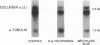Abstract
Ascorbic acid stimulates collagen gene transcription in cultured fibroblasts, and this effect is mediated through the induction of lipid peroxidation by ascorbic acid. Quiescent cultured fibroblasts in the absence of ascorbic acid have a high constitutive level of collagen production, but the mechanisms of collagen gene regulation in this unstimulated state are not known. Because lipid peroxidation also occurs in normal cells, we wondered if lipid peroxidation plays a role in the regulation of basal collagen gene expression. Inhibition of lipid peroxidation in cultured human fibroblasts with d-alpha-tocopherol or methylene blue decreased the synthesis of collagen, the steady-state levels of procollagen alpha 1(I) mRNA and the transcription of the procollagen alpha 1(I) gene. This effect on collagen gene expression was selective and not associated with cellular toxicity. Thus, these experiments suggest a role for lipid peroxidation in the modulation of constitutive collagen gene expression.
Full text
PDF
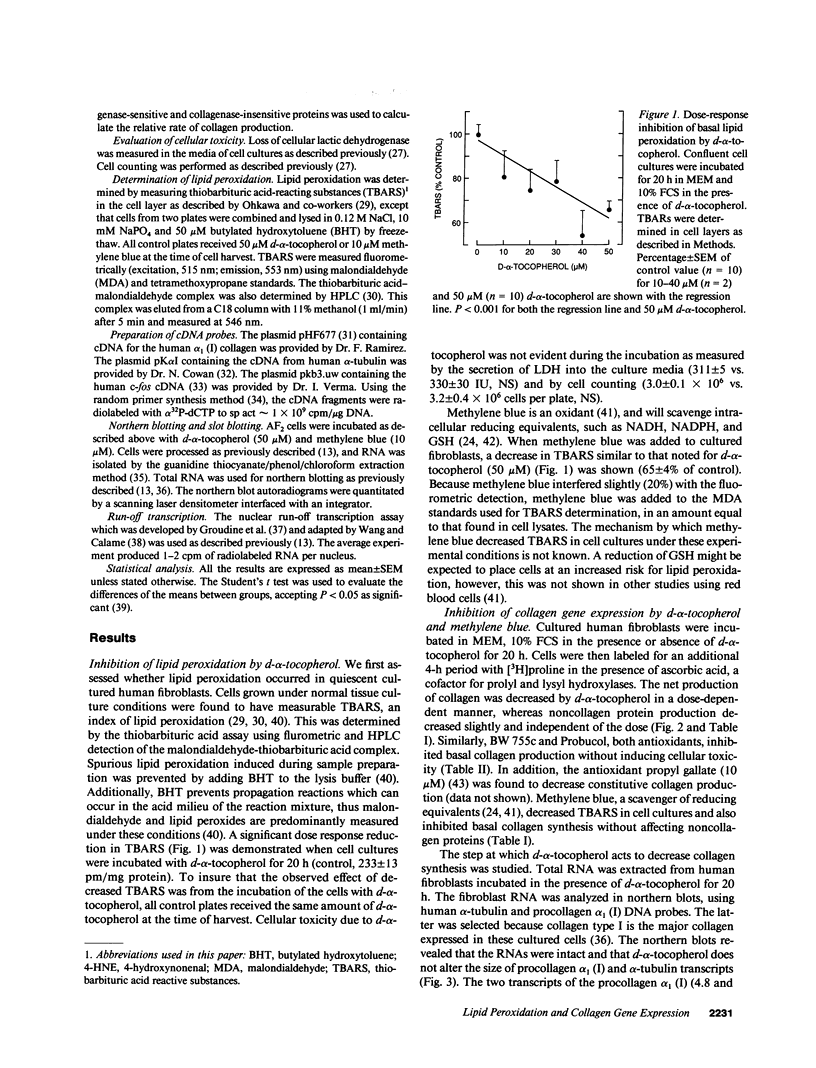
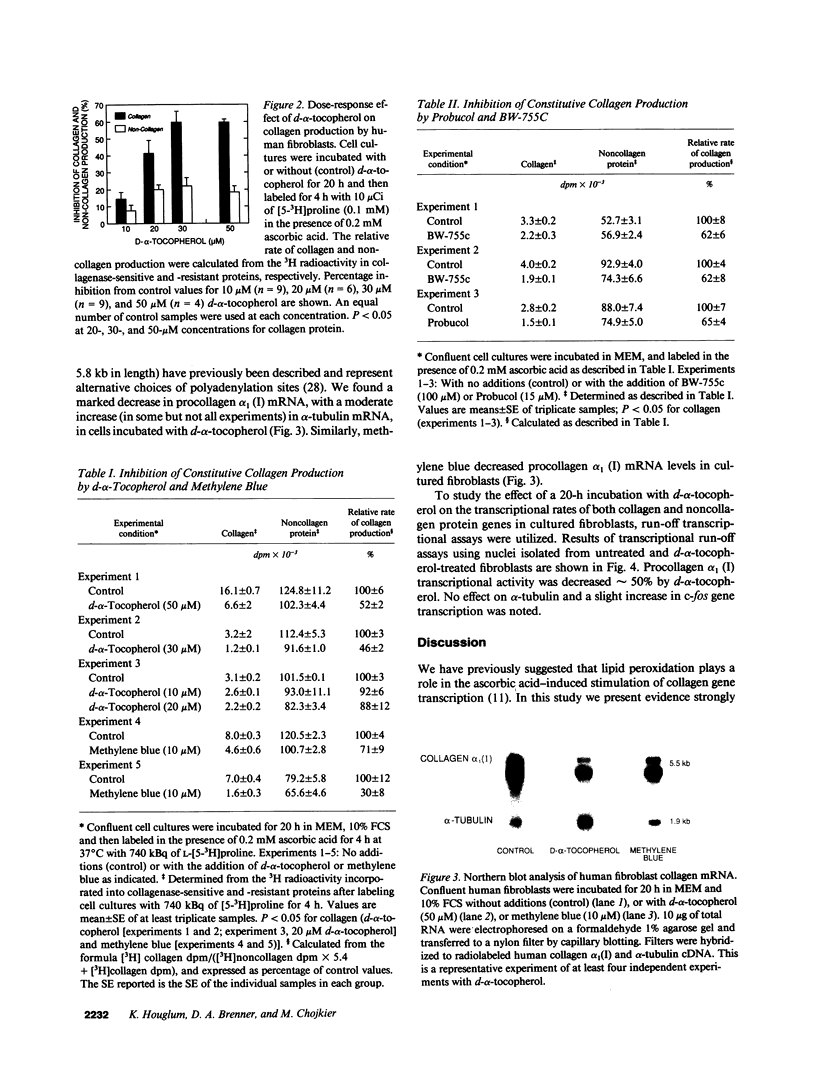
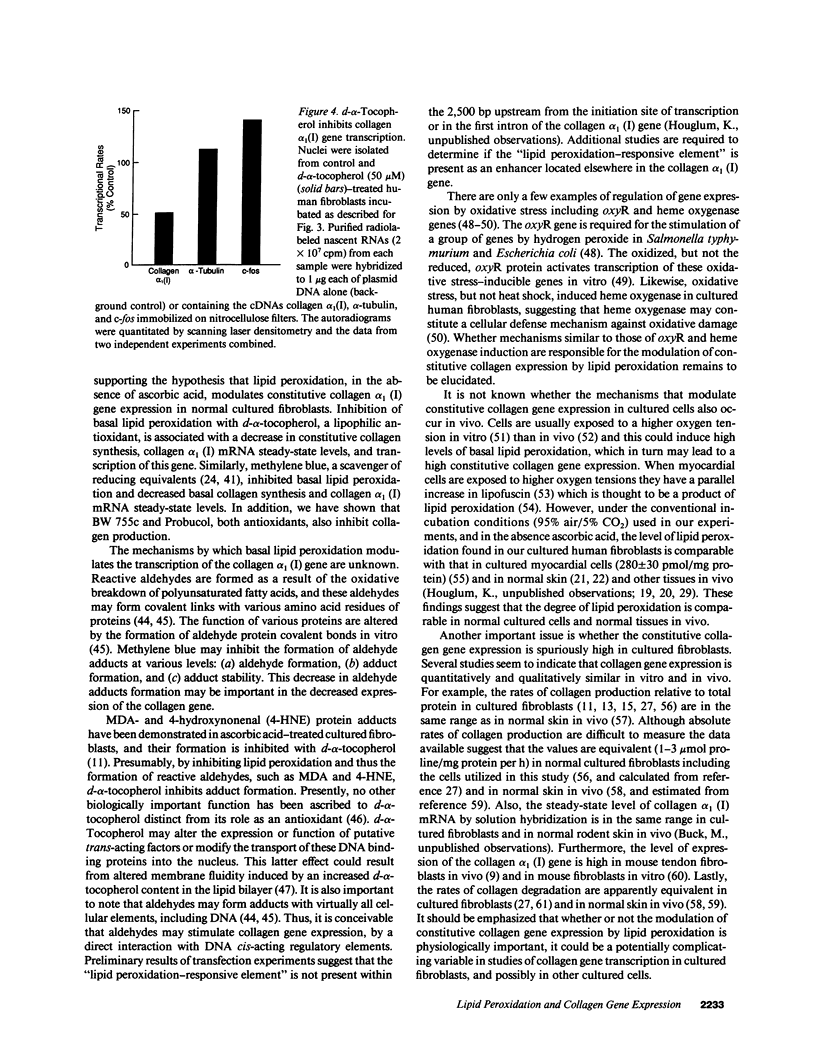


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel M. F., Ramasastry S. S., Swartz W. M., Narayanan K., Kuhns D. B., Basford R. E., Futrell J. W. The critical relationship between free radicals and degrees of ischemia: evidence for tissue intolerance of marginal perfusion. Plast Reconstr Surg. 1988 Feb;81(2):233–239. doi: 10.1097/00006534-198802000-00017. [DOI] [PubMed] [Google Scholar]
- Annerén K. G., Epstein C. J. Lipid peroxidation and superoxide dismutase-1 and glutathione peroxidase activities in trisomy 16 fetal mice and human trisomy 21 fibroblasts. Pediatr Res. 1987 Jan;21(1):88–92. doi: 10.1203/00006450-198701000-00019. [DOI] [PubMed] [Google Scholar]
- Barnes M. J. Collagens in atherosclerosis. Coll Relat Res. 1985 Jan;5(1):65–97. doi: 10.1016/s0174-173x(85)80048-0. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Ferrali M., Ciccoli L., Esterbauer H., Comporti M. Detection of carbonyl functions in phospholipids of liver microsomes in CCl4- and BrCCl3-poisoned rats. Biochim Biophys Acta. 1982 Sep 14;712(3):628–638. doi: 10.1016/0005-2760(82)90292-2. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S. Collagen degradation in human lung fibroblasts: extent of degradation, role of lysosomal proteases, and evaluation of an alternate hypothesis. J Cell Physiol. 1984 Oct;121(1):152–158. doi: 10.1002/jcp.1041210119. [DOI] [PubMed] [Google Scholar]
- Bird R. P., Draper H. H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984;105:299–305. doi: 10.1016/s0076-6879(84)05038-2. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Chojkier M. Acetaldehyde increases collagen gene transcription in cultured human fibroblasts. J Biol Chem. 1987 Dec 25;262(36):17690–17695. [PubMed] [Google Scholar]
- Chandler D. B., Butler T. W., Briggs D. D., 3rd, Grizzle W. E., Barton J. C., Fulmer J. D. Modulation of the development of bleomycin-induced fibrosis by deferoxamine. Toxicol Appl Pharmacol. 1988 Mar 15;92(3):358–367. doi: 10.1016/0041-008x(88)90176-7. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Brenner D. A. Therapeutic strategies for hepatic fibrosis. Hepatology. 1988 Jan-Feb;8(1):176–182. doi: 10.1002/hep.1840080132. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Houglum K., Solis-Herruzo J., Brenner D. A. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J Biol Chem. 1989 Oct 5;264(28):16957–16962. [PubMed] [Google Scholar]
- Chojkier M., Peterkofsky B., Bateman J. New method for determining the extent of proline hydroxylation by measuring changes in the ratio of [4-3H]:[14C]proline in collagenase digests. Anal Biochem. 1980 Nov 1;108(2):385–393. doi: 10.1016/0003-2697(80)90603-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Storz G., Ames B. N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement B., Grimaud J. A., Campion J. P., Deugnier Y., Guillouzo A. Cell types involved in collagen and fibronectin production in normal and fibrotic human liver. Hepatology. 1986 Mar-Apr;6(2):225–234. doi: 10.1002/hep.1840060212. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent damage products of lipid peroxidation. Methods Enzymol. 1984;105:337–341. doi: 10.1016/s0076-6879(84)05044-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flaherty M., Chojkier M. Selective inhibition of collagen synthesis by the Ca2+ ionophore A23187 in cultured human fibroblasts. J Biol Chem. 1986 Sep 15;261(26):12060–12065. [PubMed] [Google Scholar]
- GOLBERG L., MARTIN L. E., BATCHELOR A. Biochemical changes in the tissues of animals injected with iron. 3. Lipid peroxidation. Biochem J. 1962 May;83:291–298. doi: 10.1042/bj0830291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geesin J. C., Gordon J. S., Berg R. A. Retinoids affect collagen synthesis through inhibition of ascorbate-induced lipid peroxidation in cultured human dermal fibroblasts. Arch Biochem Biophys. 1990 May 1;278(2):350–355. doi: 10.1016/0003-9861(90)90270-9. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Quinlan G. J. Malondialdehyde formation from lipid peroxides in the thiobarbituric acid test: the role of lipid radicals, iron salts, and metal chelators. J Appl Biochem. 1983 Aug-Oct;5(4-5):293–299. [PubMed] [Google Scholar]
- Hata R., Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol. 1989 Jan;138(1):8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- Hatahara T., Seyer J. M. Isolation and characterization of a fibrogenic factor from CCl(4)-damaged rat liver. Biochim Biophys Acta. 1982 Jun 16;716(3):377–382. doi: 10.1016/0304-4165(82)90030-7. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Manzino L. Ascorbic acid, redox cycling, lipid peroxidation, and the binding of dopamine receptor antagonists. Ann N Y Acad Sci. 1987;498:63–76. doi: 10.1111/j.1749-6632.1987.tb23751.x. [DOI] [PubMed] [Google Scholar]
- Hershko C., Link G., Pinson A. Modification of iron uptake and lipid peroxidation by hypoxia, ascorbic acid, and alpha-tocopherol in iron-loaded rat myocardial cell cultures. J Lab Clin Med. 1987 Sep;110(3):355–361. [PubMed] [Google Scholar]
- Hildebran J. N., Airhart J., Stirewalt W. S., Low R. B. Prolyl-tRNA-based rates of protein and collagen synthesis in human lung fibroblasts. Biochem J. 1981 Aug 15;198(2):249–258. doi: 10.1042/bj1980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt K., Bennett M., Chojkier M. Acetaldehyde stimulates collagen and noncollagen protein production by human fibroblasts. Hepatology. 1984 Sep-Oct;4(5):843–848. doi: 10.1002/hep.1840040508. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Uzuka M., Nakajima K. The effects of vitamin E deficiency on rat skin. Br J Dermatol. 1989 Jul;121(1):43–49. doi: 10.1111/j.1365-2133.1989.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Kelner M. J., Alexander N. M. Methylene blue directly oxidizes glutathione without the intermediate formation of hydrogen peroxide. J Biol Chem. 1985 Dec 5;260(28):15168–15171. [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R., Hale B., Alexander N. M. Methylene blue competes with paraquat for reduction by flavo-enzymes resulting in decreased superoxide production in the presence of heme proteins. Arch Biochem Biophys. 1988 May 1;262(2):422–426. doi: 10.1016/0003-9861(88)90393-1. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khillan J. S., Schmidt A., Overbeek P. A., de Crombrugghe B., Westphal H. Developmental and tissue-specific expression directed by the alpha 2 type I collagen promoter in transgenic mice. Proc Natl Acad Sci U S A. 1986 Feb;83(3):725–729. doi: 10.1073/pnas.83.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons B. L., Schwarz R. I. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984 Mar 12;12(5):2569–2579. doi: 10.1093/nar/12.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty R. J., Laurent G. J. Collagen synthesis and degradation in vivo. Evidence for rapid rates of collagen turnover with extensive degradation of newly synthesized collagen in tissues of the adult rat. Coll Relat Res. 1987 Jun;7(2):93–104. doi: 10.1016/s0174-173x(87)80001-8. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Molnar J. A., Alpert N., Burke J. F., Young V. R. Synthesis and degradation rates of collagens in vivo in whole skin of rats, studied with 1802 labelling. Biochem J. 1986 Dec 1;240(2):431–435. doi: 10.1042/bj2400431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad S., Grove D., Lindberg K. A., Reynolds G., Sivarajah A., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981 May;78(5):2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ohyashiki T., Ushiro H., Mohri T. Effects of alpha-tocopherol on the lipid peroxidation and fluidity of porcine intestinal brush-border membranes. Biochim Biophys Acta. 1986 Jun 26;858(2):294–300. doi: 10.1016/0005-2736(86)90334-2. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Thrall R. S., Ward P. A. Bleomycin-induced pulmonary fibrosis in rats: biochemical demonstration of increased rate of collagen synthesis. Am Rev Respir Dis. 1980 Mar;121(3):501–506. doi: 10.1164/arrd.1980.121.3.501. [DOI] [PubMed] [Google Scholar]
- Raghow R., Gossage D., Seyer J. M., Kang A. H. Transcriptional regulation of type I collagen genes in cultured fibroblasts by a factor isolated from thioacetamide-induced fibrotic rat liver. J Biol Chem. 1984 Oct 25;259(20):12718–12723. [PubMed] [Google Scholar]
- Raghow R., Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987 Apr;79(4):1285–1288. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Rowe L. B., Schwarz R. I. Role of procollagen mRNA levels in controlling the rate of procollagen synthesis. Mol Cell Biol. 1983 Feb;3(2):241–249. doi: 10.1128/mcb.3.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryle P. R., Chakraborty J., Thomson A. D. The effect of methylene blue on the hepatocellular redox state and liver lipid content during chronic ethanol feeding in the rat. Biochem J. 1985 Dec 15;232(3):877–882. doi: 10.1042/bj2320877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Rossi P., de Crombrugghe B. Transcriptional control of the mouse alpha 2(I) collagen gene: functional deletion analysis of the promoter and evidence for cell-specific expression. Mol Cell Biol. 1986 Feb;6(2):347–354. doi: 10.1128/mcb.6.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Marzabadi M. R., Galaris D., Brunk U. T. Effect of ambient oxygen concentration on lipofuscin accumulation in cultured rat heart myocytes--a novel in vitro model of lipofuscinogenesis. Free Radic Biol Med. 1989;6(1):23–30. doi: 10.1016/0891-5849(89)90155-x. [DOI] [PubMed] [Google Scholar]
- Sokol R. J. The coming of age of vitamin E. Hepatology. 1989 Apr;9(4):649–653. doi: 10.1002/hep.1840090422. [DOI] [PubMed] [Google Scholar]
- Solis-Herruzo J. A., Brenner D. A., Chojkier M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988 Apr 25;263(12):5841–5845. [PubMed] [Google Scholar]
- Spanheimer R. G., Peterkofsky B. A specific decrease in collagen synthesis in acutely fasted, vitamin C-supplemented, guinea pigs. J Biol Chem. 1985 Apr 10;260(7):3955–3962. [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Calame K. The endogenous immunoglobulin heavy chain enhancer can activate tandem VH promoters separated by a large distance. Cell. 1985 Dec;43(3 Pt 2):659–665. doi: 10.1016/0092-8674(85)90238-7. [DOI] [PubMed] [Google Scholar]