Trinajstić, 1968). The Car—N bond formed by amine group has significantπ character (1.360 (3) Å). On the contrary, C—N bonds of the N,N-dimethylammonio groups are essentially single bonds (N2—C7 1.491 (2) Å and N2—C8 1.501 (2) Å). The values observed are in accordance with the literature data (Allen et al., 1987).
The relative orientation of the sulfothioate group to the phenyl ring is defined by the torsion angle S2—S1—C3—C4 (92.93 (13)°). The twist around Car-Nsp3 bond is described by Car—Car—Nsp3—Csp3 torsion angle of 73.97 (18)° (for the atom sequence C6—C1—N2—C8).
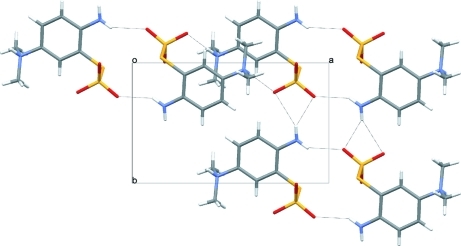
The rather complex hydrogen bond network in (I) (Table 1, Fig. 2) is characterized by the N—H···O and the C—H···O intermolecular hydrogen bonds. The atom N1 acts as double proton donor and the atoms O1 and O3 as double proton acceptors (Table 1). The C—H···O intermolecular hydrogen bonds are formed between Car-H groups along with the C8 atom of 5-N,N-dimethylammonio cation and O atoms of S—SO3- fragment.
At the unitary level antiparallel infinite chains are formed by the N1—H11N···O1i (i = x, -y, z) hydrogen bonds between amino and sulfothioate groups (Table 1, Fig. 2). The R22(8) rings are formed via N1—H11N···O1i (i = x, -y, z) and N1—H21N···O1ii (ii = 2 - x, -y, 1 - z) hydrogen bonds, thus N1 amino group participates in bifurcated hydrogen bond. The combination of these two primary motifs, chain and ring, generates a new 14-membered ring of the second level of graph-set notation: N2=R22(14) involving N+2-H···O3 hydrogen bond. Consequently, the crystal structure can be described as the two-dimensional-network in the (ab) plane.
Experimental
N,N-dimethylaniline was dissolved in aqueous HCl and nitrosilated with NaNO2 (Bennett & Bell, 1943). The resulting crude 4-nitroso-N,N-dimethylaniline hydrochloride was isolated and dissolved in aqueous acetic acid. The cold water solution of Na2S2O3 was added and the reaction mixture was stirred at 273 - 278 K for several h (Bogert & Updike, 1927), and left for two days at room temperature. The crude product was filtered off, and crystallized from water. The obtained crystals of S-2-amino-5-(dimethylammonium)phenyl sulfothioate (I) were in the form of blue prisms. Spectroscopic analysis, IR (ATR, cm-1): 3451 (m), 3342 (m), 3034 (w), 2657 (m), 1616 (s), 1504 (s), 1458 (m), 1400 (m), 1319 (w), 1242 (s), 1161 (s), 1134 (s), 1003 (s), 906 (m), 880 (w), 822 (m), 675 (w), 622 (s), 544 (m). 1H NMR (300 MHz, DMSO-d6):δ 8.99 (br s, 2H), 7.18 (s, 1H), 7.10–7.03 (m, 2H), 2.99 (s, 6H). Analysis, calculated for C8H12N2O3S2: C 38.69, H 4.87, N 11.28%; found: C 38.65, H 4.91, N 11.21%.
Refinement
Hydrogen atoms bonded to the nitrogen atoms of amino and ammonio groups were found in the difference Fourier electron-density maps and refined freely. All hydrogen atoms attached to the carbon atoms were generated at calculated positions and refined by applying the riding model (Uiso (H) = 1.2 Ueq (C) and Csp2-H distance 0.93 Å; Csp3-H 0.96 Å and Uiso (H) = 1.5 Ueq (C).
Figures
Fig. 1.
The molecular structure of (I) with the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Crystal structure of (I) viewed down the c axis. Hydrogen bonds are drawn by dashed lines.
Fig. 3.
The zwitterionic and acid forms of the title compound.
Crystal data
| C8H12N2O3S2 | F(000) = 520 |
| Mr = 248.32 | Dx = 1.524 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| a = 12.0593 (1) Å | Cell parameters from 4267 reflections |
| b = 7.3651 (1) Å | θ = 3.6–76.1° |
| c = 12.2312 (1) Å | µ = 4.41 mm−1 |
| β = 95.0766 (8)° | T = 296 K |
| V = 1082.09 (2) Å3 | Prism, blue |
| Z = 4 | 0.48 × 0.37 × 0.29 mm |
Data collection
| κ geometry Xcalibur Nova diffractometer with enhance (Cu) X-ray source and Onyx CCD | 2153 independent reflections |
| Radiation source: fine-focus sealed tube | 2006 reflections with I > 2σ(I) |
| graphite | Rint = 0.015 |
| Detector resolution: 10.4323 pixels mm-1 | θmax = 75.0°, θmin = 4.9° |
| Enhance (Cu) X–ray Source scans | h = −13→15 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2008) | k = −9→5 |
| Tmin = 0.628, Tmax = 1.000 | l = −15→14 |
| 5132 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.034 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.092 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0551P)2 + 0.3077P] where P = (Fo2 + 2Fc2)/3 |
| 2153 reflections | (Δ/σ)max < 0.001 |
| 150 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.85191 (3) | 0.04337 (6) | 0.62282 (3) | 0.04476 (15) | |
| S2 | 0.83204 (3) | 0.15312 (5) | 0.46396 (3) | 0.03773 (14) | |
| O1 | 0.92392 (11) | 0.27914 (18) | 0.46826 (12) | 0.0521 (3) | |
| O2 | 0.83442 (12) | 0.00876 (19) | 0.38531 (12) | 0.0556 (3) | |
| O3 | 0.72379 (11) | 0.24407 (18) | 0.45543 (11) | 0.0506 (3) | |
| N1 | 0.83654 (15) | −0.3588 (3) | 0.56480 (16) | 0.0531 (4) | |
| H11N | 0.832 (2) | −0.469 (4) | 0.539 (2) | 0.060 (7)* | |
| H21N | 0.891 (2) | −0.295 (3) | 0.556 (2) | 0.057 (7)* | |
| N2 | 0.43258 (11) | −0.05829 (19) | 0.68128 (11) | 0.0369 (3) | |
| H12N | 0.3836 (17) | −0.113 (3) | 0.6345 (17) | 0.042 (5)* | |
| C1 | 0.53946 (13) | −0.1301 (2) | 0.64861 (12) | 0.0354 (3) | |
| C2 | 0.63340 (13) | −0.0239 (2) | 0.65023 (13) | 0.0371 (3) | |
| H2 | 0.6306 | 0.0971 | 0.6715 | 0.045* | |
| C3 | 0.73290 (13) | −0.0974 (2) | 0.62006 (13) | 0.0375 (3) | |
| C4 | 0.73946 (14) | −0.2821 (2) | 0.59014 (13) | 0.0391 (3) | |
| C5 | 0.64152 (14) | −0.3866 (2) | 0.59039 (14) | 0.0413 (4) | |
| H5 | 0.6433 | −0.5087 | 0.5712 | 0.050* | |
| C6 | 0.54366 (14) | −0.3119 (2) | 0.61841 (13) | 0.0388 (3) | |
| H6 | 0.4799 | −0.3832 | 0.6172 | 0.047* | |
| C7 | 0.42090 (16) | 0.1431 (2) | 0.67521 (17) | 0.0491 (4) | |
| H7A | 0.4696 | 0.1979 | 0.7322 | 0.074* | |
| H7B | 0.3453 | 0.1762 | 0.6845 | 0.074* | |
| H7C | 0.4403 | 0.1848 | 0.6050 | 0.074* | |
| C8 | 0.40933 (16) | −0.1240 (3) | 0.79311 (15) | 0.0491 (4) | |
| H8A | 0.4120 | −0.2543 | 0.7948 | 0.074* | |
| H8B | 0.3368 | −0.0838 | 0.8091 | 0.074* | |
| H8C | 0.4643 | −0.0760 | 0.8470 | 0.074* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0369 (2) | 0.0545 (3) | 0.0423 (2) | −0.01012 (16) | 0.00067 (16) | 0.00048 (17) |
| S2 | 0.0361 (2) | 0.0368 (2) | 0.0409 (2) | −0.00114 (14) | 0.00620 (15) | −0.00381 (14) |
| O1 | 0.0461 (7) | 0.0505 (7) | 0.0602 (8) | −0.0109 (6) | 0.0076 (6) | 0.0049 (6) |
| O2 | 0.0643 (8) | 0.0537 (8) | 0.0503 (7) | 0.0016 (6) | 0.0127 (6) | −0.0157 (6) |
| O3 | 0.0435 (7) | 0.0476 (7) | 0.0594 (8) | 0.0071 (5) | −0.0029 (5) | −0.0075 (6) |
| N1 | 0.0463 (9) | 0.0465 (9) | 0.0690 (11) | 0.0051 (7) | 0.0192 (8) | −0.0029 (8) |
| N2 | 0.0344 (7) | 0.0419 (7) | 0.0347 (7) | −0.0012 (5) | 0.0046 (5) | −0.0001 (5) |
| C1 | 0.0344 (7) | 0.0402 (8) | 0.0320 (7) | 0.0011 (6) | 0.0046 (6) | 0.0004 (6) |
| C2 | 0.0394 (8) | 0.0361 (8) | 0.0363 (7) | −0.0021 (6) | 0.0060 (6) | −0.0030 (6) |
| C3 | 0.0354 (7) | 0.0425 (8) | 0.0348 (7) | −0.0034 (6) | 0.0047 (6) | −0.0003 (6) |
| C4 | 0.0415 (8) | 0.0425 (9) | 0.0336 (7) | 0.0026 (7) | 0.0060 (6) | 0.0006 (6) |
| C5 | 0.0487 (9) | 0.0349 (8) | 0.0409 (8) | −0.0005 (7) | 0.0067 (7) | −0.0016 (6) |
| C6 | 0.0394 (8) | 0.0393 (8) | 0.0377 (8) | −0.0060 (6) | 0.0040 (6) | 0.0011 (6) |
| C7 | 0.0486 (9) | 0.0449 (10) | 0.0551 (10) | 0.0079 (7) | 0.0114 (8) | 0.0048 (8) |
| C8 | 0.0509 (10) | 0.0567 (10) | 0.0418 (9) | −0.0005 (8) | 0.0154 (7) | 0.0067 (8) |
Geometric parameters (Å, °)
| S1—C3 | 1.7683 (16) | C2—C3 | 1.395 (2) |
| S1—S2 | 2.0986 (6) | C2—H2 | 0.9300 |
| S2—O2 | 1.4358 (13) | C3—C4 | 1.413 (2) |
| S2—O1 | 1.4428 (13) | C4—C5 | 1.410 (2) |
| S2—O3 | 1.4627 (13) | C5—C6 | 1.373 (2) |
| N1—C4 | 1.360 (2) | C5—H5 | 0.9300 |
| N1—H11N | 0.87 (3) | C6—H6 | 0.9300 |
| N1—H21N | 0.83 (3) | C7—H7A | 0.9600 |
| N2—C1 | 1.4805 (19) | C7—H7B | 0.9600 |
| N2—C7 | 1.491 (2) | C7—H7C | 0.9600 |
| N2—C8 | 1.501 (2) | C8—H8A | 0.9600 |
| N2—H12N | 0.88 (2) | C8—H8B | 0.9600 |
| C1—C2 | 1.375 (2) | C8—H8C | 0.9600 |
| C1—C6 | 1.391 (2) | ||
| C3—S1—S2 | 100.49 (5) | C2—C3—S1 | 118.90 (13) |
| O2—S2—O1 | 116.10 (8) | C4—C3—S1 | 120.36 (12) |
| O2—S2—O3 | 111.22 (8) | N1—C4—C5 | 120.80 (16) |
| O1—S2—O3 | 112.69 (8) | N1—C4—C3 | 121.86 (16) |
| O2—S2—S1 | 109.13 (7) | C5—C4—C3 | 117.32 (15) |
| O1—S2—S1 | 100.93 (6) | C6—C5—C4 | 121.37 (15) |
| O3—S2—S1 | 105.69 (6) | C6—C5—H5 | 119.3 |
| C4—N1—H11N | 116.4 (17) | C4—C5—H5 | 119.3 |
| C4—N1—H21N | 120.4 (17) | C5—C6—C1 | 120.29 (15) |
| H11N—N1—H21N | 121 (2) | C5—C6—H6 | 119.9 |
| C1—N2—C7 | 115.00 (13) | C1—C6—H6 | 119.9 |
| C1—N2—C8 | 111.61 (13) | N2—C7—H7A | 109.5 |
| C7—N2—C8 | 109.99 (14) | N2—C7—H7B | 109.5 |
| C1—N2—H12N | 102.0 (13) | H7A—C7—H7B | 109.5 |
| C7—N2—H12N | 111.7 (13) | N2—C7—H7C | 109.5 |
| C8—N2—H12N | 105.9 (13) | H7A—C7—H7C | 109.5 |
| C2—C1—C6 | 120.13 (15) | H7B—C7—H7C | 109.5 |
| C2—C1—N2 | 121.91 (14) | N2—C8—H8A | 109.5 |
| C6—C1—N2 | 117.94 (14) | N2—C8—H8B | 109.5 |
| C1—C2—C3 | 120.15 (15) | H8A—C8—H8B | 109.5 |
| C1—C2—H2 | 119.9 | N2—C8—H8C | 109.5 |
| C3—C2—H2 | 119.9 | H8A—C8—H8C | 109.5 |
| C2—C3—C4 | 120.73 (15) | H8B—C8—H8C | 109.5 |
| C3—S1—S2—O2 | −61.58 (9) | S2—S1—C3—C2 | −88.47 (13) |
| C3—S1—S2—O1 | 175.65 (8) | S2—S1—C3—C4 | 92.94 (13) |
| C3—S1—S2—O3 | 58.12 (8) | C2—C3—C4—N1 | −177.24 (16) |
| C7—N2—C1—C2 | 21.8 (2) | S1—C3—C4—N1 | 1.3 (2) |
| C8—N2—C1—C2 | −104.36 (18) | C2—C3—C4—C5 | 1.0 (2) |
| C7—N2—C1—C6 | −159.86 (15) | S1—C3—C4—C5 | 179.60 (12) |
| C8—N2—C1—C6 | 73.96 (18) | N1—C4—C5—C6 | 178.39 (17) |
| C6—C1—C2—C3 | 0.9 (2) | C3—C4—C5—C6 | 0.1 (2) |
| N2—C1—C2—C3 | 179.22 (14) | C4—C5—C6—C1 | −0.7 (2) |
| C1—C2—C3—C4 | −1.6 (2) | C2—C1—C6—C5 | 0.2 (2) |
| C1—C2—C3—S1 | 179.85 (12) | N2—C1—C6—C5 | −178.16 (14) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11N···O1i | 0.87 (3) | 2.36 (3) | 3.136 (3) | 148 (2) |
| N1—H21N···O1ii | 0.82 (2) | 2.28 (2) | 3.010 (2) | 148 (2) |
| N2—H12N···O3iii | 0.88 (2) | 1.89 (2) | 2.769 (2) | 175 (2) |
| C5—H5···O3i | 0.93 | 2.55 | 3.376 (2) | 148 |
| C8—H8A···O2iv | 0.96 | 2.41 | 3.209 (3) | 141 |
Symmetry codes: (i) x, y−1, z; (ii) −x+2, −y, −z+1; (iii) −x+1, −y, −z+1; (iv) x−1/2, −y−1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KP2216).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L. & Orpen, A. G. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bennett, G. M. & Bell, E. V. (1943). Organic Syntheses, Collected Vol. 2, p. 223. New York: John Wiley & Sons.
- Bernthasen, A. (1889). Annalen, 251, 1–97.
- Bogert, M. T. & Updike, I. A. (1927). J. Am. Chem. Soc.49, 1373–1382.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Hunger, K. (2003). Industrial Dyes: Chemistry, Properties, Application Weinheim: Wiley-VCH.
- Leventis, N., Chen, M. & Sortiriou-Leventis, C. (1997). Tetrahedron, 53, 10083–10092.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst.41, 466–470.
- Oxford Diffraction (2008). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Abingdon, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Trinajstić, N. (1968). Tetrahedron Lett.12, 1529–1532.
- Zollinger, H. (1991). Colour Chemistry, 2nd ed. Weinheim: VCH.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809016158/kp2216sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809016158/kp2216Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report