Abstract
Crystals of the title compound, C10H13N3O2, were obtained from a condensation reaction of butan-2-one and 1-(2-nitrophenyl)hydrazine. The molecule exhibits a nearly coplanar structure, except for the methyl and methylene H atoms, the largest deviations from the mean plane defined by all non-H atoms, except for the nitro group, being 0.120 (2) Å for one of the nitro O atoms. Intramolecular N—H⋯O hydrogen bonding helps to establish the molecular configuration.
Related literature
For applications of Schiff base compounds, see: Kahwa et al. (1986 ▶); Santos et al. (2001 ▶).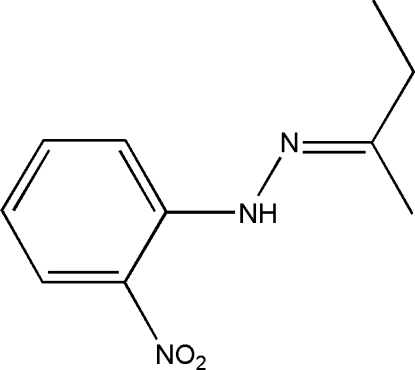
Experimental
Crystal data
C10H13N3O2
M r = 207.23
Monoclinic,

a = 7.3079 (11) Å
b = 10.2150 (17) Å
c = 14.763 (2) Å
β = 100.058 (9)°
V = 1085.1 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 296 K
0.28 × 0.21 × 0.11 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: none
7304 measured reflections
2116 independent reflections
1099 reflections with I > 2σ(I)
R int = 0.024
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.159
S = 0.93
2116 reflections
141 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.17 e Å−3
Δρmin = −0.15 e Å−3
Data collection: SMART (Bruker, 1998 ▶); cell refinement: SAINT (Bruker, 1998 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809031420/xu2581sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809031420/xu2581Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2A⋯O2 | 0.889 (16) | 1.969 (16) | 2.604 (3) | 127.2 (14) |
Acknowledgments
The authors would like to express their deep appreciation to the start-up Fund for PhDs of the Natural Scientific Research of Zhengzhou University of Light Industry (No. 2005001) and the Fund for Natural Scientific Research of Zhengzhou University of Light Industry, China (000455).
supplementary crystallographic information
Comment
The chemistry of Schiff base has attracted a great deal of interest in recent years. These compounds play an important role in the development of various proteins and enzymes (Kahwa et al., 1986; Santos et al., 2001). As part of our in the study of the coordination chemistry of Schiff bases, we synthesized the title compound and determined its crystal structure.
The molecular structure of (I) is shown in Fig. 1. The molecules is roughly planar, with the largest deviations from the mean plane defined by all non-H atoms, except the nitro group, being -0.120 (2) for atom O2.
Intramolecular N—H···O hydrogen bond is observed in compound (I), and this helps to stabilize the configuration of the molecule.
Experimental
2-Nitrophenylhydrazine (1 mmol, 0.153 g) was dissolved in anhydrous ethanol (15 ml). The mixture was stirred for several min at 351 K, then butan-2-one (1 mmol, 0.72 g) in ethanol (8 ml) was added dropwise and the mixture was stirred at refluxing temperature for 2 h. The product was isolated and recrystallized from methanol, red single crystals were obtained after 3 d.
Refinement
Imino H atom was located in a difference Fourier map and positional parameters were refined with a fixed isotropic thermal parameter of 0.08 Å2. Other H atoms were positioned geometrically and refined as riding with C—H = 0.93 (aromatic), 0.97 (methylene) and 0.96 Å (methyl), with Uiso(H) = 1.5Ueq(C) for methyl H atoms and 1.2Ueq(C) for the others.
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 30% probability level. Hydrogen bonding is shown in dashed line.
Crystal data
| C10H13N3O2 | F(000) = 440 |
| Mr = 207.23 | Dx = 1.268 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 1572 reflections |
| a = 7.3079 (11) Å | θ = 2.4–26.0° |
| b = 10.2150 (17) Å | µ = 0.09 mm−1 |
| c = 14.763 (2) Å | T = 296 K |
| β = 100.058 (9)° | Block, red |
| V = 1085.1 (3) Å3 | 0.28 × 0.21 × 0.11 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 1099 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.024 |
| graphite | θmax = 26.0°, θmin = 2.4° |
| ω scans | h = −9→8 |
| 7304 measured reflections | k = −11→12 |
| 2116 independent reflections | l = −13→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.047 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.159 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.93 | w = 1/[σ2(Fo2) + (0.0917P)2] where P = (Fo2 + 2Fc2)/3 |
| 2116 reflections | (Δ/σ)max = 0.010 |
| 141 parameters | Δρmax = 0.17 e Å−3 |
| 1 restraint | Δρmin = −0.14 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.3024 (2) | 1.04656 (17) | 0.09960 (11) | 0.0674 (5) | |
| C1 | 0.2088 (2) | 0.89169 (19) | −0.01835 (13) | 0.0551 (5) | |
| N2 | 0.2496 (2) | 1.01728 (17) | 0.00803 (12) | 0.0678 (5) | |
| C2 | 0.2183 (3) | 0.79113 (19) | 0.04780 (12) | 0.0621 (6) | |
| H2 | 0.2495 | 0.8122 | 0.1099 | 0.075* | |
| C6 | 0.1570 (2) | 0.85236 (19) | −0.11104 (12) | 0.0587 (5) | |
| C5 | 0.1221 (3) | 0.7221 (2) | −0.13432 (14) | 0.0690 (6) | |
| H5 | 0.0889 | 0.6985 | −0.1959 | 0.083* | |
| N3 | 0.1382 (3) | 0.9459 (2) | −0.18488 (14) | 0.0817 (6) | |
| C3 | 0.1830 (3) | 0.66460 (19) | 0.02310 (14) | 0.0698 (6) | |
| H3 | 0.1907 | 0.6007 | 0.0685 | 0.084* | |
| C4 | 0.1358 (3) | 0.6288 (2) | −0.06817 (15) | 0.0732 (6) | |
| H4 | 0.1136 | 0.5415 | −0.0841 | 0.088* | |
| O2 | 0.1590 (3) | 1.0630 (2) | −0.16759 (12) | 0.1089 (7) | |
| C7 | 0.3445 (3) | 1.1663 (2) | 0.11902 (16) | 0.0713 (6) | |
| O1 | 0.1019 (3) | 0.90665 (19) | −0.26427 (11) | 0.1204 (7) | |
| C8 | 0.4002 (3) | 1.2013 (2) | 0.21782 (18) | 0.0938 (8) | |
| H8A | 0.5240 | 1.2387 | 0.2266 | 0.113* | |
| H8B | 0.3166 | 1.2687 | 0.2325 | 0.113* | |
| C10 | 0.3419 (3) | 1.2749 (2) | 0.05076 (18) | 0.0974 (8) | |
| H10A | 0.4223 | 1.2531 | 0.0080 | 0.146* | |
| H10B | 0.3843 | 1.3543 | 0.0824 | 0.146* | |
| H10C | 0.2175 | 1.2869 | 0.0180 | 0.146* | |
| C9 | 0.4004 (4) | 1.0931 (3) | 0.28360 (19) | 0.1200 (10) | |
| H9A | 0.2803 | 1.0523 | 0.2740 | 0.180* | |
| H9B | 0.4286 | 1.1266 | 0.3452 | 0.180* | |
| H9C | 0.4926 | 1.0298 | 0.2745 | 0.180* | |
| H2A | 0.240 (3) | 1.0806 (15) | −0.0338 (11) | 0.080* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0685 (11) | 0.0696 (12) | 0.0636 (12) | −0.0003 (9) | 0.0106 (8) | −0.0109 (9) |
| C1 | 0.0509 (11) | 0.0590 (12) | 0.0556 (12) | 0.0053 (9) | 0.0102 (8) | 0.0030 (10) |
| N2 | 0.0764 (12) | 0.0608 (12) | 0.0652 (13) | 0.0025 (9) | 0.0095 (9) | 0.0037 (8) |
| C2 | 0.0672 (13) | 0.0693 (14) | 0.0485 (11) | 0.0010 (10) | 0.0062 (9) | 0.0024 (10) |
| C6 | 0.0557 (11) | 0.0734 (14) | 0.0468 (11) | 0.0076 (10) | 0.0081 (8) | 0.0083 (10) |
| C5 | 0.0601 (13) | 0.0917 (17) | 0.0549 (12) | −0.0007 (11) | 0.0087 (10) | −0.0156 (12) |
| N3 | 0.0826 (13) | 0.1009 (16) | 0.0613 (13) | 0.0115 (11) | 0.0116 (9) | 0.0134 (12) |
| C3 | 0.0758 (15) | 0.0658 (14) | 0.0680 (14) | −0.0028 (11) | 0.0126 (11) | 0.0065 (11) |
| C4 | 0.0764 (15) | 0.0667 (14) | 0.0777 (16) | −0.0052 (11) | 0.0169 (12) | −0.0077 (12) |
| O2 | 0.1446 (17) | 0.0932 (13) | 0.0870 (13) | 0.0062 (12) | 0.0148 (11) | 0.0327 (10) |
| C7 | 0.0523 (12) | 0.0700 (15) | 0.0934 (17) | 0.0034 (11) | 0.0173 (11) | −0.0162 (13) |
| O1 | 0.1572 (18) | 0.1516 (17) | 0.0490 (11) | 0.0180 (13) | 0.0086 (10) | 0.0100 (10) |
| C8 | 0.0826 (16) | 0.0953 (18) | 0.106 (2) | −0.0056 (13) | 0.0236 (14) | −0.0338 (16) |
| C10 | 0.0861 (18) | 0.0704 (16) | 0.136 (2) | 0.0024 (13) | 0.0191 (15) | 0.0039 (15) |
| C9 | 0.135 (2) | 0.144 (3) | 0.0799 (17) | −0.036 (2) | 0.0144 (16) | −0.0224 (18) |
Geometric parameters (Å, °)
| N1—C7 | 1.282 (2) | C3—C4 | 1.380 (3) |
| N1—N2 | 1.372 (2) | C3—H3 | 0.9300 |
| C1—N2 | 1.359 (2) | C4—H4 | 0.9300 |
| C1—C6 | 1.413 (3) | C7—C10 | 1.496 (3) |
| C1—C2 | 1.411 (2) | C7—C8 | 1.488 (3) |
| N2—H2A | 0.888 (9) | C8—C9 | 1.471 (3) |
| C2—C3 | 1.355 (2) | C8—H8A | 0.9700 |
| C2—H2 | 0.9300 | C8—H8B | 0.9700 |
| C6—C5 | 1.387 (3) | C10—H10A | 0.9600 |
| C6—N3 | 1.438 (2) | C10—H10B | 0.9600 |
| C5—C4 | 1.356 (3) | C10—H10C | 0.9600 |
| C5—H5 | 0.9300 | C9—H9A | 0.9600 |
| N3—O1 | 1.223 (2) | C9—H9B | 0.9600 |
| N3—O2 | 1.227 (2) | C9—H9C | 0.9600 |
| C7—N1—N2 | 116.33 (18) | C5—C4—H4 | 120.3 |
| N2—C1—C6 | 123.67 (17) | C3—C4—H4 | 120.3 |
| N2—C1—C2 | 120.49 (18) | N1—C7—C10 | 125.6 (2) |
| C6—C1—C2 | 115.84 (18) | N1—C7—C8 | 117.5 (2) |
| C1—N2—N1 | 119.87 (16) | C10—C7—C8 | 116.8 (2) |
| C1—N2—H2A | 120.1 (14) | C9—C8—C7 | 115.8 (2) |
| N1—N2—H2A | 120.0 (14) | C9—C8—H8A | 108.3 |
| C3—C2—C1 | 121.61 (18) | C7—C8—H8A | 108.3 |
| C3—C2—H2 | 119.2 | C9—C8—H8B | 108.3 |
| C1—C2—H2 | 119.2 | C7—C8—H8B | 108.3 |
| C5—C6—C1 | 121.30 (17) | H8A—C8—H8B | 107.4 |
| C5—C6—N3 | 117.42 (19) | C7—C10—H10A | 109.5 |
| C1—C6—N3 | 121.28 (19) | C7—C10—H10B | 109.5 |
| C4—C5—C6 | 120.57 (19) | H10A—C10—H10B | 109.5 |
| C4—C5—H5 | 119.7 | C7—C10—H10C | 109.5 |
| C6—C5—H5 | 119.7 | H10A—C10—H10C | 109.5 |
| O1—N3—O2 | 121.1 (2) | H10B—C10—H10C | 109.5 |
| O1—N3—C6 | 119.0 (2) | C8—C9—H9A | 109.5 |
| O2—N3—C6 | 119.9 (2) | C8—C9—H9B | 109.5 |
| C2—C3—C4 | 121.22 (18) | H9A—C9—H9B | 109.5 |
| C2—C3—H3 | 119.4 | C8—C9—H9C | 109.5 |
| C4—C3—H3 | 119.4 | H9A—C9—H9C | 109.5 |
| C5—C4—C3 | 119.5 (2) | H9B—C9—H9C | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2A···O2 | 0.89 (2) | 1.97 (2) | 2.604 (3) | 127 (1) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU2581).
References
- Bruker (1998). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA. AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Kahwa, I. A., Selbin, I., Hsieh, T. C. Y. & Laine, R. A. (1986). Inorg. Chim. Acta, 118, 179–185.
- Santos, M. L. P., Bagatin, I. A., Pereira, E. M. & Ferreira, A. M. D. C. (2001). J. Chem. Soc. Dalton Trans. pp. 838–844.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809031420/xu2581sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809031420/xu2581Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



