Abstract
The crystal structure of the title compound, C13H14O5, establishes (i) the (S) – rather than (R) – configuration at the acetal carbon and (ii) that both the acetal and the lactone form five- rather than six-membered rings; the absolute configuration is determined by the use of 2-C-methyl-d-ribono-1,4-lactone as the starting material. The compound consists of hydrogen-bonded chains of molecules running along the a axis; there are no unusual packing features. Only classical hydrogen bonding has been considered.
Related literature
For the synthesis of sugar lactones and their use as building blocks, see: Lundt & Madsen (2001 ▶); Hotchkiss, Soengas et al. (2007 ▶); Booth et al. (2008 ▶, 2009 ▶); Jenkinson et al. (2007 ▶); Hotchkiss, Kato et al. (2007 ▶); Chen & Joullie (1984 ▶); Dho et al. (1986 ▶); Baird et al. (1987 ▶). For the structures of benzylidene acetals, see: Baggett et al. (1985 ▶); Zinner et al. (1968 ▶).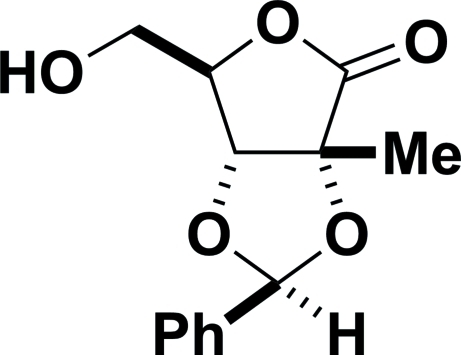
Experimental
Crystal data
C13H14O5
M r = 250.25
Orthorhombic,

a = 8.6170 (2) Å
b = 10.4615 (3) Å
c = 13.2693 (5) Å
V = 1196.18 (6) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 150 K
0.50 × 0.40 × 0.40 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (DENZO/SCALEPACK; Otwinowski & Minor, 1997 ▶) T min = 0.91, T max = 0.96
8306 measured reflections
1547 independent reflections
1369 reflections with I > 2.0σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.075
S = 0.96
1547 reflections
163 parameters
H-atom parameters constrained
Δρmax = 0.21 e Å−3
Δρmin = −0.18 e Å−3
Data collection: COLLECT (Nonius, 1997-2001 ▶).; cell refinement: DENZO/SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO/SCALEPACK; program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: CRYSTALS (Betteridge et al., 2003 ▶); molecular graphics: CAMERON (Watkin et al., 1996 ▶); software used to prepare material for publication: CRYSTALS.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809032796/lh2882sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809032796/lh2882Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O18—H181⋯O9i | 0.84 | 2.02 | 2.801 (3) | 153 |
Symmetry code: (i)  .
.
Acknowledgments
We would like to thank the Chemical Crystallography Department and ALT at Oxford University for use of the difractometers.
supplementary crystallographic information
Comment
Lactones have been widely used for the enantiospecific synthesis of complex chiral targets (Lundt & Madsen, 2001). 2-C-Methyl-D-ribono-1,4-lactone 3 (Fig. 1) has recently become a readily available starting material (Hotchkiss, Soengas et al., 2007; Booth et al., 2008) and has been used in the synthesis of doubly branched sugars (Booth et al., 2009), 2-C-methyl nucleosides (Jenkinson et al., 2007) and complex piperidines (Hotchkiss, Kato et al., 2007). D-Ribono-1,4-lactone 5 with benzaldehyde and concentrated aqueous hydrochloric acid forms a 5 ring benzylidene acetal - 6-ring lactone 6 (Fig. 1). The structure of 6 was established by X-ray crystallographic analysis (Baggett et al., 1985) which corrected the original erroneous 6 ring benzylidene acetal - 5-ring lactone structure proposed (Zinner et al., 1968). The protected 1,5-lactone 6 leaves only the C-2 OH unprotected and has been widely used as a chiron (Chen & Joullie, 1984; Dho et al., 1986; Baird et al., 1987). It was hoped that the analogous reaction with 2-C-methyl lactone 3 would form the analogous lactone 4; however, treatment of 3 with benzaldehyde and concentrated aqueous hydrochloric acid gave as the major product a mixture of epimeric 1,4-lactones 1 and 2; although it was not possible to separate 1 and 2 by chromatography, suitable crystals of the major component 1 were obtained and the structure of a 5 ring benzylidene acetal - 5-ring lactone, together with the (S) stereochemistry at the acetal carbon, was firmly established (Fig. 2).
The compound consists of H—O···H hydrogen bonded chains of molecules running along the a-axis (Fig. 3); there are no unusual packing features. Only classical hydrogen bonding has been considered.
Experimental
The title compound was recrystallized from a mixture of diethyl ether and petrol by slow evaporation: m.p. 369–372 K; [α]D18 -38.7 (c, 0.86 in CHCl3).
Refinement
In the absence of significant anomalous scattering, Friedel pairs were merged.
The H atoms were all located in a difference map, but those attached to carbon atoms were repositioned geometrically. The H atoms were initially refined with soft restraints on the bond lengths and angles to regularize their geometry (C—H in the range 0.93–0.98, O—H = 0.82 Å) and Uiso(H) (in the range 1.2–1.5 times Ueq of the parent atom), after which the positions were refined with riding constraints.
Figures
Fig. 1.
Synthetic Scheme
Fig. 2.
The title compound with displacement ellipsoids drawn at the 50% probability level. H atoms are shown as spheres of arbitary radius.
Fig. 3.
Packing diagram for the title compound projected along the c-axis. Hydrogen bonds are indicated by dotted lines.
Crystal data
| C13H14O5 | F(000) = 528 |
| Mr = 250.25 | Dx = 1.390 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 1493 reflections |
| a = 8.6170 (2) Å | θ = 5–27° |
| b = 10.4615 (3) Å | µ = 0.11 mm−1 |
| c = 13.2693 (5) Å | T = 150 K |
| V = 1196.18 (6) Å3 | Block, colourless |
| Z = 4 | 0.50 × 0.40 × 0.40 mm |
Data collection
| Nonius KappaCCD diffractometer | 1369 reflections with I > 2.0σ(I) |
| graphite | Rint = 0.036 |
| ω scans | θmax = 27.4°, θmin = 5.1° |
| Absorption correction: multi-scan (DENZO/SCALEPACK; Otwinowski & Minor, 1997) | h = −11→11 |
| Tmin = 0.91, Tmax = 0.96 | k = −13→13 |
| 8306 measured reflections | l = −17→17 |
| 1547 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.033 | H-atom parameters constrained |
| wR(F2) = 0.075 | Method = Modified Sheldrick w = 1/[σ2(F2) + ( 0.04P)2 + 0.33P] , where P = (max(Fo2,0) + 2Fc2)/3 |
| S = 0.96 | (Δ/σ)max = 0.0003 |
| 1547 reflections | Δρmax = 0.21 e Å−3 |
| 163 parameters | Δρmin = −0.18 e Å−3 |
| 0 restraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.20180 (16) | 0.58639 (12) | 0.35659 (10) | 0.0315 | |
| C2 | 0.0428 (2) | 0.53792 (18) | 0.36504 (15) | 0.0308 | |
| C3 | 0.0572 (2) | 0.41273 (17) | 0.42367 (14) | 0.0290 | |
| O4 | 0.07235 (15) | 0.30513 (13) | 0.35853 (12) | 0.0348 | |
| C5 | 0.2332 (2) | 0.27989 (17) | 0.34757 (14) | 0.0282 | |
| O6 | 0.30017 (15) | 0.31402 (12) | 0.44177 (10) | 0.0292 | |
| C7 | 0.2137 (2) | 0.42186 (16) | 0.47876 (13) | 0.0271 | |
| C8 | 0.2876 (2) | 0.54172 (17) | 0.43333 (14) | 0.0284 | |
| O9 | 0.41116 (16) | 0.58753 (14) | 0.45530 (11) | 0.0382 | |
| C10 | 0.2143 (3) | 0.4195 (2) | 0.59243 (14) | 0.0403 | |
| C11 | 0.2581 (2) | 0.14034 (17) | 0.32652 (14) | 0.0278 | |
| C12 | 0.3486 (2) | 0.10164 (19) | 0.24540 (15) | 0.0314 | |
| C13 | 0.3662 (2) | −0.0280 (2) | 0.22427 (16) | 0.0349 | |
| C14 | 0.2945 (3) | −0.11716 (19) | 0.28505 (15) | 0.0358 | |
| C15 | 0.2066 (2) | −0.07955 (18) | 0.36660 (15) | 0.0345 | |
| C16 | 0.1874 (2) | 0.04930 (19) | 0.38769 (15) | 0.0314 | |
| C17 | −0.0516 (2) | 0.63951 (19) | 0.41789 (16) | 0.0359 | |
| O18 | 0.02305 (17) | 0.66472 (14) | 0.51139 (11) | 0.0385 | |
| H21 | 0.0028 | 0.5221 | 0.2934 | 0.0368* | |
| H31 | −0.0301 | 0.4024 | 0.4712 | 0.0369* | |
| H51 | 0.2780 | 0.3352 | 0.2917 | 0.0355* | |
| H101 | 0.3207 | 0.4225 | 0.6174 | 0.0626* | |
| H103 | 0.1573 | 0.4942 | 0.6146 | 0.0621* | |
| H102 | 0.1635 | 0.3404 | 0.6145 | 0.0620* | |
| H121 | 0.3978 | 0.1652 | 0.2045 | 0.0380* | |
| H131 | 0.4316 | −0.0556 | 0.1653 | 0.0416* | |
| H141 | 0.3061 | −0.2081 | 0.2710 | 0.0428* | |
| H151 | 0.1588 | −0.1422 | 0.4102 | 0.0424* | |
| H161 | 0.1248 | 0.0765 | 0.4455 | 0.0371* | |
| H172 | −0.0541 | 0.7167 | 0.3741 | 0.0456* | |
| H171 | −0.1605 | 0.6071 | 0.4292 | 0.0454* | |
| H181 | −0.0132 | 0.7294 | 0.5413 | 0.0598* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0387 (7) | 0.0245 (6) | 0.0313 (7) | −0.0037 (6) | 0.0002 (6) | 0.0025 (5) |
| C2 | 0.0337 (9) | 0.0244 (9) | 0.0343 (10) | 0.0014 (8) | −0.0037 (8) | −0.0015 (8) |
| C3 | 0.0286 (9) | 0.0233 (9) | 0.0352 (10) | −0.0005 (8) | −0.0006 (8) | −0.0020 (8) |
| O4 | 0.0290 (7) | 0.0240 (7) | 0.0513 (9) | 0.0010 (6) | −0.0099 (7) | −0.0079 (6) |
| C5 | 0.0307 (9) | 0.0232 (9) | 0.0306 (10) | −0.0008 (7) | −0.0024 (8) | −0.0019 (7) |
| O6 | 0.0303 (6) | 0.0239 (6) | 0.0335 (7) | 0.0014 (6) | −0.0054 (6) | −0.0044 (5) |
| C7 | 0.0296 (9) | 0.0226 (8) | 0.0290 (9) | −0.0003 (8) | 0.0000 (8) | −0.0009 (7) |
| C8 | 0.0335 (10) | 0.0226 (8) | 0.0292 (9) | −0.0013 (8) | 0.0028 (8) | −0.0053 (7) |
| O9 | 0.0341 (7) | 0.0322 (7) | 0.0482 (8) | −0.0095 (6) | −0.0005 (7) | −0.0095 (7) |
| C10 | 0.0500 (12) | 0.0428 (12) | 0.0282 (10) | 0.0028 (11) | 0.0006 (9) | 0.0014 (9) |
| C11 | 0.0312 (9) | 0.0217 (8) | 0.0307 (9) | −0.0016 (7) | −0.0040 (8) | −0.0008 (7) |
| C12 | 0.0320 (9) | 0.0276 (9) | 0.0345 (10) | −0.0016 (8) | 0.0004 (8) | 0.0022 (8) |
| C13 | 0.0372 (10) | 0.0314 (10) | 0.0360 (11) | 0.0031 (9) | 0.0022 (9) | −0.0041 (8) |
| C14 | 0.0418 (11) | 0.0245 (9) | 0.0411 (11) | 0.0017 (9) | −0.0031 (10) | −0.0014 (8) |
| C15 | 0.0414 (10) | 0.0251 (9) | 0.0370 (10) | −0.0042 (9) | −0.0013 (9) | 0.0017 (8) |
| C16 | 0.0350 (10) | 0.0283 (9) | 0.0308 (9) | −0.0015 (8) | 0.0014 (8) | 0.0013 (8) |
| C17 | 0.0382 (10) | 0.0291 (10) | 0.0406 (11) | 0.0050 (9) | −0.0079 (10) | −0.0051 (9) |
| O18 | 0.0442 (8) | 0.0326 (7) | 0.0385 (8) | 0.0078 (6) | −0.0058 (7) | −0.0091 (6) |
Geometric parameters (Å, °)
| O1—C2 | 1.465 (2) | C10—H103 | 0.968 |
| O1—C8 | 1.342 (2) | C10—H102 | 0.981 |
| C2—C3 | 1.528 (3) | C11—C12 | 1.390 (3) |
| C2—C17 | 1.511 (3) | C11—C16 | 1.392 (3) |
| C2—H21 | 1.025 | C12—C13 | 1.394 (3) |
| C3—O4 | 1.425 (2) | C12—H121 | 0.957 |
| C3—C7 | 1.537 (3) | C13—C14 | 1.379 (3) |
| C3—H31 | 0.988 | C13—H131 | 1.007 |
| O4—C5 | 1.418 (2) | C14—C15 | 1.379 (3) |
| C5—O6 | 1.422 (2) | C14—H141 | 0.974 |
| C5—C11 | 1.502 (2) | C15—C16 | 1.387 (3) |
| C5—H51 | 1.016 | C15—H151 | 0.967 |
| O6—C7 | 1.438 (2) | C16—H161 | 0.981 |
| C7—C8 | 1.530 (2) | C17—O18 | 1.422 (2) |
| C7—C10 | 1.508 (2) | C17—H172 | 0.995 |
| C8—O9 | 1.203 (2) | C17—H171 | 1.009 |
| C10—H101 | 0.975 | O18—H181 | 0.844 |
| C2—O1—C8 | 109.66 (14) | C7—C10—H103 | 106.8 |
| O1—C2—C3 | 105.05 (15) | H101—C10—H103 | 110.3 |
| O1—C2—C17 | 107.19 (15) | C7—C10—H102 | 108.1 |
| C3—C2—C17 | 114.22 (17) | H101—C10—H102 | 110.2 |
| O1—C2—H21 | 107.4 | H103—C10—H102 | 111.3 |
| C3—C2—H21 | 111.2 | C5—C11—C12 | 120.49 (16) |
| C17—C2—H21 | 111.3 | C5—C11—C16 | 119.63 (17) |
| C2—C3—O4 | 112.06 (15) | C12—C11—C16 | 119.86 (17) |
| C2—C3—C7 | 105.08 (15) | C11—C12—C13 | 120.03 (18) |
| O4—C3—C7 | 104.91 (14) | C11—C12—H121 | 119.0 |
| C2—C3—H31 | 110.9 | C13—C12—H121 | 120.9 |
| O4—C3—H31 | 111.8 | C12—C13—C14 | 119.47 (19) |
| C7—C3—H31 | 111.8 | C12—C13—H131 | 119.8 |
| C3—O4—C5 | 107.37 (13) | C14—C13—H131 | 120.8 |
| O4—C5—O6 | 105.05 (15) | C13—C14—C15 | 120.82 (19) |
| O4—C5—C11 | 109.85 (15) | C13—C14—H141 | 120.1 |
| O6—C5—C11 | 110.45 (15) | C15—C14—H141 | 119.0 |
| O4—C5—H51 | 109.9 | C14—C15—C16 | 120.09 (18) |
| O6—C5—H51 | 110.1 | C14—C15—H151 | 120.7 |
| C11—C5—H51 | 111.3 | C16—C15—H151 | 119.2 |
| C5—O6—C7 | 106.65 (13) | C11—C16—C15 | 119.71 (18) |
| C3—C7—O6 | 104.08 (14) | C11—C16—H161 | 119.9 |
| C3—C7—C8 | 103.21 (15) | C15—C16—H161 | 120.4 |
| O6—C7—C8 | 107.03 (14) | C2—C17—O18 | 106.96 (16) |
| C3—C7—C10 | 118.52 (17) | C2—C17—H172 | 108.2 |
| O6—C7—C10 | 109.08 (16) | O18—C17—H172 | 111.6 |
| C8—C7—C10 | 113.95 (16) | C2—C17—H171 | 109.5 |
| C7—C8—O1 | 110.80 (15) | O18—C17—H171 | 110.7 |
| C7—C8—O9 | 126.80 (18) | H172—C17—H171 | 109.8 |
| O1—C8—O9 | 122.17 (17) | C17—O18—H181 | 113.1 |
| C7—C10—H101 | 110.0 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H103···O18 | 0.97 | 2.53 | 3.233 (3) | 130 |
| C13—H131···O9i | 1.01 | 2.58 | 3.289 (3) | 128 |
| C14—H141···O1ii | 0.97 | 2.59 | 3.340 (3) | 134 |
| O18—H181···O9iii | 0.84 | 2.02 | 2.801 (3) | 153 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x, y−1, z; (iii) x−1/2, −y+3/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH2882).
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
- Baggett, N., Buchanan, J. G., Fatah, M. V., Lachut, C. H., McCullough, K. J. & Webber, J. M. (1985). J. Chem. Soc. Chem. Commun. pp. 1826–1827.
- Baird, P. D., Dho, J. C., Fleet, G. W. J., Peach, J. M., Prout, K. & Smith, P. W. (1987). J. Chem. Soc. Perkin Trans. 1, pp. 1785–1791.
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst.36, 1487.
- Booth, K. V., da Cruz, F. P., Hotchkiss, D. J., Jenkinson, S. F., Jones, N. A., Weymouth-Wilson, A. C., Clarkson, R., Heinz, T. & Fleet, G. W. J. (2008). Tetrahedron Asymmetry19, 2417–2424.
- Booth, K. V., Jenkinson, S. F., Best, D., Nieto, F. F., Estevez, R. J., Wormald, M. R., Weymouth-Wilson, A. C. & Fleet, G. W. J. (2009). Tetrahedron Lett.50, 5088–5093.
- Chen, S. Y. & Joullie, M. M. (1984). J. Org. Chem.49, 2168–2174.
- Dho, J. C., Fleet, G. W. J., Peach, J. M., Prout, K. & Smith, P. W. (1986). Tetrahedron Lett.27, 3203–3204.
- Hotchkiss, D. J., Kato, A., Odell, B., Claridge, T. D. W. & Fleet, G. W. J. (2007). Tetrahedron Asymmetry18, 500–512.
- Hotchkiss, D. J., Soengas, R., Booth, K. V., Weymouth-Wilson, A. C., Eastwick-Field, V. & Fleet, G. W. J. (2007). Tetrahedron Lett.48, 517–520.
- Jenkinson, S. F., Jones, N. A., Moussa, A., Stewart, A. J., Heinz, T. & Fleet, G. W. J. (2007). Tetrahedron Lett.48, 4441–4445.
- Lundt, I. & Madsen, R. (2001). Top. Curr. Chem.215, 178–191.
- Nonius (1997–2001). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Watkin, D. J., Prout, C. K. & Pearce, L. J. (1996). CAMERON, Chemical Crystallography Laboratory, Oxford, UK.
- Zinner, H., Voight, H. & Voight, J. (1968). Carbohydr. Res.7, 38–55.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809032796/lh2882sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809032796/lh2882Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





