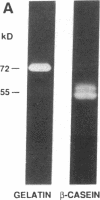
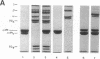
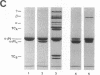
Abstract
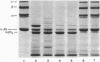
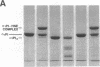
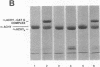
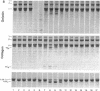
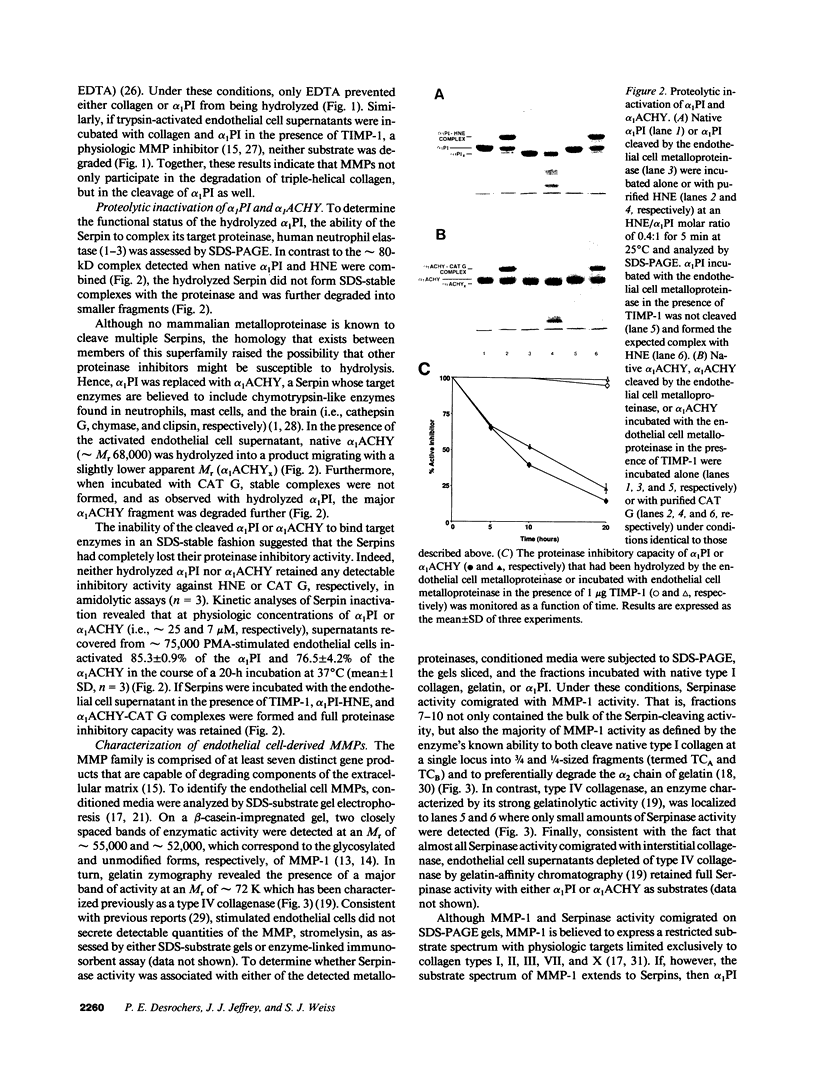
Human endothelial cells treated with either interleukin-1 beta, tumor necrosis factor-alpha, or phorbol myristate acetate secreted a metalloproteinase that hydrolyzed and inactivated the two major serine proteinase inhibitors (Serpins) found in plasma, alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin. Surprisingly, the responsible metalloproteinase was identified as human interstitial collagenase (matrix metalloproteinase-1), an enzyme whose only known physiologic substrate has heretofore been believed to be the extracellular matrix molecule, collagen. The metalloproteinase inactivated the Serpins by cleaving peptide bonds at sites unrelated to those hydrolyzed in collagenous macromolecules. NH2-terminal sequence analysis localized the cleavage sites in the Serpins to regions near their respective reactive site centers at three distinct peptide bonds on the amino-terminal side of bulky, hydrophobic residues. Together, these data indicate that matrix metalloproteinase-1 displays an expanded substrate repertoire that supports the existence of a new interface between connective tissue turnover and Serpin function.
Full text
PDF

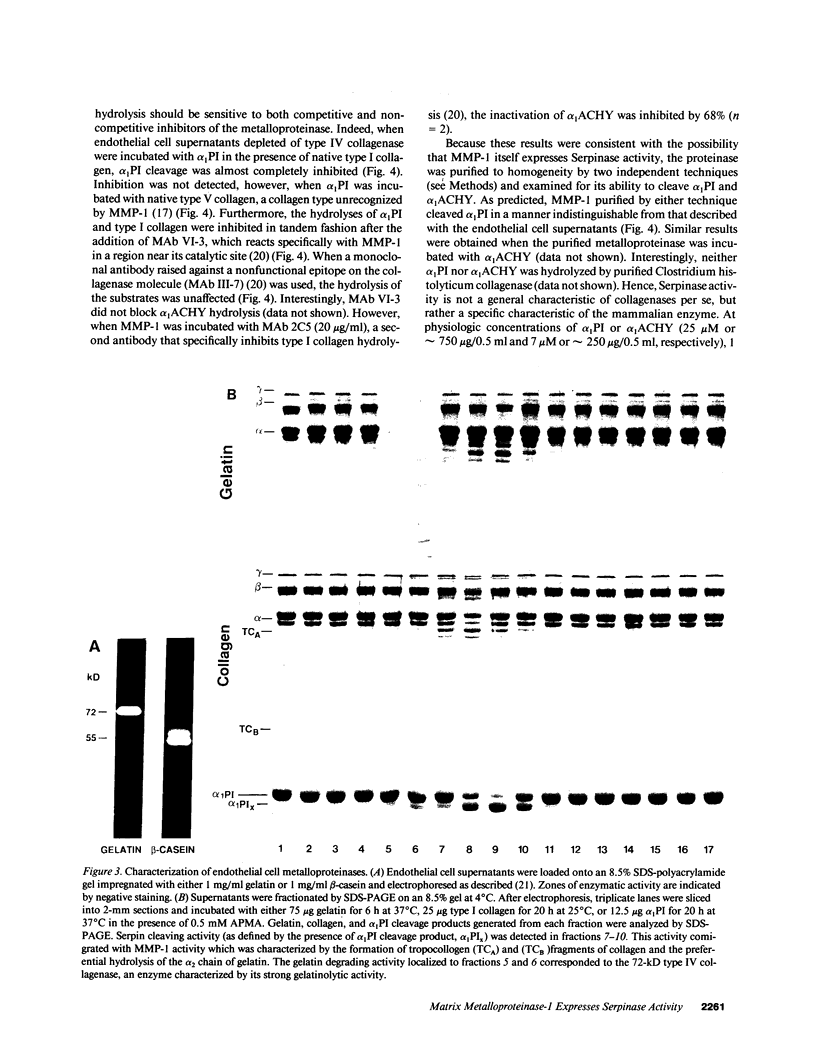
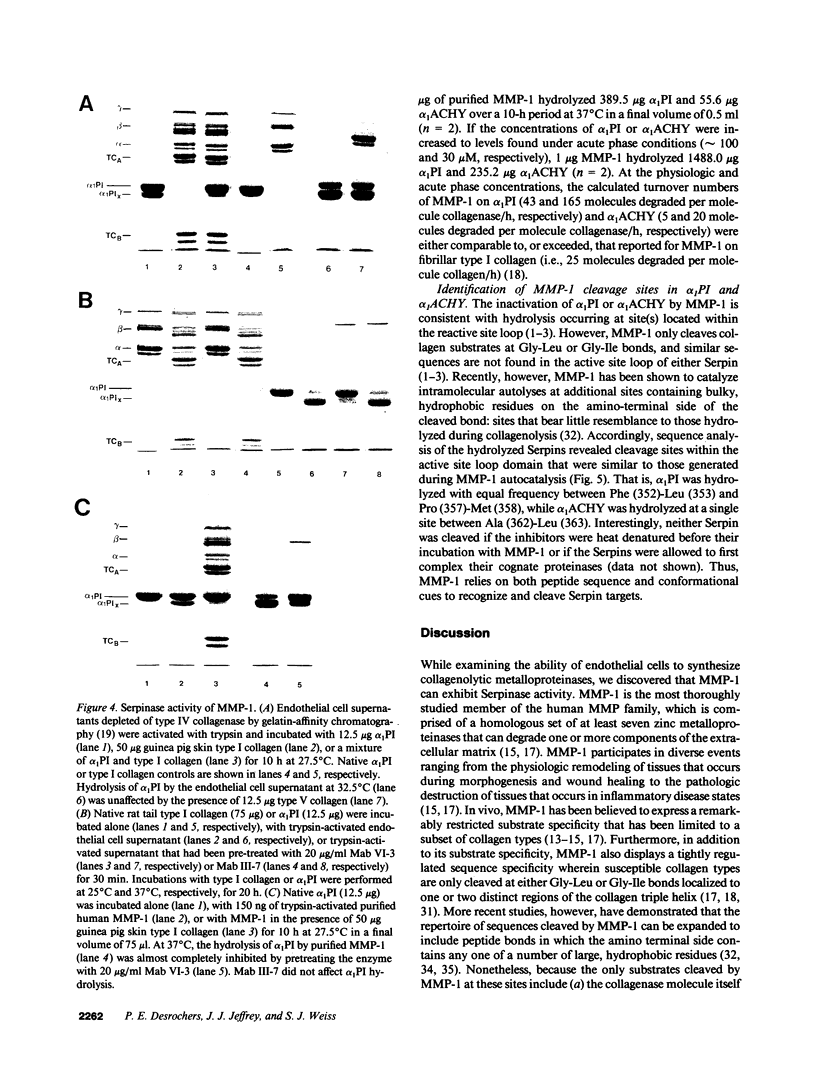



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Sinha S., Travis J. Interaction of mouse macrophage elastase with native and oxidized human alpha 1-proteinase inhibitor. J Clin Invest. 1987 May;79(5):1314–1317. doi: 10.1172/JCI112955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Rice A. G., Griffin G. L., Senior R. M. Alpha 1-proteinase inhibitor is a neutrophil chemoattractant after proteolytic inactivation by macrophage elastase. J Biol Chem. 1988 Mar 25;263(9):4481–4484. [PubMed] [Google Scholar]
- Bertozzi P., Astedt B., Zenzius L., Lynch K., LeMaire F., Zapol W., Chapman H. A., Jr Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990 Mar 29;322(13):890–897. doi: 10.1056/NEJM199003293221304. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen B., Moore W. G., Taylor R. E., Bhown A. S., Birkedal-Hansen H. Monoclonal antibodies to human fibroblast procollagenase. Inhibition of enzymatic activity, affinity purification of the enzyme, and evidence for clustering of epitopes in the NH2-terminal end of the activated enzyme. Biochemistry. 1988 Sep 6;27(18):6751–6758. doi: 10.1021/bi00418a016. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Catabolism and turnover of collagens: collagenases. Methods Enzymol. 1987;144:140–171. doi: 10.1016/0076-6879(87)44177-3. [DOI] [PubMed] [Google Scholar]
- Boswell D. R., Carrell R. W. Genetic engineering and the serpins. Bioessays. 1988 Feb-Mar;8(2):83–87. doi: 10.1002/bies.950080209. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Silverman E. K., Campbell M. A. Elastase and cathepsin G of human monocytes. Quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. J Immunol. 1989 Nov 1;143(9):2961–2968. [PubMed] [Google Scholar]
- Cawston T. E., Mercer E. Preferential binding of collagenase to alpha 2-macroglobulin in the presence of the tissue inhibitor of metalloproteinases. FEBS Lett. 1986 Dec 1;209(1):9–12. doi: 10.1016/0014-5793(86)81074-2. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Christner P., Fein A., Goldberg S., Lippmann M., Abrams W., Weinbaum G. Collagenase in the lower respiratory tract of patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985 May;131(5):690–695. doi: 10.1164/arrd.1985.131.5.690. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Cawston T. E. Fragments of human fibroblast collagenase. Purification and characterization. Biochem J. 1989 Oct 1;263(1):201–206. doi: 10.1042/bj2630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Desrochers P. E., Weiss S. J. Proteolytic inactivation of alpha-1-proteinase inhibitor by a neutrophil metalloproteinase. J Clin Invest. 1988 May;81(5):1646–1650. doi: 10.1172/JCI113500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields G. B., Netzel-Arnett S. J., Windsor L. J., Engler J. A., Birkedal-Hansen H., Van Wart H. E. Proteolytic activities of human fibroblast collagenase: hydrolysis of a broad range of substrates at a single active site. Biochemistry. 1990 Jul 17;29(28):6670–6677. doi: 10.1021/bi00480a017. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- Hasty K. A., Pourmotabbed T. F., Goldberg G. I., Thompson J. P., Spinella D. G., Stevens R. M., Mainardi C. L. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990 Jul 15;265(20):11421–11424. [PubMed] [Google Scholar]
- Heeb M. J., Griffin J. H. Physiologic inhibition of human activated protein C by alpha 1-antitrypsin. J Biol Chem. 1988 Aug 25;263(24):11613–11616. [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Huber A. R., Weiss S. J. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989 Apr;83(4):1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knäuper V., Reinke H., Tschesche H. Inactivation of human plasma alpha 1-proteinase inhibitor by human PMN leucocyte collagenase. FEBS Lett. 1990 Apr 24;263(2):355–357. doi: 10.1016/0014-5793(90)81412-h. [DOI] [PubMed] [Google Scholar]
- Lindmark B., Lilja H., Alm R., Eriksson S. The microheterogeneity of desialylated alpha 1-antichymotrypsin: the occurrence of two amino-terminal isoforms, one lacking a His-Pro dipeptide. Biochim Biophys Acta. 1989 Jul 27;997(1-2):90–95. doi: 10.1016/0167-4838(89)90139-8. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Mawatari M., Kohno K., Mizoguchi H., Matsuda T., Asoh K., Van Damme J., Welgus H. G., Kuwano M. Effects of tumor necrosis factor and epidermal growth factor on cell morphology, cell surface receptors, and the production of tissue inhibitor of metalloproteinases and IL-6 in human microvascular endothelial cells. J Immunol. 1989 Sep 1;143(5):1619–1627. [PubMed] [Google Scholar]
- Michaelis J., Vissers M. C., Winterbourn C. C. Human neutrophil collagenase cleaves alpha 1-antitrypsin. Biochem J. 1990 Sep 15;270(3):809–814. doi: 10.1042/bj2700809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Jaffe E., Rifkin D. B. Tetradecanoyl phorbol acetate stimulates latent collagenase production by cultured human endothelial cells. Cell. 1980 Jun;20(2):343–351. doi: 10.1016/0092-8674(80)90620-0. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Nelson R. B., Siman R. Clipsin, a chymotrypsin-like protease in rat brain which is irreversibly inhibited by alpha-1-antichymotrypsin. J Biol Chem. 1990 Mar 5;265(7):3836–3843. [PubMed] [Google Scholar]
- Nuijens J. H., Eerenberg-Belmer A. J., Huijbregts C. C., Schreuder W. O., Felt-Bersma R. J., Abbink J. J., Thijs L. G., Hack C. E. Proteolytic inactivation of plasma C1- inhibitor in sepsis. J Clin Invest. 1989 Aug;84(2):443–450. doi: 10.1172/JCI114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Joslin G., Nelson P., Schasteen C., Adams S. P., Fallon R. J. Endocytosis and degradation of alpha 1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (SEC) receptor. J Biol Chem. 1990 Oct 5;265(28):16713–16716. [PubMed] [Google Scholar]
- Perlmutter D. H., Pierce J. A. The alpha 1-antitrypsin gene and emphysema. Am J Physiol. 1989 Oct;257(4 Pt 1):L147–L162. doi: 10.1152/ajplung.1989.257.4.L147. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Tobin R. B., Church F. C. Interaction of heparin cofactor II with neutrophil elastase and cathepsin G. J Biol Chem. 1990 Apr 15;265(11):6092–6097. [PubMed] [Google Scholar]
- Rice W. G., Weiss S. J. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990 Jul 13;249(4965):178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- Roswit W. T., Rifas L., Gast M. J., Welgus H. G., Jeffrey J. J. Purification and characterization of human myometrial smooth muscle collagenase. Arch Biochem Biophys. 1988 Apr;262(1):67–75. doi: 10.1016/0003-9861(88)90169-5. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Sottrup-Jensen L., Birkedal-Hansen H. Human fibroblast collagenase-alpha-macroglobulin interactions. Localization of cleavage sites in the bait regions of five mammalian alpha-macroglobulins. J Biol Chem. 1989 Jan 5;264(1):393–401. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., George P. M., Bathurst I. C., Brennan S. O., Winterbourn C. C. Cleavage and inactivation of alpha 1-antitrypsin by metalloproteinases released from neutrophils. J Clin Invest. 1988 Aug;82(2):706–711. doi: 10.1172/JCI113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Fliszar C. J., Seltzer J. L., Schmid T. M., Jeffrey J. J. Differential susceptibility of type X collagen to cleavage by two mammalian interstitial collagenases and 72-kDa type IV collagenase. J Biol Chem. 1990 Aug 15;265(23):13521–13527. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Stricklin G. P., Eisen A. Z. The gelatinolytic activity of human skin fibroblast collagenase. J Biol Chem. 1982 Oct 10;257(19):11534–11539. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Stricklin G. P., Roswit W. T., Eisen A. Z. Characteristics of the action of human skin fibroblast collagenase on fibrillar collagen. J Biol Chem. 1980 Jul 25;255(14):6806–6813. [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Eisen A. Z., Teter M., Clark S. D., Kronberger A., Goldberg G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3756–3760. doi: 10.1073/pnas.83.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Agostini A., Patston P. A., Marottoli V., Carrel S., Harpel P. C., Schapira M. A common neoepitope is created when the reactive center of C1-inhibitor is cleaved by plasma kallikrein, activated factor XII fragment, C1 esterase, or neutrophil elastase. J Clin Invest. 1988 Aug;82(2):700–705. doi: 10.1172/JCI113650. [DOI] [PMC free article] [PubMed] [Google Scholar]