Abstract
During development, early-life stress, such as abuse or trauma, induces long-lasting changes that are linked to adult anxiety and depressive behavior. It has been postulated that altered expression of corticotropin-releasing hormone (CRH) can at least partially account for the various effects of stress on behavior. In accord with this hypothesis, evidence from pharmacological and genetic studies has indicated the capacity of differing levels of CRH activity in different brain areas to produce behavioral changes. Furthermore, stress during early life or adulthood causes an increase in CRH release in a variety of neural sites. To evaluate the temporal and spatial specificity of the effect of early-life CRH exposure on adult behavior, the tetracycline-off system was used to produce mice with forebrain-restricted inducible expression of CRH. After transient elevation of CRH during development only, behavioral testing in adult mice revealed a persistent anxiogenic and despair-like phenotype. These behavioral changes were not associated with alterations in adult circadian or stress-induced corticosterone release but were associated with changes in CRH receptor type 1 expression. Furthermore, the despair-like changes were normalized with antidepressant treatment. Overall, these studies suggest that forebrain-restricted CRH signaling during development can permanently alter stress adaptation leading to increases in maladaptive behavior in adulthood.
Introduction
Early-life stress has important implications for adult health and stress adaptation. Stressful events such as parental loss and physical or emotional abuse can influence or promote the development of mood disorders (for review, see Nemeroff, 2004). These early-life stress experiences are associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, the endocrine stress response (De Bellis et al., 1994; Lemieux and Coe, 1995; Heim et al., 2000, 2008). Some of the changes in HPA axis activity are detected in adulthood as evidenced by the observation that depressed men who experienced early-life trauma exhibit HPA axis hyperactivity in the corticotropin-releasing hormone (CRH)/ dexamethasone suppression test (Heim et al., 2008). In animal models, maternal deprivation stress induces an increase in anxiety and despair-like behaviors in adulthood (Aisa et al., 2008; Marco et al., 2008), mimicking the observations from humans. The precise mechanism by which early-life stress can influence major depressive disorder (MDD) in adult humans and similar behaviors in rodents is still unclear.
Although many of the long-lasting changes of early-life stress are associated with alterations in hypothalamic function, evidence from animal models has indicated that the limbic forebrain may also play a role in the long-lasting effects of stress (Brunson et al., 2001; Gross et al., 2002). In adult animals and during early postnatal life, psychological stress has been associated with an increase in CRH expression in the rodent hippocampus (Hatalski et al., 2000) and amygdala (Hatalski et al., 1998; Roozendaal et al., 2002). CRH is released at synapses throughout the CNS where it binds to CRH type 1 (CRHR1) and type 2 (CRHR2) receptors. In adults, MDD patients exhibit elevated CRH levels in their CSF following suicide (Arató and Bánki, 1989). Activity of CRH in the amygdala, hippocampus, and cortex has been hypothesized to induce anxiety-like changes related to early-life stress. Forebrain disruption of CRHR1 induces a decrease in anxiety-like behavior revealing the important role that extrahypothalamic CRH plays in the modulation of anxiety (Müller et al., 2003). CRH released during early stress events can bind to CRHR1 at presynaptic and postsynaptic sites (Chen et al., 2004a) to alter growth of hippocampal neurons (Chen et al., 2004b). CRH injected intracerebroventricularly during postnatal development induces memory deficits in adult rats without any observed HPA axis changes (Brunson et al., 2001). These data together with the established role of the limbic system in modulating affective behavior suggest that the forebrain is a potential site of early-life stress modulation of behavior in adulthood.
Present evidence suggests that forebrain CRH is involved in the mechanism by which early-life stress increases anxiety and depression in adult animals. To precisely test this hypothesis, we used the tetracycline-off system to overexpress CRH in the forebrain during development up to postnatal day 21 (P21). We found that early CRH exposure induced lasting anxiogenic and despair-like changes that were reversed with antidepressant treatment. These data suggest that limbic sites of CRH activity play an important role in the long-term effects of early-life stress.
Materials and Methods
Animals.
All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal Care and Use Committees of Washington University School of Medicine (St. Louis, MO) and Vanderbilt University (Nashville, TN). Mice were housed on a 12 h/12 h light/dark cycle [zeitgeber time (ZT) 0 denotes lights on] with ad libitum access to rodent chow and water. For control of the inducible tetracycline-off system, mice “on doxycycline” were fed doxycycline chow (200 mg doxycycline/1 kg; Research Diets) to repress transgene expression.
To produce forebrain-restricted inducible expression of CRH (FBCRHOE) mice, we constructed a plasmid (pUHCl3-3 backbone vector including the SV40 intron and polyadenylation signal at 3′ end of the insert) placing the CRH gene [excised with Sal1 (10 bp upstream of TATA box) and EcoRV (in the 3′ untranslated region)] under control of the tetracycline/doxycycline-responsive cytomegalovirus minimal promoter (tetop-CRH). The promoter and CRH gene were purified away from vector sequences and microinjected into inbred C57Bl/6 oocytes. The progeny born after pronuclear injection were screened by PCR to identify founder lines harboring the transgene. To generate mice with inducible, forebrain overexpression of CRH, we mated male tetop-CRH mice to female mice expressing the tetracycline transactivator under the control of CaMKII promotor (CaMKII-tTA mice from Jackson Laboratories) (Mayford et al., 1996). Control mice were mice positive for the tetop-CRH transgene or CaMKII-tTA transgene alone or wild-type littermates exposed to doxycycline in the same time frame as their respective overexpressing groups.
Three groups of double transgenic and three groups of control animals, all on an inbred C57Bl/6 background, were used for various studies. FBCRHOEdev mice were off doxycycline from embryonic day (E) 0 to P21 and so were exposed to CRH transiently during early development. FBCRHOElife mice were off doxycycline their entire lives and were continuously exposed to CRH overexpression. FBCRHOEon doxy mice were on doxycycline their entire lives and were therefore never exposed to CRH overexpression. Controldev mice were off doxycycline from E0 to P21 and so were exposed to doxycycline as adults. Controllife mice were off doxycycline their entire lives. Controlon doxy mice were on doxycycline their entire lives. No differences were observed between controldev mice and any of the other control groups (controllife and controlon doxy) or between controldev mice and FBCRHOEon doxy mice. Since no effect of doxycycline was seen in control mice and doxycycline efficiently suppressed overexpression in double transgenic mice, we compared FBCRHOEdev and controldev mice in most behavior and CRHR1/GR in situ hybridization experiments to take advantage of within-litter comparisons.
In situ hybridization.
For analysis of embryonic CRH overexpression, adult female mice (off doxycycline) were checked for mating plugs at ZT2 (2 h after lights on) following 12 h of breeding. Embryos were then harvested from plugged females on E12 or E15. Briefly, dams were deeply anesthetized with 2.5% avertin, and the uterus with embryos was removed into DEPC PBS. Embryos were separated from the uterus, and the placenta and the fetal membranes were removed from each fetus. Embryos were decapitated, placed in 4% DEPC paraformaldehyde (PFA) overnight, and processed as below.
For postnatal in situ hybridization experiments, male mice 8–12 weeks of age were used. For adult in situ hybridization, brains and pituitary glands were collected at ZT3–6 under nonstressful conditions and processed as previously described (Boyle et al., 2006) to evaluate CRH, glucocorticoid receptor (GR) and CRHR1 mRNA expression in matched sections from FBCRHOE and appropriate control mice. Briefly, mice were deeply anesthetized with 2.5% avertin and then transcardially perfused with DEPC PBS, followed by 4% DEPC PFA. Isolated brains were postfixed in 4% PFA for 24 h, followed by immersion in 10% sucrose in DEPC PBS. Tissues embedded in OCT (Sakura Finetek USA) were cut into 15 μm sections on a cryostat and thaw mounted onto Superfrost Plus slides (Fisher Scientific). An RNA probe complementary to mRNA for CRH (320 bp fragment from PstI to RsaI in exon 2 of CRH gene), GR (400 bp fragment targeted to 5′ end of exon 2 of the GR cDNA), or CRHR1 (fragment 25–730 bp from NM_007762) was radiolabeled with a33P-dUTP, hybridized to sections at an annealing temperature of 60°C, and washed, after hybridization, in 0.1× SSC at 65°C for 30 min. Slides were exposed for 1–6 d to Hyperfilm Max (GE Healthcare). Autoradiographic images were scanned at 3200 dots per inch on an Epson 1680 Pro scanner. Densitometric analysis of bilateral limbic (bregma = −1.32 mm) or pituitary gland in situ signal (two sections per mouse for adult sections and three sections per genotype for developmental E12-P14 sections) was performed using NIH ImageJ software. For adult sections, the in situ signal from two sections per mouse was averaged and normalized with mean control value for each individual anatomical area so that the reported “n” is the number of mice per group, not the number of total sections. For developmental sections, three sections per genotype were normalized with mean control value for each individual anatomical area that was easily identifiable. Control mice sections were a mix of single transgenic and wild-type mice. No negative controls (e.g., sense control) were used for in situ experiments as the signal detected is specific and consistent with that seen previously for these molecules (Muglia et al., 1995; Boyle et al., 2005).
FBCRHOEdev and controldev mice were treated with imipramine (16 mg/kg in 0.9% normal saline, pH 7.5) or vehicle (0.9% normal saline, pH 7.5). Mice were injected intraperitoneally daily (between ZT2 and ZT3) for 3 weeks, killed, and processed as above for CRHR1 mRNA expression 24 h after their last dose.
CRH immunohistochemistry.
Brains were collected under basal conditions. Mice were deeply anesthetized with 2.5% avertin and then transcardially perfused with PBS, followed by 4% PFA. Isolated brains were postfixed in 4% PFA for 24 h, followed by immersion in 10% sucrose in PBS. Tissues embedded in OCT were cut into 30 μm sections on a cryostat and stored in 0.1 m NaAzide/PBS at 4°C until use. Nonspecific binding for CRH was blocked with 3% NGS in PBS. Sections were incubated with CRH primary antibody (1:500, Peninsula Laboratories, RGG-8561 rabbit anti-CRH) overnight at 4°C, washed with PBS, incubated with secondary antibody for 60 min at room temperature (1:250 biotinylated goat anti-rabbit IgG), incubated in avidin/biotin complex reagent for 60 min, washed with Tris saline, and incubated in a DAB reagent. As a negative control, a section was stained with no primary antibody. In this section, no signal was detected (data not shown). Similar results were seen with the RC-12 CRH antibody (Muglia et al., 1995).
Behavioral analysis.
All behavioral analyses were performed by an observer blinded to genotype and treatment. Behavioral tests were done in the morning (ZT3–6). For behavioral analysis, adult male mice 8–16 weeks of age were used. As no differences were observed in pilot studies or in the present studies between single transgenic or wild-type mice, we grouped these mice into a single “control” cohort for each experiment.
Open field.
Our open-field apparatus consisted of a Plexiglas box (76 × 76 × 30 cm). Each mouse was placed in a corner of the open field under low light conditions. Lighting consisted of a single 100 W incandescent light bulb placed in the corner of the testing room 2.5 m from the testing arena. Each trial lasted for 10 min with one trial per mouse. Between sessions, the maze was rinsed with 70% ethanol and dried with paper towels. Latency to enter the center square (31 × 31 cm), time in center square, distance traveled in center square, and total distance traveled in all the areas were analyzed using Any-Maze software (Stoelting).
Light/dark preference.
As previously described (Boyle et al., 2006), our light/dark (L:D) preference apparatus consisted of a two-compartment standard shuttle box (20.3 × 15.9 × 21.3 cm; Med Associates) with compartments of equal size and a stainless steel bar floor. The compartments were separated by a 3 × 4 cm sliding door built into the separating wall. Light was generated by a 100 W incandescent light bulb placed 22 cm over the floor of the light compartment. In this mildly aversive test, mice were placed in the dark compartment and allowed to acclimate for 1 min. The slide door was then opened, and the latency to enter the light compartment, the total time spent in the light compartment, and the total number of entries into the light compartment were recorded for a 10 min trial.
Rotorod.
We used the Ugo Basile Accela Roto-Rod apparatus (model 7650, Jones and Roberts). Each mouse was tested on a stationary rod, continuously rotating rod (2.5 rpm) and accelerating rotating rod (2.5–14 rpm) with two trials on the continuous and accelerating tests. Time spent on the rod was measured for each animal (maximum time for stationary and continuous was 60 s; maximum time for accelerating rod was 180 s).
Sensory-motor battery.
The general sensory-motor capabilities of the mice were evaluated using the walk initiation, ledge, platform, 60° and 90° inclined screens, and the inverted screen test (Ho et al., 2000). For the walk initiation test, mice were placed in the middle of a square outlined by white tape (21 × 21 cm) on a smooth black surface of a large table top. The time it took each mouse to leave the square (place all four paws outside of the tape) was recorded, with a maximum time of 60 s allowed. For the ledge test, each mouse was timed for how long it could maintain its balance on a narrow (0.75 cm thick) Plexiglas ledge without falling (60 s maximum). In the platform test, each mouse was timed for how long it remained on an elevated (47 cm above the floor) circular platform (1.0 cm thick, 3.0 cm diameter). A maximum score of 60 s was assigned if the mouse remained on the platform for the duration of that time or if it could climb down, without falling, on a very thin pole that supported the platform. The inclined screen test involved placing mice on an elevated wire mesh grid (16 squares per 10) with an aluminum frame that was 15 × 52 cm and inclined to 60° or 90°. Each mouse was placed in the middle of the screen with its head oriented downward and scored for either how long it remained on the screen or how long it took to climb to the top or bottom of the apparatus. A maximum score of 60 s was given if an animal did not fall. In the inverted screen, mice were placed on screen at 90° before the screen was moved into the inverted position. Time spent on the screen was measured (60 s maximum).
Tail suspension test.
As previously described (Boyle et al., 2005), the tail suspension apparatus consisted of a cubicle made of 1.2 cm white Plexiglas with inside dimensions of 33 × 33 × 32 cm. Mice were suspended by the distal end of their tails from the tail hanger with tape. Activity was scored continuously for immobility behavior across an entire 5 min trial. Immobility was defined as the lack of all motion except respiration. Graphs were generated by calculating the amount of time each mouse was active.
Forced swim test.
As previously described (Boyle et al., 2005), mice were placed in a 2 L beaker with 1.3 L of water (18−20°C). The level of the water prevented the animals from escaping or from reaching the bottom of the container. Mice were continuously monitored for immobility behavior from 1–6 min of a 6 min trial. Immobility was defined as the lack of all motion except respiration and that required to keep the mouse afloat. At the end of the trial, the animal was removed from the water, dried, and returned to its home cage. Graphs were generated by calculating the amount of time each mouse was active during the trial, and the initial latency to float was defined as the first float lasting 3 consecutive seconds.
Antidepressant treatment for behavioral analysis.
FBCRHOEdev and controldev mice were treated with imipramine (16 mg/kg in 0.9% normal saline, pH 7.5) or vehicle (0.9% normal saline, pH 7.5). Mice were injected intraperitoneally daily (between ZT2 and ZT3) for 3 weeks and tested in the tail suspension test (TST) 24 h after their last dose. Mice were then given an additional 5 d of imipramine followed by testing in the forced swim test (FST) 24 h after the last dose. For acute studies, mice were injected with imipramine or vehicle 30 min before testing in the TST or FST. In a separate cohort, mice were injected intraperitoneally daily (between ZT2 and ZT3) for 3 weeks and tested in the open field 24 h after their last dose.
Radioimmunoassay. Corticosterone and adrenocorticotropic hormone (ACTH) were analyzed by radioimmunoassay as previously described (Boyle et al., 2005).
Circadian analysis.
Adult mice were singly or doubly housed at least 1 week before testing [note: housing due to space constraints; no differences were seen within genotypes between single- and double-housed mice, consistent with previous literature showing no effect of housing on basal HPA axis activity (Misslin et al., 1982; Holson et al., 1991)]. In adult mice, plasma was collected at circadian nadir (ZT1) and circadian peak (ZT11) by retro-orbital phlebotomy. Collection at nadir and peak was done in the same mice separated by 1 week between bleeds. In group-housed juvenile mice, plasma was collected at circadian nadir (ZT1) by rapid decapitation.
Stress analysis.
All restraint stress was performed by placing a mouse in a ventilated 50 ml conical tube for 30 min at ZT1. Plasma was obtained immediately following and 90 min after the end of the stressor.
Data analysis.
Results are expressed as the mean ± SEM. Student's t test was used to compare pairs of means. In cases of more than two groups, two-way ANOVA was used followed by Bonferroni post hoc tests when appropriate. A one-way ANOVA was used for comparison of the three control groups. A p value ≤ 0.05 was considered statistically significant. All statistical comparisons were done with Prism 4 software (GraphPad).
Results
Generation of inducible forebrain-specific CRH overexpressing mutant mice
To produce an inducible forebrain CRH overexpressing mouse line, we used the tetracycline-off system, the concept of which has been previously described (Mayford et al., 1996). This system involves two transgenes giving spatial specificity with the CaMKII promoter and reversible repression with the doxycycline-sensitive tetracycline transactivator (tTA). In this system, double transgenic mice given doxycycline should exhibit no transgenic CRH overexpression. Mice expressing both transgenes (FBCRHOE) have been screened for endogenous CRH expression and forebrain CRH overexpression using in situ hybridization and immunohistochemistry.
Evaluation of CRH expression in FBCRHOE mice on and off doxycycline
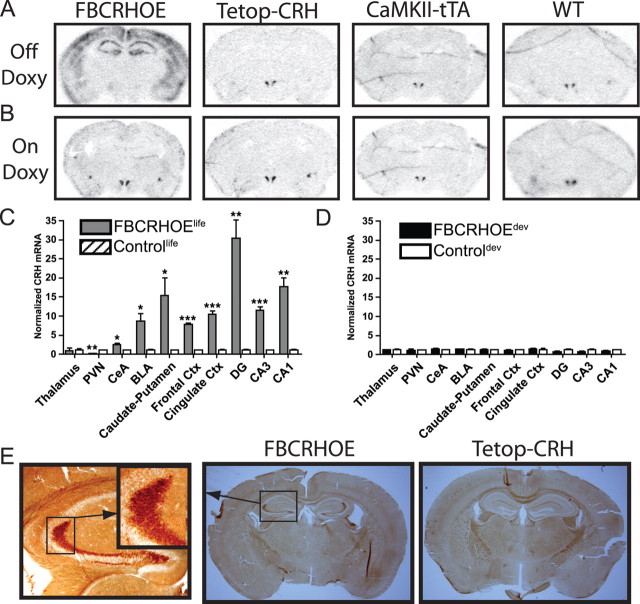
In adult brains, robust CRH mRNA signal was detected throughout the forebrain of lifetime FBCRHOE mice (FBCRHOElife, off doxycycline E0–adult so mice are exposed to transgenic CRH throughout life). CRH expression was increased in FBCRHOElife mice in the dorsal hippocampus [Student's t test; dentate gyrus (DG): t(4) = 6.14, p = 0.004; CA1: t(4) = 7.77, p = 0.002; CA3: t(4) = 11.62, p = 0.0003], caudate/putamen (t(4) = 3.07, p = 0.04), cingulate cortex (t(4) = 10.01 p = 0.0006), and somatosensory cortex (t(4) = 29.52, p < 0.0001), basolateral nucleus of the amygdala (BLA) (t(4) = 3.70, p = 0.02), and central nucleus of the amygdala (CeA) (t(4) = 3.52, p = 0.02) compared with control mice (either single transgenic or wild-type mice; controllife, off doxycycline E0-adult) but no differences were detected between FBCRHOElife and controllife mice in the thalamus (t(4) = 0.04, p = 0.97) (Fig. 1A,C). Interestingly, CRH mRNA expression was significantly reduced in the paraventricular nucleus of the hypothalamus (PVN) (t(4) = 6.85, p = 0.002) in FBCRHOElife compared with controllife mice (Fig. 1A,C). To assess whether the CRH mRNA translated into more CRH protein, we evaluated expression of CRH in FBCRHOElife mice and controllife mice (Fig. 1E). Focusing on the hippocampus, there is a clear upregulation in CRH protein in the FBCRHOElife mice (Fig. 1E, inset).
Figure 1.
Expression of CRH in FBCRHOE and control mice on and off doxycycline (doxy). A, B, Representative sections showing CRH mRNA expression from FBCRHOE, Tetop-CRH, CaMKII-tTA, and wild-type (WT) mice off (A) or on (B) doxy chow. C, Quantitation of CRH mRNA expression in FBCRHOElife (off doxy entire lives) and controllife mice (off doxy entire lives) in the thalamus, PVN, CeA, BLA, caudate/putamen, somatosensory cortex (frontal Ctx), cingulate cortex (cingulate Ctx), DG, CA1, and CA3. In situ signal is normalized with mean control value for each individual anatomical area. D, Quantitation of CRH mRNA expression in FBCRHOEdev (on doxy P21–P56 when tested) and controldev mice (on doxy P21–P56 when tested). E, Sections from FBCRHOElife and Tetop-CRH mice off doxy showing CRH immunohistochemical staining. Left panel, 10× magnification of hippocampus in FBCRHOE section with inset magnification of granule cell axon staining. FBCRHOElife, Double transgenic mice off doxy entire lives; controllife, control mice off doxy entire lives; FBCRHOEdev, double transgenic mice off doxy E0–P21 and on doxy >P21; controldev, control mice off doxy E0–P21 and on doxy >P21. Student's t test; *p < 0.05; **p < 0.01; ***p < 0.001 FBCRHOElife vs controllife.
Although the tTA expressing transgene had been previously validated (Mayford et al., 1996), we also verified that CRH overexpression is inhibited in adult FBCRHOE mice that are fed doxycycline chow. We performed in situ hybridization for CRH on adult (>P56) FBCRHOE mice transiently exposed to early CRH (FBCRHOEdev, off doxycycline E0–P21 so mice are exposed to transgenic CRH only during early development) and corresponding control mice (controldev, off doxycycline E0–P21). No significant differences were seen in CRH mRNA expression between groups in any of the areas quantitated above (DG: t(4) = 1.50, p = 0.21; CA1: t(4) = 1.34, p = 0.25; CA3: t(4) = 1.44, p = 0.22; caudate/putamen: t(4) = 0.57, p = 0.60; cingulate cortex: t(4) = 0.57, p = 0.60; somatosensory cortex: t(4) = 0.13, p = 0.90; BLA: t(4) = 0.60, p = 0.58; CeA: t(4) = 0.83, p = 0.45; thalamus: t(4) = 0.05, p = 0.96; PVN: t(4) = 0.06, p = 0.96) (Fig. 1B,D).
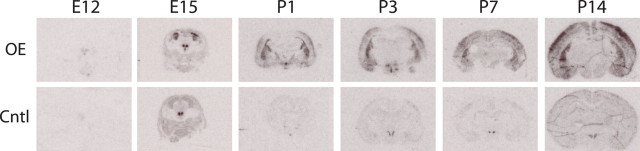
To determine the developmental expression of the CRH transgene, we performed in situ hybridization on FBCRHOEdev and controldev brains from E12–P14 mice that were off doxycycline (i.e., overexpression on). In young mice, we found a clear upregulation of the CRH mRNA signal at all time points except in E12 brains (Fig. 2, Table 1). CRH expression was increased in FBCRHOEdev mice in the amygdala at P1–P14 (Student's t test; P1: t(4) = 5.21, p = 0.007; P3: t(4) = 8.37, p = 0.001; P7: t(4) = 9.94, p = 0.0006; P14: t(4) = 3.89, p = 0.02), in the caudate/putamen at E15–P14 (E12: t(4) = 0.30, p = 0.78; E15: t(4) = 7.91, p = 0.001; P1: t(4) = 7.87, p = 0.001; P3: t(4) = 10.24, p = 0.0005; P7: t(4) =7.29, p = 0.002; P14: t(4) =5.12, p = 0.007), in the frontal cortex at P1–P14 (P1: t(4) = 3.94, p = 0.02; P3: t(4) = 26.49, p < 0.0001; P7: t(4) =5.26, p = 0.006; P14: t(4) = 6.72, p = 0.003), and in the hippocampus at P14 only (P1: t(4) = 0.53, p = 0.63; P3: t(4) = 1.25, p = 0.28; P7: t(4) = 1.73, p = 0.16; P14: t(4) = 2.87, p = 0.05) compared with controldev mice (Table 1). There were no significant differences between FBCRHOEdev and controldev mice at any time point analyzed in the thalamus (E15: t(4) = 0.58, p = 0.59; P1: t(4) = 0.37, p = 0.73; P3: t(4) = 0.73, p = 0.50; P7: t(4) = 0.56, p = 0.61; P14: t(4) = 1.56, p = 0.19) or PVN (E15: t(4) = 0.45, p = 0.67; P1: t(4) = 2.38, p = 0.08; P3: t(4) = 0.66, p = 0.54; P7: t(4) = 0.88, p = 0.43; P14: t(4) = 1.86, p = 0.14) (Table 1). This developmental time frame of transgenic expression is consistent with reports showing activation of the CaMKII promoter during the last 2 weeks of rodent gestation (Burgin et al., 1990) but is different from other reports using the CaMKII promoter in a transgenic manner (Wei et al., 2004; Lu et al., 2008).
Figure 2.
Expression of CRH in FBCRHOEdev and controldev mice during development. Representative sections showing CRH mRNA expression from FBCRHOEdev (OE) and controldev (Cntl) mice at E12, E15, P1, P3, P7 and P14. All mice were off doxycycline when analyzed. Overexpression of CRH likely occurs between E12 and E15.
Table 1.
Expression of CRH mRNA during development
| Developmental age | Brain area |
|||||
|---|---|---|---|---|---|---|
| Thalamus | PVN | Amygdala | Caudate/putamen | Frontal cortex | Hippocampus | |
| E12 | n.i. | n.i. | n.i. | 0.70 ± 0.49 | n.i. | n.i. |
| E15 | 0.79 ± 0.26 | 1.58 ± 0.95 | n.i. | 12.36 ± 1.41*** | n.i. | n.i. |
| P1 | 0.90 ± 0.11 | 4.98 ± 2.74 | 12.46 ± 2.18** | 25.98 ± 3.17*** | 10.27 ± 2.32* | 0.35 ± 1.07 |
| P3 | 0.68 ± 0.32 | 0.74 ± 0.11 | 19.00 ± 2.15*** | 33.86 ± 3.17*** | 11.64 ± 0.31*** | 1.67 ± 0.50 |
| P7 | 0.74 ± 0.30 | 1.55 ± 0.62 | 5.37 ± 0.37*** | 7.11 ± 0.81** | 3.84 ± 0.23** | 3.76 ± 1.54 |
| P14 | 0.68 ± 0.16 | 0.19 ± 0.07 | 9.64 ± 2.20* | 11.05 ± 1.95** | 9.03 ± 1.13** | 3.36 ± 0.81* |
Values are reported as mean ± SEM for FBCRHOE mRNA fold change over controldev signal. Mice were analyzed at E12, E15, P1, P3, P7, and P14. All mice were off doxycycline. Overexpression of CRH likely occurs between E12 and E15. n.i., Not identifiable.
*p ≤ 0.05;
**p ≤ 0.01;
***p ≤ 0.001 compared to controldev; Student's t test.
Lifetime CRH expression induces a Cushingoid-like phenotype
As an activator peptide in the HPA axis, CRH overexpression can induce strong activation of the HPA axis leading to pathological levels of glucocorticoids. Previous models of global CRH overexpression exhibited truncal obesity, muscle wasting, hair loss, and thinning of the skin (Stenzel-Poore et al., 1992). To evaluate the possibility of a similar Cushingoid-like phenotype in FBCRHOE mice, we compared lifetime overexpressors (FBCRHOElife) and transient overexpressors (FBCRHOEdev mice) with corresponding control mice for each group of OE mice. At weaning (i.e., P21), mice from all groups looked outwardly similar. However, by 8 weeks of age FBCRHOElife mice developed a Cushingoid-like phenotype including truncal obesity, hair loss, and thinning of the skin compared with controllife mice (supplemental Fig. S1A, available at www.jneurosci.org as supplemental material). Consistent with our in situ data showing suppression of CRH overexpression with doxycycline, FBCRHOEdev mice showed no Cushingoid-like phenotype at 8 weeks compared with controldev mice. To quantify the Cushingoid-like phenotype, we compared the adult weights for all groups. FBCRHOElife mice exhibited reduced weight at adulthood compared with controllife mice (Student's t test; t(32) = 2.52, p = 0.02) (supplemental Fig. S1B, available at www.jneurosci.org as supplemental material). Adult weights of FBCRHOEdev mice were indistinguishable from controldev mice (t(22) = 0.39, p = 0.70) (supplemental Fig. S1B, available at www.jneurosci.org as supplemental material).
Circadian HPA axis activity in mice with lifetime CRH overexpression
The Cushingoid-like phenotype in FBCRHOElife mice suggested that these mice may exhibit elevated glucocorticoid levels. To evaluate circadian HPA axis activity, we measured corticosteroid levels at circadian nadir and peak from FBCRHOElife and controllife mice. FBCRHOElife mice show increased corticosterone (Student's t test; t(16) = 3.63, p = 0.002) (supplemental Fig. S1C, available at www.jneurosci.org as supplemental material) and ACTH at circadian nadir (nadir: controllife, 62.34 ± 13.77 pg/ml, n = 9; FBCRHOElife, 123.4 ± 23.40, n = 8; t(15) = 2.311, p = 0.04), but no differences at circadian peak (corticosterone, t(16) = 0.43, p = 0.67) (supplemental Fig. S1C, available at www.jneurosci.org as supplemental material) (ACTH controllife, 83.80 ± 16.98 pg/ml, n = 8; FBCRHOElife, 63.17 ± 7.61, n = 8; t(14) = 1.11, p = 0.29).
HPA axis activity in mice with transient early-life CRH overexpression
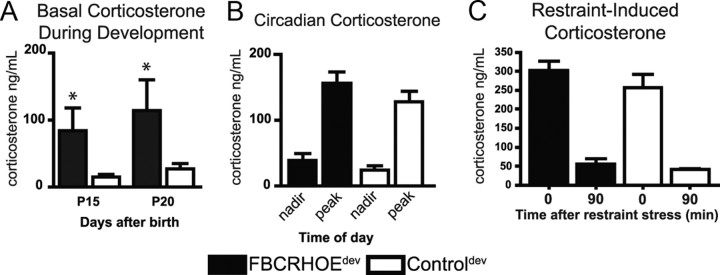
A change in the endocrine system is an important component in the hypothesized effects of early-life stress on long-term molecular and behavioral changes. To evaluate the role of HPA axis activity in developmentally exposed FBCRHOE mice, we obtained basal plasma samples from P15 and P20 FBCRHOEdev mice and controldev mice. During this early period of transient CRH overexpression (i.e., E15-P21) FBCRHOEdev mice show elevated corticosterone at P15 (Student's t test; t(6) = 2.00, p = 0.05) and P20 (t(8) = 1.88, p = 0.05) compared with controldev mice (Fig. 3A).
Figure 3.
Examination of corticosterone in mice exposed to CRH during development only. A, Basal corticosterone is increased in P15 and P20 transient overexpressors (FBCRHOEdev) mice (n = 4–5) compared with controldev mice (n = 4–5). B, No differences are seen between adult FBCRHOEdev (n = 16) and controldev mice (n = 16) in circadian corticosterone. C, Adult FBCRHOEdev mice (n = 9) show no alterations in plasma corticosterone 0 or 90 min following restraint stress compared with controldev mice (n = 9). OE, FBCRHOEdev; Cntl, controldev. Student's t test; *p = 0.05 compared with controldev at specified postnatal day.
After CRH overexpression is turned off, comparing adult FBCRHOEdev and controldev mice, we found a main effect of time in circadian corticosterone (two-way ANOVA, F(1,59) = 68.7, p < 0.0001) and ACTH levels (F(1,56) = 7.03, p = 0.01) but no main effect of genotype or time × genotype for corticosterone (genotype: F(1,59) = 2.59, p = 0.11; time × genotype: F(1,59) = 0.19, p = 0.66) or ACTH (genotype: F(1,56) = 2.98, p = 0.09; time × genotype: F(1,56) = 0.00, p = 0.97). Bonferroni post hoc tests revealed that adult FBCRHOEdev overexpressing mice show no significant differences in circadian corticosterone (nadir: t = 0.82, p > 0.05; peak: t = 1.46, p > 0.05) (Fig. 3B) or ACTH (nadir: controldev, 79.98 ± 10.29 pg/ml, n = 14; FBCRHOEdev, 107.9 ± 13.12 pg/ml, n = 15, t = 1.23, p > 0.05; peak: controldev, 122.6 ± 20.99 pg/ml, n = 15; FBCRHOEdev, 149.2 ± 16.01, n = 16, t = 1.21, p > 0.05) compared with controldev mice.
To investigate the stress-activated HPA axis in adult FBCRHOEdev mice, we obtained plasma from these mice immediately following and 90 min after 30 min of restraint stress. Comparing FBCRHOEdev to controldev mice, we found a main effect of time for corticosterone (two-way ANOVA, F(1,18) = 118.9, p < 0.0001) and ACTH (F(1,35) = 123.0, p < 0.0001) but no main effect for genotype (corticosterone: F(1,18) = 1.56, p = 0.22; ACTH: F(1,35) = 0.12, p = 0.74) or genotype × time (corticosterone: F(1,18) = 0.5, p = 0.49; ACTH: F(1,35) = 0.41, p = 0.53). Bonferroni post hoc tests revealed that FBCRHOEdev mice show no differences either immediately following or 90 min following restraint stress in corticosterone (0 min: t = 1.34, p > 0.05; 90 min: t = 0.43, p > 0.05) (Fig. 3C) or ACTH (0 min: controldev, 368.1 ± 39.5 pg/ml; FBCRHOEdev, 342.4 ± 29.7; t = 0.70, p > 0.05; 90 min: controldev, 59.60 ± 8.6 pg/ml; FBCRHOEdev, 67.4 ± 11.5; t = 0.21, p > 0.05) compared with controldev mice. Thus, the behavioral changes described below in FBCRHOEdev mice are not likely due directly to a change in the absolute level of corticosterone seen acutely in the brain during behavioral testing.
Forebrain CRH overexpression results in altered anxiety-like behavior
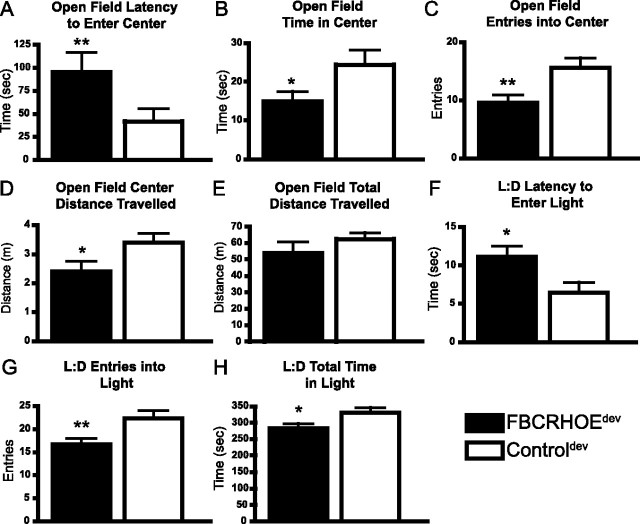
As an increase in CRH early in life has been associated with changes in adult behavior, we examined anxiety-like behaviors in mice exposed to early forebrain CRH (FBCRHOEdev) and lifetime overexpressors (FBCRHOElife). The open-field test opposes a mouse's innate curiosity to explore a novel area with its aversion to center open spaces. We evaluated the latency to enter the center, total time in the center, number of entries into the center, distance traveled in the center and total distance traveled in the open-field test. Consistent with an increase in anxiety-like behavior, FBCRHOEdev mice show increased latency to enter the center of the field (Student's t test; t(20) = 2.85, p = 0.01) (Fig. 4A), reduced time in the center zone (t(20) = 2.05, p = 0.05) (Fig. 4B), reduced number of entries in the center zone (t(20) = 2.68, p = 0.01) (Fig. 4C), and reduced distance traveled in the center (t(20) = 2.05, p = 0.05) (Fig. 4D) compared with controldev mice. As a measure of locomotor output, FBCRHOEdev mice show equivalent distance traveled in the open field (t(20) = 1.19, p = 0.25) (Fig. 4E) compared with controldev mice.
Figure 4.
FBCRHOEdev mice show increased anxiety-like behavior. A–D, In the open-field test, FBCRHOEdev (n = 11) mice show increased latency to enter the center of the open field (A), decreased time in the center of the open field (B), decreased number of entries center of the open field (C), and decreased distance traveled in the center of the open field compared with controldev mice (n = 11) (D). E, FBCRHOEdev show equivalent total distance traveled in open-field testing compared with controldev mice. F–H, In the L:D preference task, FBCRHOEdev mice (n = 11) show increased latency to enter the light (F), decreased number of entries into the light (G), and decreased time in the light compared with controldev mice (n = 11) (H). Student's t test; *p ≤ 0.05; **p ≤ 0.01 compared with controldev mice.
Although our in situ data in FBCRHOEdev adult mice suggested that doxycycline repressed transgenic CRH expression appropriately, we wanted to test the efficacy of doxycycline further by comparing the behavior of controldev mice to lifetime non-overexpressing FBCRHOE mice (FBCRHOEon doxy, on doxycycline E0–>P56, so mice were never exposed to transgenic CRH). We reasoned that if doxycycline repressed transgenic CRH in FBCRHOEon doxy mice, their behavior would be indistinguishable from controldev mice. Supporting this hypothesis, we found no significant differences between controldev mice and lifetime non-overexpressing FBCRHOEon doxy mice in latency to enter the center (controldev: 42.42 ± 8.30 s, n = 11; FBCRHOEon doxy: 46.42 ± 6.18 s, n = 6; Student's t test; t(15) = 0.32, p = 0.76), time in center square (controldev: 24.37 ± 3.82 s, n = 11; FBCRHOEon doxy: 24.70 ± 1.41 s, n = 6; t(15) = 0.06, p = 0.95), entries into the center (controldev: 15.55 ± 1.77 entries, n = 11; FBCRHOEon doxy: 15.33 ± 2.22 entries, n = 6; t(15) = 0.07, p = 0.94), distance in center square (controldev: 3.34 ± 0.33 m, n = 11; FBCRHOEon doxy: 3.26 ± 0.52 m, n = 6; t(15) = 0.21, p = 0.83), or total distance traveled (controldev: 62.40 ± 3.90 m, n = 11; FBCRHOEon doxy: 59.08 ± 10.12 m, n = 6; t(15) = 0.37, p = 0.72).
One potential caveat of the tetracycline-off system is that doxycycline treatment could induce behavioral changes independent of genotype. To evaluate possible nonspecific effects of doxycycline treatment, we compared controldev mice (on doxy P21-adult), controlon doxy mice (on doxy E0-adult) and controllife mice (off doxy E0-adult). In open-field testing, using a one-way ANOVA we found no significant differences between controldev mice, controlon doxy mice and controllife mice in latency to enter the center (controldev: 42.42 ± 8.30 s, n = 11; controlon doxy: 66.38 ± 20.12, n = 6; controllife: 47.56 ± 15.3, n = 9; F(2,23) = 0.66, p = 0.53), time in center square (controldev: 24.37 ± 3.82 s, n = 11; controlon doxy: 17.02 ± 3.70, n = 6; controllife: 18.33 ± 2.69, n = 9; F(2,23) = 1.22, p = 0.31), entries into the center (controldev: 15.55 ± 1.77 entries, n = 11; controlon doxy: 17.0 ± 3.18, n = 6; controllife: 17.25 ± 1.9, n = 9; F(2,23) = 0.20, p = 0.82), distance in center square (controldev: 3.34 ± 0.33 m, n = 11; controlon doxy: 3.60 ± 0.67, n = 6; controllife: 3.71 ± 0.55, n = 9; F(2,23) = 0.13 p = 0.88), or total distance traveled (controldev: 62.40 ± 3.90 m, n = 11; controlon doxy: 56.04 ± 5.02, n = 6; controllife: 66.40 ± 5.07, n = 9; F(2,23) = 1.06, p = 0.36).
We next analyzed anxiety and locomotor behavior in FBCRHOElife mice using the open-field test. Similar to FBCRHOEdev mice, FBCRHOElife mice show increased latency to enter the center of the field (controllife, 47.56 ± 15.34 s; FBCRHOElife, 177.5 ± 49.58 s; Student's t test; t(16) = 2.50, p = 0.02), a trend toward reduced time in the center zone (controllife, 18.33 ± 2.69 s; FBCRHOElife, 11.94 ± 2.31 s; t(16) = 1.80, p = 0.09), reduced number of entries in the center zone (controllife, 15.89 ± 2.18 entries; FBCRHOElife, 9.00 ± 2.30 entries; t(16) = 2.18, p = 0.04), and reduced distance traveled in the center (controllife, 3.71 ± 0.56 m; FBCRHOElife, 1.95 ± 0.56 m; t(16) = 2.21, p = 0.04) compared with controllife mice. However, confounding these anxiety-like measures, FBCRHOElife mice showed decreased distance traveled in the open field compared with controllife mice (controllife, 66.52 ± 5.07 m; FBCRHOElife, 44.51 ± 4.89 m; t(16) = 3.12, p = 0.007). Due to this locomotor change, the Cushingoid-like phenotype of FBCRHOElife mice and the established data on other models of lifetime CRH overexpression (Stenzel-Poore et al., 1992, 1994), we did not test these mice in any other anxiety or despair-like behavioral tests.
To validate the anxiogenic phenotype of FBCRHOEdev mice in open-field testing, we tested mice exposed to CRH during development in L:D preference. L:D preference is a common test for anxiety-like behavior that pits an animal's instinct for exploration against the animal's aversion to bright spaces. In L:D preference, mice start in a dark chamber and are allowed to cross over to a light chamber. The latency to enter the light chamber and time spent in the chamber are measures of anxiety-like behavior. FBCRHOEdev mice show increased latency to enter the light compartment (Student's t test; t(20) = 2.41, p = 0.03) (Fig. 4F), decreased number of entries into the light (t(20) = 2.77, p = 0.01) (Fig. 4G) and reduced total time spent in the light compartment compared with controldev mice (t(20) = 2.21, p = 0.04) (Fig. 4H). We found no significant differences between controldev mice and FBCRHOEon doxy in latency to enter the light (controldev: 6.45 ± 1.30 s, n = 11; FBCRHOEon doxy: 5.83 ± 0.87 s, n = 6; Student's t test; t(15) = 0.33, p = 0.75), number of entries into the light (controldev: 22.36 ± 1.61 entries, n = 11; FBCRHOEon doxy: 22.83 ± 1.33 entries, n = 6; t(15) = 0.19, p = 0.84), or total time spent in the light (controldev: 329.9 ± 16.23 s, n = 11; FBCRHOEon doxy: 340.5 ± 8.44 s, n = 6; t(15) = 0.45, p = 0.65). In L:D preference, using a one-way ANOVA, we found no significant differences between controldev mice, controlon doxy mice and controllife mice in latency to enter the light (controldev: 6.45 ± 1.30 s, n = 11; controlon doxy: 6.50 ± 1.15 s, n = 6; controllife: 4.78 ± 1.61 s, n = 9; F(2,23) = 0.41, p = 0.67), number of entries into the light (controldev: 22.36 ± 1.61 entries, n = 11; controlon doxy: 24.33 ± 1.12 entries, n = 6; controllife: 25.78 ± 2.62 entries, n = 9; F(2,23) = 0.87, p = 0.43), or total time spent in the light (controldev: 329.9 ± 16.23 s, n = 11; controlon doxy: 322.0 ± 11.97 s, n = 6; controllife: 307.4 ± 25.53 s, n = 9; F(2,23) = 0.98, p = 0.39).
Although our behavioral testing indicated an increase in anxiogenic behavior in FBCRHOEdev mice, it also revealed a possible motoric change in L:D preference with FBCRHOEdev mice showing decreased number of light entries. To determine the significance of any motoric deficit, we tested FBCRHOEdev in the rotorod test and a sensory-motor battery. In all tests, FBCRHOEdev mice failed to show any statistically significant differences compared with controldev mice (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
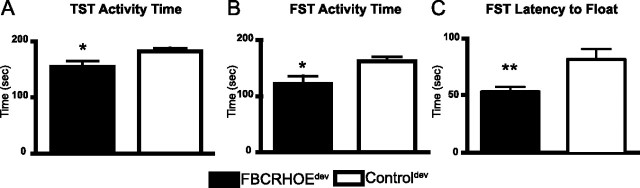
Forebrain CRH overexpression results in increased depression-like behavior
MDD is associated with a variety of changes in behavior including loss of energy, sleep alterations, learning and memory impairments, anhedonia, and helplessness (despair). To evaluate the role of CRH in despair-like behavior, we tested FBCRHOEdev mice in the TST and FST. In both tests, reduced activity is associated with an increase in despair-like behavior as a variety of antidepressants increase activity in these tests in mice and rats. In the TST, FBCRHOEdev mice show reduced activity (increase in despair-like behavior) compared with controldev mice (t(22) = 2.12, p = 0.05) (Fig. 5A). Consistent with their depression-like phenotype in the TST, FBCRHOEdev mice show reduced activity in the FST compared with controldev mice (t(18) = 2.52, p = 0.02) (Fig. 5B). Furthermore, FBCRHOEdev mice exhibit decreased latency to float in the FST compared with controldev mice (t(18) = 3.07, p = 0.01) (Fig. 5C). We found no significant differences between controldev mice and lifetime non-overexpressing FBCRHOEon doxy mice in TST activity (controldev: 181.30 ± 6.69 s, n = 12; FBCRHOEon doxy: 195.8 ± 8.55 s, n = 6; Student's t test; t(16) = 1.29, p = 0.21), FST activity (controldev: 161.2 ± 8.24 s, n = 11; FBCRHOEon doxy: 173.4 ± 11.67 s, n = 6; t(13) = 0.79, p = 0.44), or FST latency to float (controldev: 78.19 ± 10.05 s, n = 11; FBCRHOEon doxy: 112.5 ± 20.96 s, n = 6; t(13) = 1.67, p = 0.12). Using a one-way ANOVA we found no significant differences between controldev mice, controlon doxy mice and controllife mice in TST activity (controldev: 181.30 ± 6.69 s, n = 12; controlon doxy: 210.9 ± 17.88 s, n = 6; controllife: 183.2 ± 13.50 s, n = 8; Student's t test; F(2,23) = 1.73, p = 0.20). In the FST, using Student's t test we found no difference between controldev mice and controlon doxy mice in FST activity (controldev: 161.2 ± 8.24 s, n = 11; controlon doxy, 160.9 ± 10.60 s, n = 5; t(14) = 002, p = 0.99) or FST latency to float (controldev: 78.19 ± 10.05 s, n = 11; controlon doxy: 102.0 ± 9.70, n = 5; t(14) = 1.44, p = 0.17) (note: controllife mice were never tested in FST).
Figure 5.
FBCRHOEdev mice exhibit despair-like behavior in the TST and FST. A, In the TST, FBCRHOEdev mice (n = 12) show reduced activity compared with controldev mice (n = 12). B, C, In the FST, FBCRHOEdev mice (n = 9) show reduced activity (B) and reduced latency to float (C) compared with controldev mice (n = 11). Student's t test; *p ≤ 0.05; **p = 0.01 compared with controldev mice.
Treatment with the tricyclic antidepressant imipramine reverses depression-like behavior in FBCRHOE mice
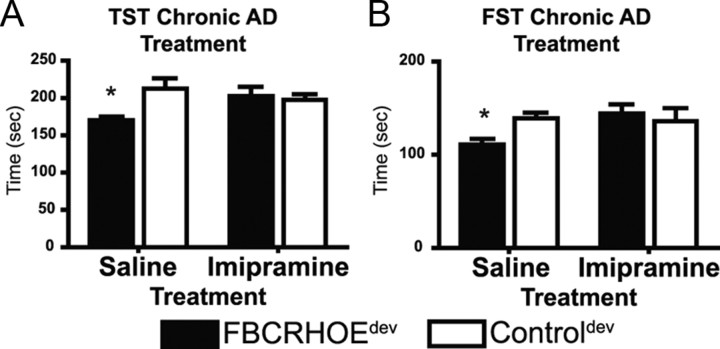
To determine the effect of antidepressant activity on behavioral deficits in FBCRHOE mice exposed to CRH early in life, we treated FBCRHOEdev and controldev mice with the tricyclic antidepressant, imipramine, or vehicle, chronically. We treated FBCRHOEdev and controldev mice with daily imipramine or saline for 3–4 weeks before performing TST and FST. In the TST, we found a significant interaction of genotype × treatment but no main effect of treatment or genotype (two-way ANOVA genotype × treatment: F(1,20) = 5.99, p = 0.02; treatment: F(1,20) = 0.82, p = 0.38; genotype: F(1,20) = 3.77, p = 0.07). Bonferroni post hoc tests revealed a significant difference between FBCRHOEdev saline-treated mice and saline-treated controldev mice (t = 3.10, p < 0.05) (Fig. 6A). Similarly, in the FST, we found a significant interaction of genotype × treatment but no main effect of treatment or genotype (genotype × treatment: F(1,19) = 8.13, p = 0.01; treatment: F(1,19) = 2.89, p = 0.11; genotype: F(1,19) = 1.40, p = 0.25). Bonferroni post hoc tests revealed a significant difference between FBCRHOEdev saline-treated mice and saline-treated controldev mice in the FST (t = 2.79, p < 0.05) (Fig. 6B). However, imipramine normalized the activity level in FBCRHOEdev mice in both the TST (t = 0.36, p > 0.05) (Fig. 6A) and FST (t = 1.21, p > 0.05) (Fig. 6B) compared with imipramine-treated controldev mice. Using an acute treatment schedule, we found similar results in both the TST and FST compared with those reported above for chronic treatment with imipramine (supplemental Fig. S3A,B, available at www.jneurosci.org as supplemental material). In slight contrast, in the acute FST, imipramine caused a significant increase in activity in both controldev and FBCRHOEdev mice (supplemental Fig. S3B, available at www.jneurosci.org as supplemental material).
Figure 6.
Effects of chronic imipramine [antidepressant (AD)] on FBCRHOEdev despair-like behavior. A, FBCRHOEdev mice (n = 6) treated with chronic imipramine show no difference in TST activity compared with controldev mice (n = 6) treated with imipramine. B, FBCRHOEdev mice (n = 6) treated with chronic imipramine show no difference in FST activity compared with controldev mice (n = 6) treated with imipramine. Saline (vehicle)-treated FBCRHOEdev continued to show despair-like behavior compared with controldev saline-treated mice in the TST (A) and FST (B). Bonferroni test; *p < 0.05 versus saline-treated controldev.
The reversal of despair-like behavior after imipramine treatment suggests a specific mode of action regarding the pharmacological effects of imipramine on despair. However, it is possible that imipramine is nonspecifically increasing locomotor behavior causing an apparent normalization of the despair-like behavior. To address this question and to determine the effect of imipramine on anxiety-like behavior, we treated FBCRHOEdev and controldev mice with imipramine, or vehicle, chronically and then tested the mice in the open field (supplemental Fig. S3C–G, available at www.jneurosci.org as supplemental material). We found no evidence for an effect of imipramine on total distance traveled (two-way ANOVA main effect, treatment × genotype: F(1,12) = 0.68, p = 0.43; treatment: F(1,12) = 0.14, p = 0.71; genotype: F(1,12) = 0.49, p = 0.50) (supplemental Fig. S3G, available at www.jneurosci.org as supplemental material) suggesting that our previous results in despair-like behavior were not confounded by a nonspecific increase in locomotor output with imipramine treatment. Surprisingly, testing the ability of imipramine to normalize anxiety-like behavior in FBCRHOEdev mice, we found no main effects of treatment × genotype, treatment or genotype for latency to enter the center (treatment × genotype: F(1,12) = 0.04, p = 0.84; treatment: F(1,12) = 0.39, p = 0.56; genotype: F(1,12) = 0.01, p = 0.93) (supplemental Fig. S3C, available at www.jneurosci.org as supplemental material), time in the center (treatment × genotype: F(1,12) = 0.14, p = 0.71; treatment: F(1,12) = 0.01, p = 0.99; genotype: F(1,12) = 1.43, p = 0.25) (supplemental Fig. S3D, available at www.jneurosci.org as supplemental material), or distance traveled in the center (treatment × genotype: F(1,12) = 0.19, p = 0.67; treatment: F(1,12) = 0.03, p = 0.88; genotype: F(1,12) = 1.74, p = 0.21) (supplemental Fig. S3F, available at www.jneurosci.org as supplemental material). We found a significant main effect of genotype and treatment for the number of entries into the center (treatment × genotype: F(1,12) = 1.19, p = 0.30; treatment: F(1,12) = 6.00, p = 0.03; genotype: F(1,12) = 9.78, p = 0.01) (supplemental Fig. S3E, available at www.jneurosci.org as supplemental material). Bonferroni post hoc tests revealed that FBCRHOEdev saline-treated mice make fewer entries into the center compared with controldev saline-treated mice (t = 2.98, p < 0.05) but that FBCRHOEdev mice treated with imipramine make equivalent number of entries into the center compared with imipramine-treated controldev mice (t = 1.44, p > 0.05). Overall, these data suggest that daily injections (of imipramine or saline) have no effect on total locomotor output but may have a nonspecific effect on anxiety that masks many of the basal differences between genotypes.
Analysis of GR and CRHR1 expression after early exposure to CRH
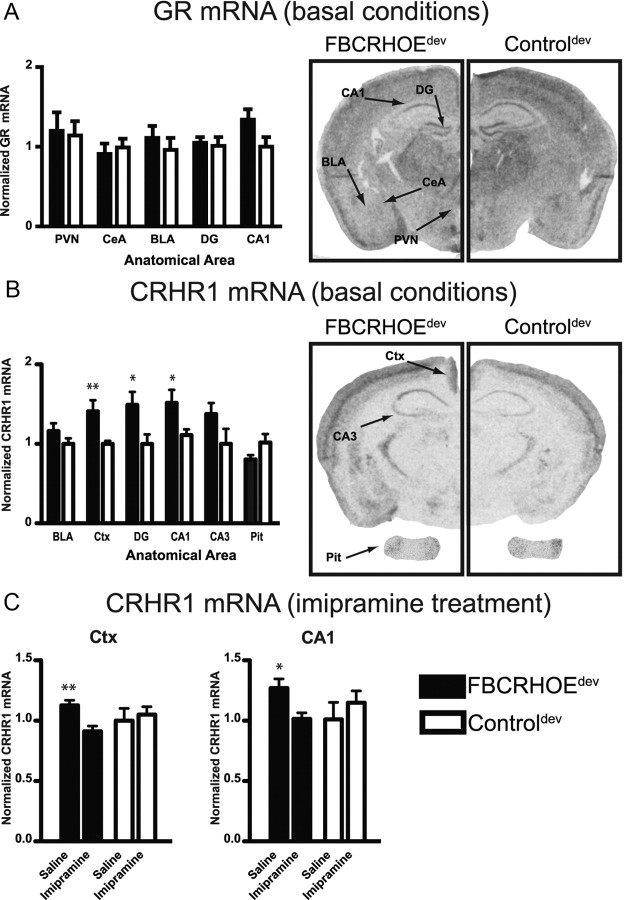
To investigate the mechanism of the behavioral deficits in FBCRHOEdev mice, we evaluated GR and CRHR1 mRNA expression in adult developmental animals. Both of these molecules are related to the effects of early-life stress and both have the capacity to be regulated by CRH and corticosterone signaling. We evaluated GR mRNA expression by in situ hybridization in the PVN, CeA, BLA, DG and CA1. FBCRHOEdev mice show no significant changes in GR mRNA compared with controldev mice in the PVN (Student's t test; t(16) = 0.20, p = 0.85), CeA (t(16) = 0.69, p = 0.50), BLA (t(16) = 0.50, p = 0.63), or DG (t(16) = 0.35, p = 0.74) although we observed a trend toward increased CA1 GR mRNA in FBCRHOEdev mice (t(16) = 1.92, p = 0.07) (Fig. 7A). We also evaluated CRHR1 mRNA expression by in situ hybridization in the BLA, cingulate cortex, DG, CA1, CA3, and the pituitary gland. In contrast to the GR results, FBCRHOEdev mice expressed increased CRHR1 mRNA in the cingulate cortex (t(16) = 2.82, p = 0.01), DG (t(16) = 2.44, p = 0.03), and CA1 (t(16) = 2.27, p = 0.04) with equivalent CRHR1 in the BLA (t(16) = 1.34, p = 0.20), area CA3 (t(16) = 1.61, p = 0.13), and the pituitary gland (t(5) = 1.58, p = 0.17) compared with controldev mice (Fig. 7B). Observing this basal CRHR1 change and the behavioral normalization of TST and FST with imipramine, we wanted to assess whether imipramine treatment influences CRHR1 expression in either the FBCRHOEdev or controldev mice. After chronic treatment with imipramine, we found a significant reduction in CRHR1 signal comparing saline-treated FBCRHOEdev and imipramine-treated FBCRHOEdev mice in the cingulate cortex (t(6) = 3.54, p = 0.01) and CA1 (t(6) = 2.80, p = 0.03) (Fig. 7C). In contrast, we found no significant difference between saline and imipramine-treated controldev mice in the cingulate cortex (t(6) = 0.42, p = 0.69) or CA1 (t(6) = 0.81, p = 0.45) in CRHR1 mRNA expression. No differences were seen within genotypes between saline and imipramine treatment for the BLA, DG or CA3 (data not shown).
Figure 7.
FBCRHOEdev mice show changes in CRHR1 mRNA expression that are reversed by imipramine treatment. A, Quantitation of GR mRNA from in situ hybridization of FBCRHOEdev mice and controldev mice in the PVN, CeA, BLA, DG, and CA1. FBCRHOEdev mice (n = 9) show equivalent GR expression compared with controldev mice (n = 9). Right, Representative sections showing GR in situ signal. B, Quantitation of basal CRHR1 mRNA in FBCRHOEdev and controldev mice. FBCRHOEdev mice (n = 3–9) show increased CRHR1 expression in the cingulate cortex (Ctx), DG and CA1 compared with controldev mice (n = 4–9). On right, representative sections showing CRHR1 in situ signal in brain and pituitary gland (Pit). C, Chronic imipramine reduces CRHR1 expression in the Ctx and CA1 in FBCRHOEdev mice (n = 4 per treatment). No differences were observed between controldev mice treated with saline (n = 4) or imipramine (n = 4) for either area. Student's t test; *p < 0.05; **p = 0.01 compared with controldev (B) or imipramine FBCRHOEdev (C).
Discussion
Although multiple lines of evidence have suggested that CRH is an important component in the pathogenesis of MDD, the spatial specificity of CRH action and the role of CRH in mediating the effects of early-life stress remain unresolved. Here, we report that elevation of CRH during early development induces long-lasting changes in adult anxiety in L:D preference and open-field behavior and despair-like activity in the TST and FST. Furthermore, the behavioral changes were accompanied by changes in CRHR1 expression. Finally, our observations that the despair-like behavioral deficits were normalized with imipramine treatment has important implications for understanding the role of an altered stress adaptation system in the treatment of MDD associated with early-life abuse.
The tetracycline-off system described here is a useful model to investigate the role of CRH and the HPA axis in modulating the long-term effects of early postnatal stress because this system allows us to look at stress-associated molecules without actually subjecting the mice to an external stressor. Although our temporal targeting from E15 to P21 of elevated CRH is an extension beyond the normal period for early-life stress studies in rodents, we believe that our ability to induce long-term behavioral changes in the absence of external stress makes our system a novel system to study the biochemical components of early-life stress. Importantly, in this study, we did not find any significant effects of doxycycline treatment on behavior in control mice. This suggests that differences observed between FBCRHOEdev mice and controldev are a result of the unique intersection of double transgenic genotype and time of overexpression. We find that transient early biochemical changes similar to those seen with early-life stress are likely to impact later behavioral and molecular sequelae. Of note, we do not know whether transient, but robust, elevation of CRH during adulthood would similarly lead to long-term behavioral sequelae.
The molecular changes we observed in FBCRHOEdev mice illustrate the dynamic nature of HPA axis-controlled molecules. First, we found no significant changes in GR mRNA in FBCRHOEdev mice. This finding is interesting because early-life stress in rodents is associated with decreased GR expression, altered HPA axis activity and behavioral changes in adult animals (Aisa et al., 2008). Nonetheless, our results are consistent with the observation that during early-life stress disruption of forebrain CRHR1 does not influence GR mRNA expression despite increases in corticosterone in CRHR1 knockout (KO) mice (Schmidt et al., 2006). These and our data suggest that GR changes associated with maternal deprivation occur via a limbic CRH/CRHR1-independent pathway. In addition, we see normal HPA axis activity under circadian conditions and after restraint stress in adult FBCRHOEdev mice that cannot be accounted for by any observed changes in CRHR1 expression in the pituitary gland. These results suggest that the effects of early-life stress on behavior and HPA axis function can be dissociated. Specifically, our results with FBCRHOEdev mice suggest that early exposure to CRH in extrahypothalamic areas coupled with increased glucocorticoid levels during development are important factors in the long-term development of behavioral changes associated with depression and anxiety with reduced impact on adult HPA axis changes normally observed in a early-life stress models.
Our inducible system shows overexpression in the adult mouse in a variety of important limbic areas including the amygdala, hippocampus, and cortex. However, there exists the possibility that some of the endocrine and behavioral changes observed arose from ectopic expression of CRH. Although the areas of highest endogenous CRH expression are the PVN and CeA, the other areas targeted in FBCRHOE mice do show the presence of endogenous CRH (Keegan et al., 1994) and CRHR (Aguilera et al., 2004), and/or respond to CRH activity under a variety of conditions (Brunson et al., 2001; Chen et al., 2001). Our observed increase in anxiety-like behavior is consistent with lowered anxiety-like behavior observed in forebrain restricted CRHR1 KO mice (Müller et al., 2003).
Furthermore, our results do not provide the only confirmation of a forebrain-restricted role for the effects of early life stress on adult function. There is evidence that forebrain expressed serotonin 1A receptor (5-HT1AR) can modulate long-term anxiety-like behavior (Gross et al., 2002). Using the tetracycline-off system, researchers were able to show that expression of the 5-HT1AR in the forebrain (hippocampus, cortex, and amygdala) during early postnatal life is necessary and sufficient to rescue the anxiogenic behavior in conventional 5-HT1AR KO mice (Heisler et al., 1998; Parks et al., 1998; Gross et al., 2002).
Interestingly, we found reduced CRH expression in FBCRHOElife mice in the PVN compared with endogenous levels in the control mice. It is likely that this reduction in CRH is an adaptive response to elevated glucocorticoids (Yi et al., 1993). The reason for the increase in FBCRHOElife HPA axis activity despite a decrease in PVN CRH is unknown and could involve a number of factors. First, the levels of CRH overexpression in the FBCRHOElife transgenic mice may be high enough that there is some leakage of CRH into the hypothalamic portal system. Another possibility may be related to the hypothesized role of CeA localized CRH in promoting HPA axis drive (Redgate and Fahringer, 1973) through induction of other peptides, such as vasopressin.
Notably, CRH overexpression in double transgenic mice was efficiently suppressed with doxycycline administration. Mice exposed to CRH from E15 to P21 show no changes in adult CRH expression in any area quantitated. Doxycycline suppression of transgenic overexpression is further supported by our finding that FBCRHOE mice on doxycycline from E0 to adulthood (i.e., FBCRHOEon doxy) show no behavioral differences compared with controldev mice. Furthermore, FBCRHOEdev mice show no significant changes in circadian HPA axis activity as adults. Changes in circadian corticosterone in FBCRHOEdev mice could have significantly hampered our ability to determine whether any changes in behavioral output are a result of long-term changes associated with early CRH exposure or merely continued HPA axis dysfunction.
Understanding the mechanisms of depression and anxiety can provide a novel framework to explore pharmacological treatment of these disorders. It has been hypothesized that CRH signaling through CRHR1 might be an important component of these mechanisms (Zobel et al., 2000). Between the two CRH receptors, CRHR1 is thought to contribute to increased behavioral disturbance (Claes, 2004). Disruption of CRHR1 throughout the mouse (Smith et al., 1998; Timpl et al., 1998) or restricted to the limbic forebrain (Müller et al., 2003) results in a decrease in anxiety-like behavior. As such, the increase in CRHR1 observed in FBCRHOEdev mice may be involved in some of the behavioral changes that we observed especially considering the finding that our early CRH overexpression overlaps with the normal spatial and developmental pattern of CRHR1 expression (Vazquez et al., 2006). Interestingly, we found that imipramine treatment caused a decrease in CRHR1 mRNA expression in FBCRHOEdev mice in the cingulate cortex and area CA1. These data suggest that imipramine-induced CRHR1 expression changes may have played a role in the antidepressant reversal of the FBCRHOEdev TST and FST despair-like phenotype. Supporting this link between CRHR1 and despair-like behavior, recent evidence has suggested that polymorphisms in CRHR1 may be related to the effect of early-life stress on human adult depression (Bradley et al., 2008).
It is likely that our similar results using acute versus chronic imipramine reflect a common mechanism between treatment schedules. We do, in general, find a stronger effect of antidepressant treatment in the FBCRHOEdev mice compared with the controldev mice, suggesting a specific antidepressant effect rather than a nonspecific change in locomotion. Of note, we found a difference between chronic and acute imipramine treatment in the FST. Although both treatment schedules increased activity of FBCRHOEdev mice, in the acute test, imipramine also increased activity in the controldev mice compared with saline-treated controldev mice. These data are consistent with previous research showing different effects of chronic and acute treatment in the FST (Detke et al., 1997; Boyle et al., 2005).
In contrast to the effects of chronic imipramine on the TST and FST, we were unable to show a reversal of the anxiety-like phenotype of FBCRHOEdev mice in open-field behavior with antidepressant treatment. However, daily handling associated with vehicle treatment seemed to increase anxiety in controldev mice to the point that for all measures except for the number of center zone entries, no differences were detected between saline-treated FBCRHOEdev mice and saline-treated controldev mice.
The observed long-lasting effects of early CRH exposure may be related to the role of CRH in hippocampal differentiation (Yan et al., 1998). Research in CRHR1 KO mice has suggested that early effects of increased CRH might induce long-term changes in neuronal connectivity (Chen et al., 2004b). Specifically, CRHR1 KO mice exhibit increased growth of dendritic processes during the early postnatal period. We found an increase in basal CRHR1 mRNA expression in the hippocampus and cortex in adult animals exposed to CRH early in life. Future research looking at hippocampal growth in FBCRHOEdev juvenile and adult mice should be informative in determining what role changes in hippocampal growth may play in any memory-related changes in these mice.
In humans, a variety of early stressors including physical abuse, sexual abuse, and parental loss have a significant impact on the development of adult endocrine and behavioral changes (Nemeroff, 2004). Our results suggest that some of the effects of stress in humans may be mediated by CRH activity in forebrain areas. Overall, we have demonstrated that increased CRH expression in the forebrain of mice induces an increase in anxiety-like and despair-like behavioral changes. These effects of CRH appear to be set early in development as animals exposed to CRH before weaning maintain behavioral changes as adults. The behavioral effects are associated with long-term changes in CRHR1 mRNA expression and can be reversed with imipramine. These results have important implications for understanding the role of the neuroendocrine system in MDD. The observation that early exposure to CRH in the forebrain causes long-term behavioral changes suggests that researchers and clinicians should explore the use of pharmacological blockade of CRH receptors in a prophylactic manner before high-risk patients develop psychiatric illness. The FBCRHOEdev mice should provide a useful model to begin testing this scenario.
Footnotes
This work was supported by grants from the National Institutes of Health to B.J.K. (F31MH075250), M.P.B. (F31MH067374), and L.J.M. (AG18876). We thank Maria Elena Morales for manuscript editing.
References
- Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:319–329. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Arató M, Bánki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25:355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004a;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004b;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes SJ. CRH, stress, and major depression: a psychobiological interplay. Vitam Horm. 2004;69:117–150. doi: 10.1016/S0083-6729(04)69005-4. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotoni1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, Muglia LM, Wozniak DF, Nardi A, Arvin KL, Holtzman DM, Linden DJ, Zhuo M, Muglia LJ, Chatila TA. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Scallet AC, Ali SF, Turner BB. “Isolation stress” revisited: isolation-rearing effects depend on animal care methods. Physiol Behav. 1991;49:1107–1118. doi: 10.1016/0031-9384(91)90338-o. [DOI] [PubMed] [Google Scholar]
- Keegan CE, Herman JP, Karolyi IJ, O'Shea KS, Camper SA, Seasholtz AF. Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M, Rubenstein JL, Stalla GK, Singewald N, Holsboer F, Wotjak CT, Wurst W, Deussing JM. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Llorente R, Laviola G, Viveros MP. Detrimental psychophysiological effects of early maternal deprivation in adolescent and adult rodents: altered responses to cannabinoid exposure. Neurosci Biobehav Rev. 2008;33:498–507. doi: 10.1016/j.neubiorev.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Misslin R, Herzog F, Koch B, Ropartz P. Effects of isolation, handling and novelty on the pituitary–adrenal response in the mouse. Psychoneuroendocrinology. 1982;7:217–221. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kühn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgate ES, Fahringer EE. A comparison of the pituitary adrenal activity elicited by electrical stimulation of preoptic, amygdaloid and hypothalamic sites in the rat brain. Neuroendocrinology. 1973;12:334–343. doi: 10.1159/000122182. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Deussing JM, Oitzl MS, Ohl F, Levine S, Wurst W, Holsboer F, Müller MB, de Kloet ER. Differential disinhibition of the neonatal hypothalamic- pituitary-adrenal axis in brain-specific CRH receptor 1-knockout mice. Eur J Neurosci. 2006;24:2291–2298. doi: 10.1111/j.1460-9568.2006.05121.x. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing's syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, López JF, Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Brain Res Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Künzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]