Summary
The creation of an oral drug delivery platform to administer chemotherapeutic agents effectively can not only increase patient compliance, but also potentially diminish drug toxicity. A microfabricated device offers advantages over conventional drug delivery technology. Here we describe the development of a multi-layered polymeric drug-loaded microfabricated device (microdevice) for the oral delivery of therapeutics, which offers unidirectional release of multiple therapeutics. The imaging and release of therapeutics from the multi-layered device was performed with three different fluorescently labeled albumins. The release of insulin and chemotherapeutic camptothecin was also observed to be released in a controlled manner over the course of 180 minutes in vitro. Furthermore, asymmetric delivery was shown to concrete drug at the device/cell interface, wherein 10 times more drug permeated an intestinal epithelial cell monolayer, compared to unprotected drug-loaded hydrogels. The bioactivity of the released chemotherapeutic was shown with cytostasis of colorectal adenocarcinoma cells. Cytostasis of drug loaded hydrogels was significantly higher than control empty hydrogel laden microdevices. Our results conclude that microfabrication of a hydrogel laden microdevice leads to a viable oral delivery platform for chemotherapeutics.
Keywords: Microfabrication, Microdevice, Cancer, Poly (ethylene glycol), micropatterning
Introduction
Several obstacles remain for delivery of therapeutic drugs in vivo, such as physical barriers to prevent adequate absorption, or non-specific delivery of drug. In particular, oral delivery presents a unique set of obstacles such as low drug permeability through the gastrointestinal epithelium and limited drug retention at the epithelial interface. Development of a delivery vehicle that can overcome many of the obstacles presented with oral delivery requires a high level of design control, which can be achieved using microfabrication. We have previously reported on the development of a microfabricated drug delivery vehicle that is precisely manufactured to have increased contact with the intestinal wall, while minimizing shear disturbances and allowing for unidirectional drug release from a protected reservoir1. Here we report on the development of a drug eluting microdevice specifically designed for the delivery of chemotherapeutics.
The development of a drug-eluting microdevice has advantages over current oral drug delivery strategies. Many conceptual approaches to improve oral delivery vehicles include polymer based protection of the drug reservoir from enzymatic degradation, targeting of the drug delivery vehicle to a specific regions in the intestine, and increased drug stability and permeability2. The most common oral drug delivery vehicles are microparticles that can be targeted to specific areas. Microparticles can be made from a variety of natural and synthetic polymers as well as possess advantageous properties such as biodegradation3, 4, and pH5, 6 and temperature7, 8 sensitivity. However, the method employed to synthesize these particles9, such as emulsion chemistry, solvent evaporation, or spray-drying, leads to inherent polydispersity of size, variance in drug-loading, and limited stability in the harsh GI microenvironment. Additionally, the geometry of spherical particles leads to sparse contact with the microvilliated apical cell surface (Fig. 1 A).
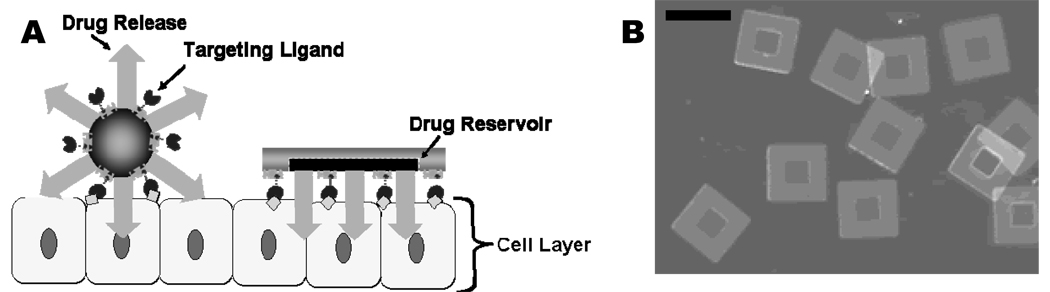
Figure 1.
A) Schematic representation of spherical particles and microdevice interface with intestinal epithelial cell surface. This illustration displays the advantages of a microfabricated drug delivery particle over traditional spherical particles: asymmetric release of drug, multi-site targeting for flow stability, and drug reservoir protection can be engineered into the design of the microdevice. B) Light micrograph of detached SU-8 microdevices without hydrogel. The scale bar represents 150 microns.
Micromachining allows for control over particle size, shape, aspect ratio, and surface features, facilitating the development of an engineered particle for oral drug delivery that can incorporate the advantages of microparticles while avoiding their design flaws1, 10–13. The microdevices, are engineered to overcome the barriers associated with oral delivery. To facilitate multi-cell and multi-site attachment, the active area of these devices is 22,500 square microns (Fig. 1 A, B). The increased number of attachment sites and the minimal height, on the order of 10 microns1, are engineered to reduce the shear forces, per mass, which can dislodge a particle and disrupt therapeutic release. A reservoir is patterned into the microdevice that allows for asymmetric delivery of the therapeutics, leading to a higher drug concentration at the cellular surface. Additionally, the reservoir protects the drug from enzymatic degradation (Fig. 1 B). The development of the microdevice body has been thoroughly reported1, 11–14; however an effective technique to fill the microdevice reservoir and achieve controlled release has not yet been demonstrated.
One promising route to provide effective control of drug loading and release is the incorporation of a hydrogel into the microdevice. Hydrogels are attractive for controlled release of therapeutics as they are a well studied system in tissue engineering15, 16 and drug delivery3, 15, 17–19. Orally delivered pH sensitive hydrogels, in the form of spherical particles, have been used to diffuse therapeutic proteins, such as insulin, directly into the gastrointestinal tract5, 18. The micropatterning of hydrogels, outside of spherical forms, has been accomplished through photoreaction injection20, 21, photolithography21–23, microfluidic patterning24–26 and micro-molding27, 28. Hydrogels have been photolithographically added to many lab-on-a-chip type devices, combining the hydrogel polymer with silicon or other polymers, such as SU-8. Many of these processes end with the hydrogel separated from the original substrate. One such process produces high aspect ratio micron scale hydrogels of poly(e-caprolactone)-co-polyethylene glycol (PCL-b-PEG-b-PCL-diacrylate) by releasing the particles from a polydimethylsiloxane (PDMS) mold. 21 In a related micro-molding process, methacrylated hyperbranched polyglycol microgels were patterned from SU-8 masters.29 One process where the hydrogel is not separated from the SU-8 involves a poly-2-hydroxyethylmethacrylate (p-HEMA) cross-linked with tertaethylene glycol dimethacrylate (TEGMA) hydrogel being patterned onto SU-8 by initially grafting photoinitiator 1-hydroxycyclohexyl phenyl ketone (HCPK) onto the surface, cross linking it with light, and applying the hydrogel through photolithography.23 More simply, acrylated PEG polymers can be covalently bound to free radicals present on a SU-8 surface. Wang et al. report that the photoacid generator in SU-8 forms free radicals which can act as a source to initiate UV-mediated grafting of acrylated polymers to the surface.30 Here we report the incorporation of micro-patterned poly (ethylene glycol) dimethacrylate (PEGMA) into SU-8 microdevices for the controlled elution of chemotherapeutics.
Results and Discussion
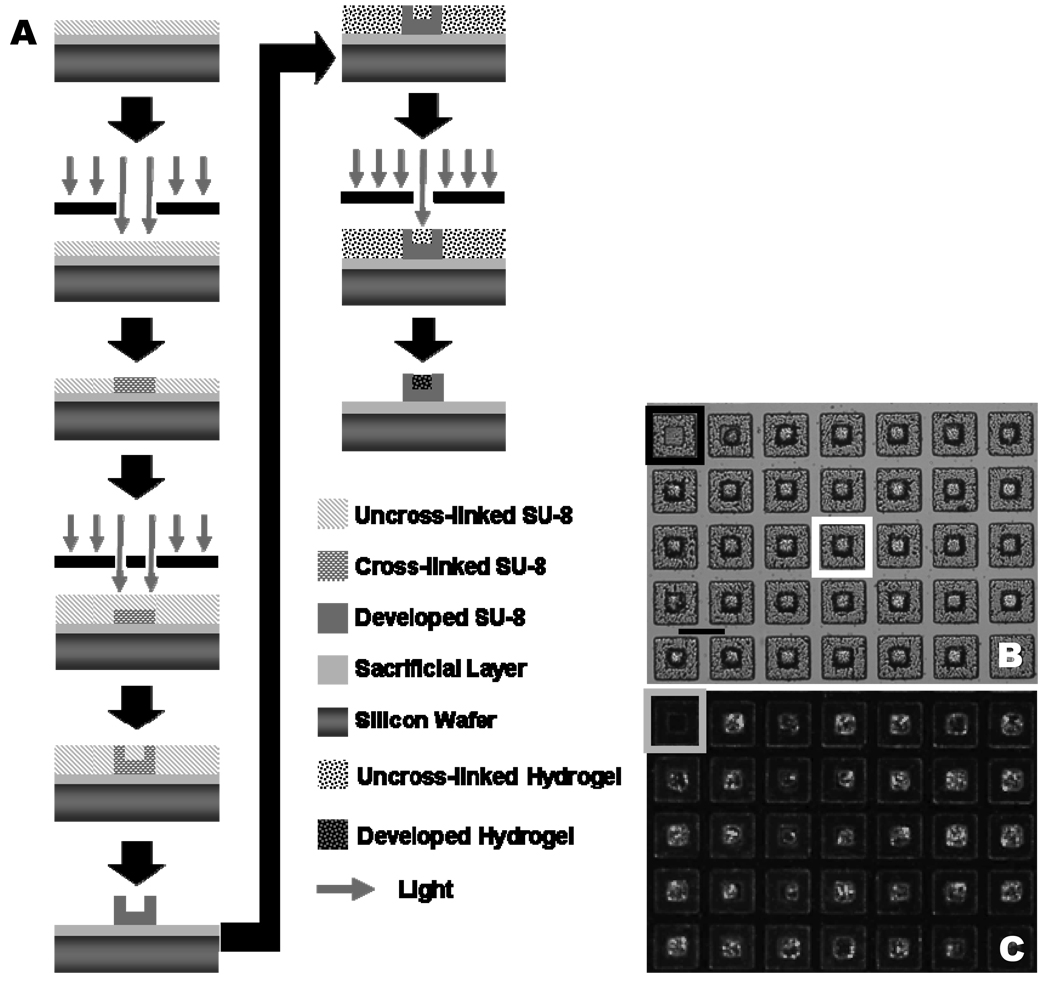
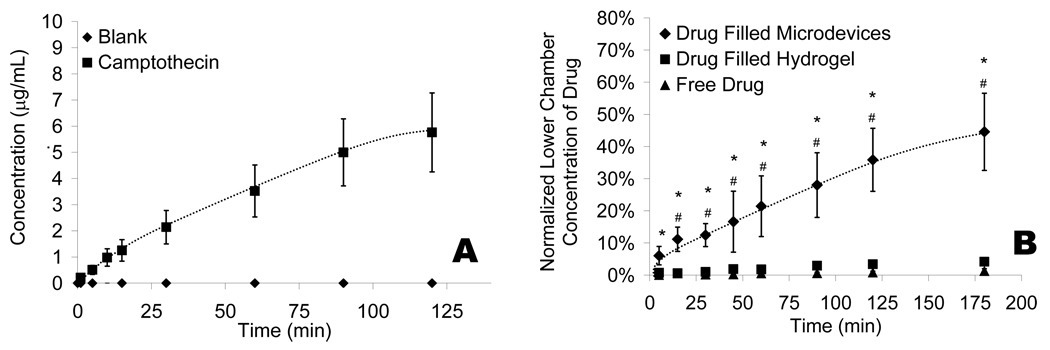
The hydrogels are introduced into the developed SU-8 microdevices through the same photolithographic process in which the microdevices are fabricated (Fig. 2 A). To confirm the covalent attachment of the PEGMA to the photoacid free radicals, the microdevices were agitated in phosphate buffer solution (PBS). After three days in PBS under agitation (250 rpm) the difference between the initial and final number of hydrogel filled microdevices was not significant (p-value > 0.65). By varying the photoinitiator solution concentration (cross linker and photoinitiator) in PEGMA from 1.5% (v/v) (34.1 ± 24.7% filled) to 2.9% (56.3 ± 12.5%) and finally 5.7% (94.3 ± 4.60%) the percent of hydrogel filled particles can be optimized to near complete filling. Filled microdevices were visualized with optical and fluorescent microscopy (Fig. 2 B, C). Figure 2 C displays the uniform filling of the microdevices with fluorescently labeled bovine serum albumin (BSA). To measure the drug elution kinetics of the microdevices, release of the auto-fluorescent chemotherapeutic camptothecin was monitored, in vitro, from a hydrogel laden microdevice (Fig. 3 A). It can be estimated that approximately 2.15 ± 0.56 nanograms of camptothecin are released over 120 minutes from an individual microdevice. Additional in vitro studies were performed wherein camptothecin loaded microdevices were added to the apical side of a caco-2 monolayer and drug concentration was measured in the basal media. Caco-2 monolayers were grown using established methods to simulate tight-junctions seen in vivo, resulting in a close model for the permeation of drug.31 The trans-monolayer studies conclude that under ideal conditions, the release of chemotherapeutics from the microdevice limits the amount of free drug available to affect surrounding tissue (Fig. 3 B). Furthermore, the microdevices seem to concentrate drug release at the cell interface, resulting in increased permeability of drug through the caco-2 monolayer. We have shown PEG hydrogels can be easily incorporated into SU-8 microdevices and both camptothecin and BSA can be encapsulated and released from the device in a controlled manner.
Figure 2.
A) Process flow overview for fabrication of single layer PEGMA laden SU-8 microdevice. B) A light micrograph of hydrogel laden microdevices. The black box represents an unfilled microdevice. A white box represents a filled microdevice. The line represents 150 microns. C) A fluorescent micrograph of hydrogel laden microdevices with bovine serum albumin conjugated to fluorescein isothiocyanate encapsulated in the hydrogel. The gray box represents an unfilled microdevice.
Figure 3.
A) The release of auto-fluorescent chemotherapeutic camptothecin was measured in PBS with a fluorescent spectrometer and reported as concentration in microgram per milliliter. The blank hydrogel represents the amount of camptothecin release for a non-drug loaded hydrogel in a microdevice. B) The permeation of camptothecin through a caco-2 epithelial monolayer on collagen treated Transwells®. The concentration in the bottom well of the Transwell® was normalized to that of the top well. An * indicates statistical significance with respect to the free drug conditions. A # represents significance with respect to drug filled hydrogels. All data is presented as average ± standard deviation.
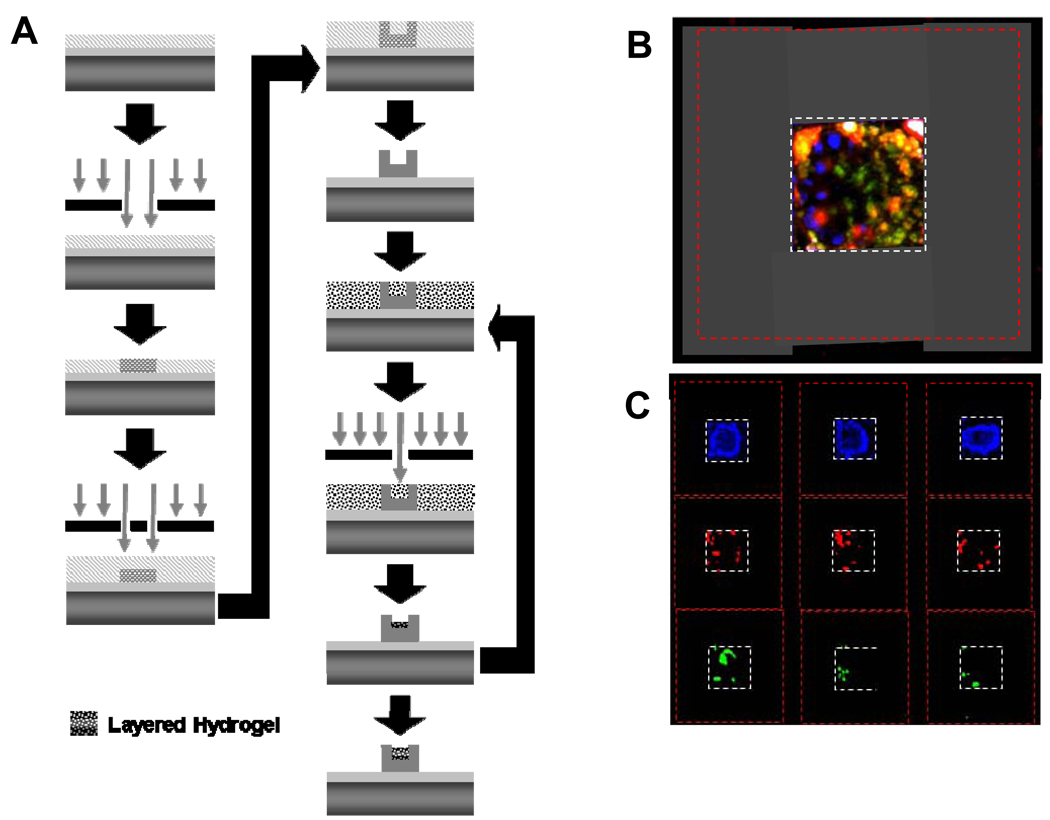
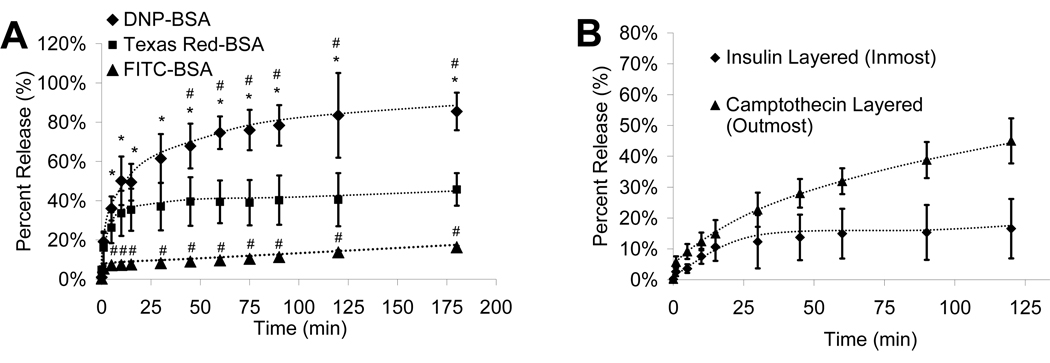
By varying the UV light exposure time and/or initiator concentration, the hydrogel solution can be layered through successive spin-coating and photolithography, resulting in the loading of one or more unique therapeutic agents (Fig. 4 A). The multi-layer process was imaged fluorescently using three different fluorphores conjugated to BSA (Figure 3 B, C). The release of BSA linked to three unique fluorphores demonstrates that although the agents are of near equal molecular weight their release characteristics can be controlled and altered significantly (Fig. 4 A). Additionally, a therapeutic protein (insulin) as well as a chemotherapeutic agent (camptothecin) can simultaneously be released from the same microdevice (Fig. 4 B). Multi-layer incorporation can lead to multi-drug incorporation, which is very advantageous in chemotherapeutic delivery. The standard of care for cancer therapy is multi-drug therapy, and this hydrogel laden microdevice allows for single point delivery of at least three therapeutics.
Figure 4.
A) Process flow overview for fabrication of multi-layer PEGMA laden SU-8 microdevice. B) A fluorescent micrograph composite of a layered hydrogel prepared with DNP-BSA, FITC-BSA and Texas-red-BSA (from outmost layer to inmost). The grey dotted-line box highlights the reservoir area and the red dotted-line box the outer area of the microdevice. C) A fluorescent micrograph of each individual filter for the labeled BSA is presented for three unique hydrogel-filled microdevices.
The bioactivity of the delivered chemotherapeutic was tested and is reported in Table 1. The camptothecin loaded hydrogel microdevices placed in direct contact with HT-29 cells or those in indirect contact with HT-29s through a caco-2 cell monolayer, significantly impacted both the viability and number of cells. Cell death studies that report the number of dead to live cells noted that the caco-2 monolayer did not contain a significantly increased number of dead cells when in contact with drug loaded microdevices (P-value > 0.90). Maintaining a live monolayer indicates that the structure of the monolayer was not breeched, although the MTT measured viability was decreased (Table 1). Also, camptothecin loaded microdevices were compared to non-drug loaded hydrogel-laden microdevices. The viability, but not number, of cells in contact with the empty hydrogel is significantly different from the tissue culture polystyrene (TCPS) condition when the hydrogels are placed directly on the HT-29 cell layer. Residual cross-linker or photoinitiator could account for this decreased viability. Further studies would need to be performed with other photoinitiators and cross linkers that have been shown to be more bio-friendly24 to determine the causative agent. The factor that inhibits the viability when in direct contact with the cells appears to not permeate the cell monolayer since lower well cells are not as affected in the trans-monolayer studies. Camptothecin released from the hydrogel laden microdevices appears to arrest the growth of cancer cells whether in direct contact with the cells or through a cell monolayer, suggesting that the released drug is indeed bioactive.
Table 1.
Viability of in vitro colon cancer cell (HT-29 and caco-2) layers exposed to camptothecin loaded hydrogel-laden microdevices (Camptothecin), hydrogels without drug (Empty Hydrogel) and unexposed to any microdevices or drug (Tissue Culture Polystyrene). “Directly on HT-29” labels the cell count or viability of the cell layer in direct contact with the microdevices. “Trans-Monolayer Studies” corresponds to the same measurement techniques with a HT-29 cell layer under a caco-2 cell monolayer grown on a Transwell(R) filter. The microdevices are placed on the apical side of the monolayer, therefore measuring the chemotherapeutic effect on a monolayer and the effect of chemotherapeutic permeating the monolayer. All data is presented as average normalized to tissue culture polystyrene negative control. The range given is that of the standard deviation.
| Camptothecin | Empty Hydrogel | Tissue Culture Polystyrene |
|||
|---|---|---|---|---|---|
| MTT Viability Assay | Directly on HT-29 | 20% ± 2% *# | 71% ± 15% * | 100% ± 8% | |
| Trans-Monolayer Studies | Upper Well (Caco-2) | 76% ± 3% # | 122% ± 19% | 100% ± 26% | |
| Lower Well (HT-29) | 3% ± 2% *# | 97% ± 17% | 100% ± 10% | ||
| Cell Count | Directly on HT-29 | 5% ± 1% *# | 87% ± 2% | 100% ± 28% | |
| Trans-Monolayer Studies | Lower Well (HT-29) | 3% ± 1% *# | 71% ± 39% | 100% ± 19% | |
An * signifies significance with respect to the value for tissue culture polystyrene.
A # corresponds to significances with respect to the value for the empty hydrogel case.
Here we report the development of a highly engineered hydrogel-incorporated microdevice. Through simple microfabrication techniques, the microdevice body is designed and fabricated to increase contact with a cell monolayer and form a protected drug-loaded reservoir.1, 14, 32 With the same techniques, a PEG hydrogel can be incorporated into the microdevice, resulting in a near 100% incorporation of hydrogel into empty microdevices. From the hydrogel, both proteins and chemotherapeutics can be released in a controlled manner. Furthermore, the microdevices concentrate the release of therapeutics at the cell interface, allowing more drug to pass through epithelial tight junctions to the vasculature. Layers of hydrogels with unique therapeutics allow for distinct controlled release of each agent from the microdevice. The drug released from the hydrogel-laden microdevices is bioactive and capable of acting in a therapeutic fashion. The expansion of simple microfabrication techniques has been used to create a multi-polymeric and multi-layered microdevice for medical application in oral drug delivery. In addition to drug delivery applications, the integration of a micro-scaled hydrogel within a micro-scaled SU-8 device can impact sensor development, BioMEMs fabrication, tissue engineering applications and many other applied microfabrication technologies.
Experimental
Hydrogel-laden microdevice development
A detail method of microdevice body development is provided elsewhere.14 Briefly, 4 mL of SU-8 2005 (Microchem; Newton, MA) were spin-coated on a 3” silicon wafer at 500 r.p.m. for 5–10 seconds with an acceleration of 100 r.p.m./sec and then at 2000 r.p.m. for 30 seconds with acceleration of 300 r.p.m./sec. After a soft-bake, the wafer was placed in mask aligner (Karl Seuss MJB 3;Garching Germany) and exposed to 405 nm light, for total of 100 mJ/cm2 of exposure through a square base silver mask printed on polyethylene terephthalate (CAD Art Services; Bandon, OR). A post-bake is performed and a second layer of epoxy resin is applied. After a soft-bake, the wafer is placed in the mask aligner and exposed to light through square frame mask. The surface is then post-baked and developed as per manufacturer’s directions.
After microdevice development, the hydrogel precursor solution is spun into the empty wells. The precursor solution consists of photoinitiator solution (cross-linker vinyl-2-pyrrolidone (Sigma, St. Louis, MO) in photoinitiator 2,2-dimethyloxy-2-phenylacetophenone (DMPA; Sigma) at a concentration of 60 mg/mL, poly(ethylene glycol) dimethacrylate (PEGMA; MW 750, Sigma) and PBS. The therapeutic is dissolved in the PBS prior to mixing, then only chemotherapeutics are sonicated separately for 30 minutes at room temperature (VWR Model 75T;West Chester, PA). Upon preparing all ingredients for hydrogel precursor solution, the final mixture is sonicated for 30 minutes at room temperature to ensure equal distribution of initiator, cross-linker, and drug. The hydrogel precursor solution is then spun onto the microdevices with a spin coater at 500 r.p.m. with an acceleration of 100 r.p.m/sec for 10 seconds and then at 1,500 r.p.m. with an acceleration of 300 r.p.m./sec for 30 seconds. The precursor-coated microdevices were aligned on a mask aligner. Near-UV light (350–400 nm) was exposed (450 mJ/cm2) to the hydrogel surface through a mask, allowing light to only permeate to areas within the reservoir. The hydrogel microdevices were developed with water. The wafer was dried with nitrogen after rinsing with isopropyl alcohol. Approximately 0.5 mL of hydrogel precursor solution was required to fill 10,700 microdevices.
For single layer hydrogel microdevices (Fig. 2 A) a solution of 8.3% vol/vol photoinitiator solution and 8.3% PBS in PEGMA was applied to the surface by spin coating. The hydrogel solution was initiated with UV light (9 mW/cm2) passed through a high-resolution transparency mask for 90 seconds. The hydrogel was then developed by rinsing with water and isopropanol. Bovine serum albumin conjugated to fluorescein isothiocyanate at a concentration of 1 mg/mL PEGMA was added to the hydrogel for fluorescent imaging of the devices.
For multi-layered hydrogel microdevices (Fig. 4 A) a hydrogel precursor solution of 5.6% photoinitiator solution and 8.3% PBS in PEGMA was spun onto a developed SU-8 microdevice surface. After UV light exposure (9 W/cm2 for 75 sec) of layer one of the hydrogel, the surface was developed with water and a second layer is spun onto the surface. This process was repeated to form three layers. Fluorphores conjugated to albumin, 2,4-dinitrophenylated (DNP)-bovine serum albumin (BSA), fluorescein isothiocyanate (FITC)-BSA and Texas-red-BSA, were added to the hydrogel precursor solution at a concentration of 3.33 mg/mL PEGMA solution for fluorescent imaging and release studies.
Release studies
All in vitro release studies were performed in PBS while agitating at approximately 250 r.p.m. in polystyrene 6-well plates. Drug release was measured with a fluorescent spectrophotometer (Fluorolog FL3-22; Horiba Jobin Yvon; Edison, NJ; use generously donated by J. Fréchet; University of California, Berkeley). Camptothecin (Sigma; Excitation: 365 nm Emission: 430 nm) was added to the hydrogel precursor solution at a concentration of 0.67 mg/mL of PEGMA. Fluorescently labeled bovine serum albumin (BSA) (FITC-BSA, Sigma, Ex: 494 nm Em: 520 nm; DNP-BSA, Ex: 360 nm Em: 385 nm; and Texas Red-BSA, Invitrogen, Ex: 596 nm Em: 615 nm was added at a concentration of 3.33 mg/mL of PEGMA. Texas-red conjugated insulin (Sigma, Ex: 596 nm Em: 615 nm) was prepared at a concentration of 3.33 mg/mL PEGMA.
Direct Contact Studies
Human colorectal adenocarcinoma cells (HT-29; ATCC Manassas, VA) were seeded at 20,000 cells per a 6-well plate area (962 mm2) and grown to sub-confluency for one day. Ethanol sterilized microdevices were placed on the cell surface with 1 mL of McCoy’s 5a complete media for four hours. After four hours the microdevices were removed and cells were maintained at culture conditions for three days. After three days, viability and cell counting assays were performed. Cell viability was measured with an MTT assay, as per manufacturer’s directions. Cell counting was performed by detaching the cells and counting them with a hemacytometer.
Monolayer studies
Human colorectal adenocarcinoma epithelial cells (caco-2; ATCC) were grown to confluency on collagen (Type 1; Becton Dickinson, Franklin Lakes, NJ) treated 6-well Transwell(R) filters (BD). Prior to seeding, caco-2 cells were maintained in Modified Eagle’s Media (MEM) with 20% fetal bovine serum (Invitrogen), 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 2.5 g/L glucose, 10 mM HEPES, 1.0 mM sodium pyruvate and 1 mg/mL penicillin/streptomycin for 3 days or more. For monolayer permeation studies, discrete time samples were taken from both the upper and lower chamber of the Transwell(R) and observed for fluorescence in a fluorimeter. For bioactivity studies colorectal adenocarcinoma cells (HT-29) were used. One day prior to bioavailability studies HT-29 cells were added to the lower chamber of the transwell at a density of 20,000 cells per a well and a volume of 2 mL, and cultured separately from the caco-2 cells. Prior to introduction of the microdevices, media was aspirated from both cell layers and replaced with 1 mL of complete MEM without sodium pyruvate. Ethanol sterilized microdevices were incubated face down on the apical side of the caco-2 monolayer for four hours. After four hours of incubation the Transwells(R) containing HT-29 cells with caco-2 monolayers were placed in the incubator for seventy-two hours. As outlined above, an MTT assay and cell counting was used to measure drug bioactivity. For live/dead staining, the cells were then stained with CellTracker™ and propidium iodide (Invitrogen).
Statistical analysis
All data values are an average of three or more points. Data is presented as average plus or minus standard deviation. Comparison between two groups of data was compared with a two tail student t-test. A p-value of 0.05 or less was considered statistically significant.
Conclusion
Oral delivery of therapeutics is a preferred route over other options such as intravenous injection. The Desai Laboratory has developed a micro-patterned device with a protected drug reservoir that can asymmetrically deliver therapeutics in a concentrated fashion at the device and cell surface. To fill the reservoir with therapeutics, a photolithographically patterned poly (ethylene glycol) dimethacrylate hydrogel laden with a protein or chemotherapeutic was introduced into the multi-layer SU-8 microdevice. Within the microdevice, up to three layers of hydrogel, each with a unique therapeutic agent can be incorporated. The release characteristics of the hydrogel as well as the bioactivity of the released therapeutic were measured. Controlled release of chemotherapeutic was shown to be capable of cytostasis. These results indicate that bioactive, therapeutic loaded hydrogels can be easily incorporated into the micro-patterned device, thus forming a viable device for oral delivery.
Figure 5.
A) The release of fluorescently labeled BSA from a layered hydrogel was prepared with DNP-BSA, FITC-BSA and Texas-red-BSA. A * indicates significance with respect to the FITC-BSA release, and a # with respect to Texas-red-BSA. B) The release of both therapeutic proteins (insulin) and small chemicals (camptothecin) is shown over time. All data is presented as average ± standard deviation.
Acknowledgements
This work was supported by the NIH, The T. Gary and Kathleen Rogers Family Foundation and Bridging the Gap Award and Sandler Foundation. Also, National Science Foundation Research Experience for Undergraduates.
Bibliographic References
- 1.Tao SL, Desai TA. Advanced Materials. 2005;17:1625. [Google Scholar]
- 2.Clark MA, Hirst BH, Jepson MA. Adv Drug Deliv Rev. 2000;43:207. doi: 10.1016/s0169-409x(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 3.Brannon-Peppas L, Blanchette JO. Adv Drug Deliv Rev. 2004;56:1649. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Proc Natl Acad Sci U S A. 2004;101:9534. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppas NA, Wood KM, Blanchette JO. Expert Opin Biol Ther. 2004;4:881. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 6.Standley SM, Kwon YJ, Murthy N, Kunisawa J, Shastri N, Guillaudeu SJ, Lau L, Frechet JM. Bioconjug Chem. 2004;15:1281. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa H, Fukumori Y. Yakugaku Zasshi. 2007;127:813. doi: 10.1248/yakushi.127.813. [DOI] [PubMed] [Google Scholar]
- 8.Klouda L, Mikos AG. Eur J Pharm Biopharm. 2008;68:34. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arayne MS, Sultana N, Noor Us S. Pak J Pharm Sci. 2007;20:251. [PubMed] [Google Scholar]
- 10.Ahmed A, Bonner C, Desai TA. Journal of Controlled Release. 2002;81:291. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 11.Tao SL, Lubeley MW, Desai TA. Journal of Controlled Release. 2003;88:215. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 12.Tao SL, Desai TA. Journal of Controlled Release. 2005;109:127. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Tao SL, Lubeley MW, Desai TA. Journal of Biomedical Materials Research Part A. 2003;67A:369. doi: 10.1002/jbm.a.10047. [DOI] [PubMed] [Google Scholar]
- 14.Tao SL, Popat K, Desai TA. Nat Protoc. 2006;1:3153. doi: 10.1038/nprot.2006.451. [DOI] [PubMed] [Google Scholar]
- 15.Chung HJ, Park TG. Adv Drug Deliv Rev. 2007;59:249. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Tessmar JK, Gopferich AM. Adv Drug Deliv Rev. 2007;59:274. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Heller J. Crit Rev Ther Drug Carrier Syst. 1984;1:39. [PubMed] [Google Scholar]
- 18.Kim SW, Bae YH, Okano T. Pharm Res. 1992;9:283. doi: 10.1023/a:1015887213431. [DOI] [PubMed] [Google Scholar]
- 19.Ziaie B, Baldi A, Lei M, Gu Y, Siegel RA. Adv Drug Deliv Rev. 2004;56:145. doi: 10.1016/j.addr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Tissue Eng. 1999;5:267. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 21.Zhu AP, Chan-Park MB, Gao JX. J Biomed Mater Res B Appl Biomater. 2006;76:76. doi: 10.1002/jbm.b.30348. [DOI] [PubMed] [Google Scholar]
- 22.Bryant SJ, Cuy JL, Hauch KD, Ratner BD. Biomaterials. 2007;28:2978. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Henthorn D, Kim C. paper presented at the IEEE Sensors. Korea: Daegu; 2006. [Google Scholar]
- 24.Koh WG, Itle LJ, Pishko MV. Anal Chem. 2003;75:5783. doi: 10.1021/ac034773s. [DOI] [PubMed] [Google Scholar]
- 25.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, Khademhosseini A. Lab Chip. 2007;7:756. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 26.Zguris JC, Itle LJ, Koh WG, Pishko MV. Langmuir. 2005;21:4168. doi: 10.1021/la0470176. [DOI] [PubMed] [Google Scholar]
- 27.Bouwstra JB, Spoelstra EC, De Waard P, Leeflang BR, Kamerling JP, Vliegenthart JF. Eur J Biochem. 1990;190:113. doi: 10.1111/j.1432-1033.1990.tb15553.x. [DOI] [PubMed] [Google Scholar]
- 28.Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. J Control Release. 2007;121:10. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oudshoorn MH, Penterman R, Rissmann R, Bouwstra JA, Broer DJ, Hennink WE. Langmuir. 2007;23:11819. doi: 10.1021/la701910d. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Bachman M, Sims CE, Li GP, Allbritton NL. Langmuir. 2006;22:2719. doi: 10.1021/la053188e. [DOI] [PubMed] [Google Scholar]
- 31.Meunier V, Bourrie M, Berger Y, Fabre G. Cell Biol Toxicol. 1995;11:187. doi: 10.1007/BF00756522. [DOI] [PubMed] [Google Scholar]
- 32.Tao SL, Desai TA. Drug Discovery Today. 2005;10:909. doi: 10.1016/S1359-6446(05)03489-6. [DOI] [PubMed] [Google Scholar]