Abstract
In the title compound, C12H11FO4S, the O atom and the methyl group of the methylsulfinyl substituent lie on opposite sides of the plane of the benzofuran fragment [O—S—C—C and C—S—C—C torsion angles = 126.70 (13) and −123.55 (13)°, respectively]. The crystal structure is stabilized by weak non-classical intermolecular C—H⋯O hydrogen-bond interactions. The crystal structure also exhibits aromatic π–π stacking interactions between furan/benzene and benzene/benzene rings of adjacent benzofuran ring systems [centroid–centroid distances = 3.8258 (9) and 3.8794 (9) Å] and a weak intermolecular C—H⋯π ring interaction.
Related literature
For crystal structures of similar methyl 2-(5-halo-3-methylsulfinyl-1-benzofuran-2-yl)acetate derivatives. see: Choi et al. (2008a
▶,b
▶). For the pharmacological properties of benzofuran compounds, see: Howlett et al. (1999 ▶); Twyman & Allsop (1999 ▶).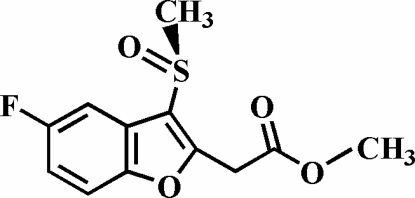
Experimental
Crystal data
C12H11FO4S
M r = 270.27
Triclinic,

a = 7.7799 (5) Å
b = 8.5609 (6) Å
c = 10.5592 (7) Å
α = 73.834 (1)°
β = 80.178 (1)°
γ = 67.486 (1)°
V = 622.36 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.28 mm−1
T = 273 K
0.60 × 0.40 × 0.40 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: none
5368 measured reflections
2667 independent reflections
2389 reflections with I > 2σ(I)
R int = 0.014
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.089
S = 1.08
2667 reflections
165 parameters
H-atom parameters constrained
Δρmax = 0.28 e Å−3
Δρmin = −0.31 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 1998 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809030451/jj2003sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809030451/jj2003Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O4i | 0.93 | 2.39 | 3.303 (2) | 166 |
| C9—H9A⋯O4ii | 0.97 | 2.22 | 3.179 (2) | 168 |
| C9—H9B⋯O1iii | 0.97 | 2.54 | 3.489 (2) | 166 |
| C12—H12A⋯O3iv | 0.96 | 2.60 | 3.478 (2) | 152 |
| C11—H11A⋯Cg2v | 0.96 | 2.97 | 3.93 | 173 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  . Cg2 is the centroid of the C2–C7 ring.
. Cg2 is the centroid of the C2–C7 ring.
supplementary crystallographic information
Comment
Molecules containing the benzofuran skeleton have received considerable attention related to their pharmacological properties (Howlett et al., 1999; Twyman & Allsop, 1999). This work is related to our communications on the synthesis and crystal structures of methyl 2-(5-halo-3-methylsulfinyl-1-benzofuran-2-yl)acetate derivatives, viz. methyl 2-(5-chloro-3-methylsulfinyl-1-benzofuran-2-yl)acetate (Choi et al., 2008a), and methyl 2-(5-bromo-3-methylsulfinyl-1-benzofuran-2-yl)acetate (Choi et al., 2008b). Here we report the crystal structure of the title compound, methyl 2-(5-fluoro-3-methylsulfinyl-1-benzofuran-2-yl)acetate (Fig. 1).
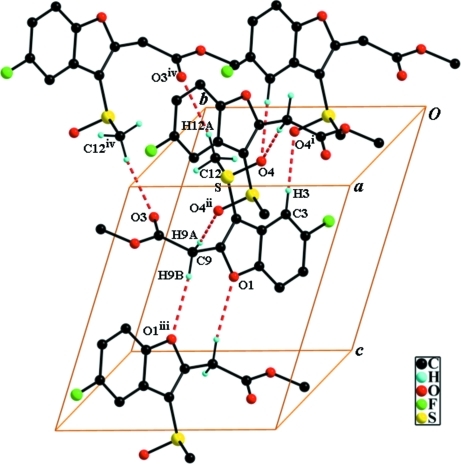
The benzofuran unit is essentially planar, with a mean deviation of 0.006 (1) ° from the least-squares plane defined by the nine constituent atoms (Fig. 1). In the title compound, all of the bond angles and bond distances are in the normal range of related molecules (Choi et al., 2008a, b). The oxygen atom and the methyl group of the methylsulfinyl substituent lie on opposite sides of the plane of the benzofuran fragment [O4–S–C1–C8 and C12–S–C1–C8 torsional angles = 126.70 (13)° and -123.55 (13) °, respectively]. Crystal packing is stabilized by weak intermolecular C–H···O hydrogen bond interactions involving the S═O unit with the benzofurn ring (C3–H3···O4i) and a methylene H atom (C9–H9A···O4ii), between the furan ring and methylene H atom (C9–H9B···O1iii) as well as between a methyl H atom of the methylsulfinyl substituent and the C═O unit (C12–H12A···O3iv), respectively (Fig. 2, Table 1). Crystal packing also exhibits aromatic π–π stacking interactions between the furan/benzene and the benzene/benzene rings of neighbouring molecules [Cg1···Cg2vii = 3.8258 (9)Å and Cg2···Cg2vi = 3.8794 (9)Å, where Cg1 and Cg2 are centroids of the furan (C1/C2/C7/O1/C8) and benzene (C2-C7) rings, respectively, Fig. 3]. The molecular packing is further stabilized by a weak, intermolecular C–H···π ring interaction between a methyl H atom of the methoxy group and a benzene ring of a neighbouring molecule (C11–H11A···Cg2v; = 3.927 (3)Å, Table 1).
Experimental
The 77% 3-chloroperoxybenzoic acid (247 mg, 1.1 mmol) was added in small portions to a stirred solution of methyl 2-(5-fluoro-3-methylsulfanyl-1-benzofuran-2-yl)acetate (254 mg, 1.0 mmol) in dichloromethane (30 ml) at 273 K. After being stirred for 4 h at room temperature, the mixture was washed with saturated sodium bicarbonate solution and the organic layer was separated, dried over magnesium sulfate, filtered and concentrated in vacuum. The residue was purified by column chromatography (hexane-ethyl acetate,1:2 v/v) to afford the title compound as a colorless solid [yield 79%, m.p. 370-371 K; Rf = 0.4 (hexane-ethyl acetate, 1;2 v/v )]. Single crystals suitable for X-ray diffraction were prepared by slow evaporation of a solution of the title compound in benzene at room temperature.
Refinement
All H atoms were geometrically positioned and refined using a riding model, with C–H = 0.93 Å for the aryl, 0.97 Å for the methylene, and 0.96 Å for the methyl H atoms. Uiso(H) = 1.2 Ueq(C) for the aryl and methylene H atoms, and 1.5 Ueq(C) for methyl H atoms.
Figures
Fig. 1.
The molecular structure of the title compound with the atom numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms are presented as a small cycles of arbitrary radius.
Fig. 2.
Weak, C–H···O hydrogen bond interactions (dotted lines) in the title compound. [Symmetry code: (i) - x + 1, - y + 1, - z; (ii) - x + 2, - y + 1, - z; (iii) - x + 2, - y + 1, - z + 1; (iv) - x+1, - y + 2, - z.]
Fig. 3.
Weak, π–π stacking and C–H···π ring intermoleclar interactions (dotted lines) in the title compound. Cg1 and C2 denote ring centroids for C1/C2/C7/O1/C8 and C2-C7, respectively.[Symmetry code: (v) x, y + 1, z; (vi) - x + 1, - y + 2 , - z + 1; 1-x, 2-y, (vii) - x + 1, - y + 1, - z + 1.]
Crystal data
| C12H11FO4S | Z = 2 |
| Mr = 270.27 | F(000) = 280 |
| Triclinic, P1 | Dx = 1.442 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.7799 (5) Å | Cell parameters from 3896 reflections |
| b = 8.5609 (6) Å | θ = 2.7–27.5° |
| c = 10.5592 (7) Å | µ = 0.28 mm−1 |
| α = 73.834 (1)° | T = 273 K |
| β = 80.178 (1)° | Block, colorless |
| γ = 67.486 (1)° | 0.60 × 0.40 × 0.40 mm |
| V = 622.36 (7) Å3 |
Data collection
| Bruker SMART CCD diffractometer | 2389 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.014 |
| graphite | θmax = 27.0°, θmin = 2.0° |
| Detector resolution: 10.0 pixels mm-1 | h = −9→9 |
| φ and ω scans | k = −10→10 |
| 5368 measured reflections | l = −13→13 |
| 2667 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.089 | H-atom parameters constrained |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0432P)2 + 0.227P] where P = (Fo2 + 2Fc2)/3 |
| 2667 reflections | (Δ/σ)max = 0.001 |
| 165 parameters | Δρmax = 0.28 e Å−3 |
| 0 restraints | Δρmin = −0.31 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S | 0.73212 (5) | 0.63256 (5) | 0.04928 (3) | 0.03039 (12) | |
| F | 0.30035 (14) | 0.21264 (13) | 0.37311 (11) | 0.0513 (3) | |
| O1 | 0.84250 (14) | 0.43881 (13) | 0.42317 (9) | 0.0288 (2) | |
| O2 | 1.04499 (16) | 0.88742 (15) | 0.21928 (13) | 0.0436 (3) | |
| O3 | 0.75373 (16) | 0.90527 (15) | 0.20563 (13) | 0.0449 (3) | |
| O4 | 0.75098 (17) | 0.49556 (16) | −0.02040 (11) | 0.0415 (3) | |
| C1 | 0.73869 (19) | 0.53270 (18) | 0.21918 (13) | 0.0263 (3) | |
| C2 | 0.63718 (19) | 0.42498 (17) | 0.29942 (13) | 0.0261 (3) | |
| C3 | 0.4978 (2) | 0.37061 (19) | 0.27953 (15) | 0.0309 (3) | |
| H3 | 0.4480 | 0.4036 | 0.1981 | 0.037* | |
| C4 | 0.4390 (2) | 0.26551 (19) | 0.38775 (17) | 0.0352 (3) | |
| C5 | 0.5098 (2) | 0.2096 (2) | 0.51151 (16) | 0.0375 (4) | |
| H5 | 0.4641 | 0.1377 | 0.5804 | 0.045* | |
| C6 | 0.6489 (2) | 0.26217 (19) | 0.53113 (15) | 0.0342 (3) | |
| H6 | 0.7000 | 0.2268 | 0.6124 | 0.041* | |
| C7 | 0.70799 (19) | 0.36995 (18) | 0.42381 (14) | 0.0275 (3) | |
| C8 | 0.85804 (19) | 0.53640 (18) | 0.29708 (13) | 0.0264 (3) | |
| C9 | 0.9967 (2) | 0.62392 (19) | 0.27115 (14) | 0.0290 (3) | |
| H9A | 1.0886 | 0.5816 | 0.2024 | 0.035* | |
| H9B | 1.0611 | 0.5919 | 0.3506 | 0.035* | |
| C10 | 0.9129 (2) | 0.82024 (19) | 0.22925 (14) | 0.0296 (3) | |
| C11 | 0.9865 (3) | 1.0754 (2) | 0.1780 (3) | 0.0657 (6) | |
| H11A | 0.9333 | 1.1147 | 0.0941 | 0.099* | |
| H11B | 1.0924 | 1.1099 | 0.1701 | 0.099* | |
| H11C | 0.8950 | 1.1261 | 0.2425 | 0.099* | |
| C12 | 0.4904 (2) | 0.7705 (2) | 0.04906 (17) | 0.0386 (4) | |
| H12A | 0.4625 | 0.8393 | −0.0388 | 0.058* | |
| H12B | 0.4656 | 0.8458 | 0.1077 | 0.058* | |
| H12C | 0.4139 | 0.7000 | 0.0782 | 0.058* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S | 0.0313 (2) | 0.0392 (2) | 0.02016 (18) | −0.01352 (15) | −0.00616 (13) | −0.00224 (14) |
| F | 0.0428 (6) | 0.0440 (6) | 0.0702 (7) | −0.0247 (5) | −0.0127 (5) | 0.0003 (5) |
| O1 | 0.0340 (5) | 0.0313 (5) | 0.0211 (5) | −0.0111 (4) | −0.0076 (4) | −0.0034 (4) |
| O2 | 0.0324 (6) | 0.0323 (6) | 0.0661 (8) | −0.0123 (5) | −0.0036 (5) | −0.0104 (5) |
| O3 | 0.0323 (6) | 0.0356 (6) | 0.0588 (8) | −0.0086 (5) | −0.0120 (5) | 0.0019 (5) |
| O4 | 0.0454 (7) | 0.0528 (7) | 0.0277 (6) | −0.0129 (5) | −0.0069 (5) | −0.0154 (5) |
| C1 | 0.0278 (7) | 0.0284 (7) | 0.0209 (6) | −0.0074 (5) | −0.0053 (5) | −0.0046 (5) |
| C2 | 0.0270 (7) | 0.0249 (6) | 0.0237 (6) | −0.0050 (5) | −0.0036 (5) | −0.0065 (5) |
| C3 | 0.0294 (7) | 0.0285 (7) | 0.0334 (7) | −0.0070 (6) | −0.0071 (6) | −0.0069 (6) |
| C4 | 0.0295 (7) | 0.0278 (7) | 0.0477 (9) | −0.0100 (6) | −0.0051 (6) | −0.0065 (6) |
| C5 | 0.0389 (8) | 0.0275 (7) | 0.0382 (8) | −0.0103 (6) | 0.0018 (6) | 0.0000 (6) |
| C6 | 0.0403 (8) | 0.0299 (7) | 0.0261 (7) | −0.0082 (6) | −0.0043 (6) | −0.0017 (6) |
| C7 | 0.0283 (7) | 0.0267 (7) | 0.0261 (7) | −0.0065 (5) | −0.0045 (5) | −0.0070 (5) |
| C8 | 0.0276 (7) | 0.0264 (7) | 0.0217 (6) | −0.0055 (5) | −0.0048 (5) | −0.0041 (5) |
| C9 | 0.0265 (7) | 0.0315 (7) | 0.0285 (7) | −0.0078 (6) | −0.0080 (5) | −0.0061 (5) |
| C10 | 0.0302 (7) | 0.0338 (7) | 0.0238 (7) | −0.0112 (6) | −0.0019 (5) | −0.0055 (5) |
| C11 | 0.0528 (12) | 0.0333 (9) | 0.1083 (19) | −0.0182 (9) | −0.0025 (12) | −0.0103 (10) |
| C12 | 0.0349 (8) | 0.0362 (8) | 0.0382 (8) | −0.0074 (6) | −0.0134 (6) | 0.0001 (6) |
Geometric parameters (Å, °)
| S—O4 | 1.500 (2) | C4—C5 | 1.391 (2) |
| S—C1 | 1.760 (1) | C5—C6 | 1.385 (2) |
| S—C12 | 1.797 (2) | C5—H5 | 0.9300 |
| F—C4 | 1.365 (2) | C6—C7 | 1.383 (2) |
| O1—C8 | 1.374 (2) | C6—H6 | 0.9300 |
| O1—C7 | 1.382 (2) | C8—C9 | 1.484 (2) |
| O2—C10 | 1.335 (2) | C9—C10 | 1.514 (2) |
| O2—C11 | 1.450 (2) | C9—H9A | 0.9700 |
| O3—C10 | 1.200 (2) | C9—H9B | 0.9700 |
| C1—C8 | 1.355 (2) | C11—H11A | 0.9600 |
| C1—C2 | 1.444 (2) | C11—H11B | 0.9600 |
| C2—C7 | 1.398 (2) | C11—H11C | 0.9600 |
| C2—C3 | 1.398 (2) | C12—H12A | 0.9600 |
| C3—C4 | 1.374 (2) | C12—H12B | 0.9600 |
| C3—H3 | 0.9300 | C12—H12C | 0.9600 |
| O4—S—C1 | 106.58 (7) | C6—C7—C2 | 123.8 (1) |
| O4—S—C12 | 106.22 (8) | C1—C8—O1 | 111.2 (1) |
| C1—S—C12 | 98.29 (7) | C1—C8—C9 | 132.4 (1) |
| C8—O1—C7 | 106.3 (1) | O1—C8—C9 | 116.5 (1) |
| C10—O2—C11 | 116.0 (1) | C8—C9—C10 | 114.0 (1) |
| C8—C1—C2 | 107.3 (1) | C8—C9—H9A | 108.8 |
| C8—C1—S | 123.2 (1) | C10—C9—H9A | 108.8 |
| C2—C1—S | 129.4 (1) | C8—C9—H9B | 108.8 |
| C7—C2—C3 | 119.5 (1) | C10—C9—H9B | 108.8 |
| C7—C2—C1 | 104.8 (1) | H9A—C9—H9B | 107.7 |
| C3—C2—C1 | 135.8 (1) | O3—C10—O2 | 124.1 (1) |
| C4—C3—C2 | 115.9 (1) | O3—C10—C9 | 126.4 (1) |
| C4—C3—H3 | 122.1 | O2—C10—C9 | 109.5 (1) |
| C2—C3—H3 | 122.1 | O2—C11—H11A | 109.5 |
| F—C4—C3 | 117.7 (1) | O2—C11—H11B | 109.5 |
| F—C4—C5 | 117.5 (1) | H11A—C11—H11B | 109.5 |
| C3—C4—C5 | 124.8 (2) | O2—C11—H11C | 109.5 |
| C6—C5—C4 | 119.4 (1) | H11A—C11—H11C | 109.5 |
| C6—C5—H5 | 120.3 | H11B—C11—H11C | 109.5 |
| C4—C5—H5 | 120.3 | S—C12—H12A | 109.5 |
| C7—C6—C5 | 116.6 (1) | S—C12—H12B | 109.5 |
| C7—C6—H6 | 121.7 | H12A—C12—H12B | 109.5 |
| C5—C6—H6 | 121.7 | S—C12—H12C | 109.5 |
| O1—C7—C6 | 125.7 (1) | H12A—C12—H12C | 109.5 |
| O1—C7—C2 | 110.5 (1) | H12B—C12—H12C | 109.5 |
| O4—S—C1—C8 | 126.70 (13) | C5—C6—C7—C2 | −0.8 (2) |
| C12—S—C1—C8 | −123.55 (13) | C3—C2—C7—O1 | −179.47 (12) |
| O4—S—C1—C2 | −48.22 (14) | C1—C2—C7—O1 | 0.62 (15) |
| C12—S—C1—C2 | 61.53 (14) | C3—C2—C7—C6 | 0.3 (2) |
| C8—C1—C2—C7 | −0.35 (15) | C1—C2—C7—C6 | −179.62 (13) |
| S—C1—C2—C7 | 175.19 (11) | C2—C1—C8—O1 | −0.04 (16) |
| C8—C1—C2—C3 | 179.77 (15) | S—C1—C8—O1 | −175.92 (9) |
| S—C1—C2—C3 | −4.7 (2) | C2—C1—C8—C9 | −179.96 (14) |
| C7—C2—C3—C4 | 0.6 (2) | S—C1—C8—C9 | 4.2 (2) |
| C1—C2—C3—C4 | −179.54 (15) | C7—O1—C8—C1 | 0.42 (15) |
| C2—C3—C4—F | 178.65 (12) | C7—O1—C8—C9 | −179.65 (12) |
| C2—C3—C4—C5 | −1.0 (2) | C1—C8—C9—C10 | 61.1 (2) |
| F—C4—C5—C6 | −179.15 (14) | O1—C8—C9—C10 | −118.82 (13) |
| C3—C4—C5—C6 | 0.5 (2) | C11—O2—C10—O3 | 0.5 (2) |
| C4—C5—C6—C7 | 0.4 (2) | C11—O2—C10—C9 | 179.20 (16) |
| C8—O1—C7—C6 | 179.59 (14) | C8—C9—C10—O3 | −6.6 (2) |
| C8—O1—C7—C2 | −0.65 (14) | C8—C9—C10—O2 | 174.75 (12) |
| C5—C6—C7—O1 | 178.93 (13) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O4i | 0.93 | 2.39 | 3.303 (2) | 166 |
| C9—H9A···O4ii | 0.97 | 2.22 | 3.179 (2) | 168 |
| C9—H9B···O1iii | 0.97 | 2.54 | 3.489 (2) | 166 |
| C12—H12A···O3iv | 0.96 | 2.60 | 3.478 (2) | 152 |
| C11—H11A···Cg2v | 0.96 | 2.97 | 3.93 | 173 |
Symmetry codes: (i) −x+1, −y+1, −z; (ii) −x+2, −y+1, −z; (iii) −x+2, −y+1, −z+1; (iv) −x+1, −y+2, −z; (v) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JJ2003).
References
- Brandenburg, K. (1998). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2001). SAINT and SMART Bruker AXS Inc., Madison, Wisconsin, USA.
- Choi, H. D., Seo, P. J., Son, B. W. & Lee, U. (2008a). Acta Cryst. E64, o2139. [DOI] [PMC free article] [PubMed]
- Choi, H. D., Seo, P. J., Son, B. W. & Lee, U. (2008b). Acta Cryst. E64, o2397. [DOI] [PMC free article] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Howlett, D. R., Perry, A. E., Godfrey, F., Swatton, J. E., Jennings, K. H., Spitzfaden, C., Wadsworth, H., Wood, S. J. & Markwell, R. E. (1999). Biochem. J 340, 283–289. [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Twyman, L. J. & Allsop, D. (1999). Tetrahedron Lett 40, 9383–9384.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809030451/jj2003sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809030451/jj2003Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report