Abstract
The asymmetric unit of the title compound, C10H10O4, which has been isolated from rhizoma Polygonum Cuspidatum, a Chinese folk medicine, contains two crystallographically independent molecules. The molecules are essentially planar, with a maximum deviation of 0.061 (2) Å from the best planes. The crystal packing is stabilized by weak intermolecular C—H⋯O hydrogen-bonding interactions, with a stacking direction of the molecules parallel to [101].
Related literature
For the synthesis of 5,7-dimethoxyphthalide, see: Talapatra & Monoj (1980 ▶); Dang et al. (1999 ▶); Orito et al. (1995 ▶). For the title compound as an intermediate, see: Zuo et al. (2008 ▶); Lee et al. (2001 ▶). For the title compound as a byproduct, see: Fürstner et al. (2000 ▶).
Experimental
Crystal data
C10H10O4
M r = 194.18
Monoclinic,
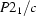
a = 8.532 (3) Å
b = 25.877 (10) Å
c = 8.374 (3) Å
β = 104.322 (6)°
V = 1791.5 (11) Å3
Z = 8
Mo Kα radiation
μ = 0.11 mm−1
T = 293 K
0.12 × 0.12 × 0.10 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.987, T max = 0.989
7489 measured reflections
3216 independent reflections
1766 reflections with I > 2σ(I)
R int = 0.062
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.131
S = 0.93
3216 reflections
258 parameters
H-atom parameters constrained
Δρmax = 0.18 e Å−3
Δρmin = −0.19 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809031183/wm2246sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809031183/wm2246Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6A—H6A⋯O1Bi | 0.93 | 2.51 | 3.397 (3) | 161 |
| C8A—H8A1⋯O2Bii | 0.97 | 2.53 | 3.337 (3) | 140 |
| C6B—H6B⋯O1Aiii | 0.93 | 2.44 | 3.325 (3) | 159 |
Symmetry codes: (i) 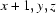 ; (ii)
; (ii) 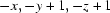 ; (iii)
; (iii) 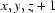 .
.
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (20872179) and the Science and Technology Commission of Shanghai Municipality (STCSM) (08DZ1971504).
supplementary crystallographic information
Comment
The compound 5, 7-dimethoxyphthalide has been previously reported. It could be obtained by different synthetic strategies, e.g. from 5,7-dihydroxyphthalide (Talapatra & Monoj, 1980), 6-iodo-3-methoxybenzyl alcohols (Dang et al., 1999) or 3,5-dimethoxybenzyl alcohol (Orito et al.,1995). It could act as an intermediate product in the process of synthesizing some significant compounds, such as 5,7-dimethoxy-4-methylphthalide and 5,7-dihydroxy-4-methylphthaIide (Zuo et al., 2008), or mycophenolic acid and its analogs (Lee et al., 2001). It was also reported as a byproduct in the synthesis of zearalenone and lasiodiplodin (Fürstner et al., 2000). However, no structural details were provided. In this study, 5,7-dimethoxyphthalide was isolated from the rhizoma Polygonum cuspidatum as colorless prismatic crystals.
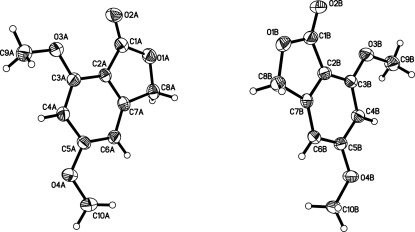
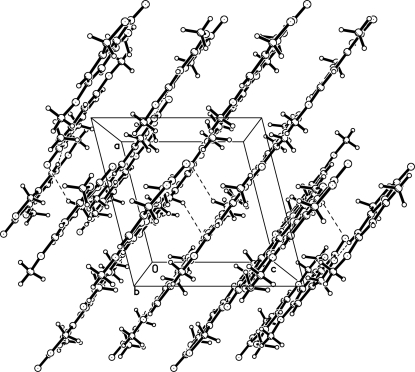
The molecule (Fig. 1 ) is essentially planar with a maximum deviation of 0.061 (2) Å from the best planes. The crystal packing is stabilized by weak intermolecular C—H···O hydrogen-bonding interactions with a stacking direction of the molecules parallel to [101] (Fig. 2 ).
Experimental
The slices of the dried roots of P. cuspidatum (10 kg) were extracted with 60% aqueous acetone 3 times (24 h each) at room temperature. The solvent was evaporated in vacuo and some hydrophobic substances precipitated which were filtered off. The filtrate was concentrated to a suitable volume, then chromatographed on a Sephadex LH-20 column eluted with H2O, aqueous MeOH (10%-70%) and 50% acetone successively to give five fractions. The fraction eluated by 10% MeOH was subjected to MCI gel chromatography eluted with gradient aqueous MeOH solvent. The 30% aqueous MeOH eluate from the MCI column afforded the compound 5,7-dimethoxyphthalide as an amorphous powder. The powder was recrystallized in acetone and produced colourless prismatic crystals.
Refinement
The H atoms were refined at calculated positions riding on the parent carbon atoms (C–H = 0.95–0.99 Å) with isotropic displacement parameters Uiso(H) = 1.2U(Ceq) or 1.5U(–CH3). All CH3 hydrogen atoms were allowed to rotate but not to tip.
Figures
Fig. 1.
The molecular structure of 5,7-dimethoxyphthalide, showing the atom-labelling scheme. H atoms are shown as small spheres of arbitrary radius. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Molecular packing in the crystal, viewed along the b axis. Dashed lines indicate intermolecular C—H···O hydrogen bonds.
Crystal data
| C10H10O4 | F(000) = 816 |
| Mr = 194.18 | Dx = 1.440 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 715 reflections |
| a = 8.532 (3) Å | θ = 2.6–21.3° |
| b = 25.877 (10) Å | µ = 0.11 mm−1 |
| c = 8.374 (3) Å | T = 293 K |
| β = 104.322 (6)° | Prism, colourless |
| V = 1791.5 (11) Å3 | 0.12 × 0.12 × 0.10 mm |
| Z = 8 |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 3216 independent reflections |
| Radiation source: fine-focus sealed tube | 1766 reflections with I > 2σ(I) |
| graphite | Rint = 0.062 |
| φ and ω scans | θmax = 25.2°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −7→10 |
| Tmin = 0.987, Tmax = 0.989 | k = −30→31 |
| 7489 measured reflections | l = −10→8 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.052 | H-atom parameters constrained |
| wR(F2) = 0.131 | w = 1/[σ2(Fo2) + (0.053P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.93 | (Δ/σ)max < 0.001 |
| 3216 reflections | Δρmax = 0.18 e Å−3 |
| 258 parameters | Δρmin = −0.19 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0026 (5) |
Special details
| Experimental. The powder of 5,7-dimethoxyphthalide was solved in acetone and produced colorless crystal. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.3975 (2) | 0.42391 (7) | 0.1615 (2) | 0.0627 (6) | |
| O2A | 0.2605 (3) | 0.35976 (8) | 0.0110 (2) | 0.0674 (6) | |
| O3A | 0.4002 (2) | 0.25798 (7) | 0.1709 (2) | 0.0571 (6) | |
| O4A | 0.8571 (2) | 0.28610 (7) | 0.6142 (2) | 0.0628 (6) | |
| C1A | 0.3670 (4) | 0.37248 (11) | 0.1270 (3) | 0.0518 (8) | |
| C2A | 0.4830 (3) | 0.34270 (10) | 0.2496 (3) | 0.0417 (6) | |
| C3A | 0.5069 (3) | 0.28902 (10) | 0.2724 (3) | 0.0470 (7) | |
| C4A | 0.6341 (3) | 0.27237 (10) | 0.3971 (3) | 0.0471 (7) | |
| H4A | 0.6529 | 0.2372 | 0.4141 | 0.057* | |
| C5A | 0.7359 (3) | 0.30831 (11) | 0.4992 (3) | 0.0487 (7) | |
| C6A | 0.7112 (3) | 0.36054 (10) | 0.4795 (3) | 0.0486 (7) | |
| H6A | 0.7780 | 0.3842 | 0.5478 | 0.058* | |
| C7A | 0.5835 (3) | 0.37640 (10) | 0.3545 (3) | 0.0452 (7) | |
| C8A | 0.5296 (3) | 0.43027 (10) | 0.3046 (3) | 0.0542 (8) | |
| H8A1 | 0.4942 | 0.4478 | 0.3920 | 0.065* | |
| H8A2 | 0.6164 | 0.4500 | 0.2782 | 0.065* | |
| C9A | 0.4125 (4) | 0.20351 (11) | 0.2069 (4) | 0.0644 (9) | |
| H9A1 | 0.5177 | 0.1914 | 0.2025 | 0.097* | |
| H9A2 | 0.3312 | 0.1853 | 0.1270 | 0.097* | |
| H9A3 | 0.3968 | 0.1976 | 0.3150 | 0.097* | |
| C10A | 0.9664 (4) | 0.31963 (13) | 0.7237 (4) | 0.0764 (10) | |
| H10A | 1.0202 | 0.3415 | 0.6614 | 0.115* | |
| H10B | 1.0451 | 0.2994 | 0.7999 | 0.115* | |
| H10C | 0.9072 | 0.3406 | 0.7833 | 0.115* | |
| O1B | −0.0885 (2) | 0.46776 (7) | 0.6553 (2) | 0.0582 (5) | |
| O2B | −0.2380 (2) | 0.53224 (8) | 0.5190 (2) | 0.0674 (6) | |
| O3B | −0.0891 (2) | 0.63386 (7) | 0.6714 (2) | 0.0551 (5) | |
| O4B | 0.3667 (2) | 0.60524 (7) | 1.1150 (2) | 0.0539 (5) | |
| C1B | −0.1230 (3) | 0.51938 (12) | 0.6263 (3) | 0.0522 (8) | |
| C2B | −0.0013 (3) | 0.54864 (11) | 0.7460 (3) | 0.0454 (7) | |
| C3B | 0.0172 (3) | 0.60196 (10) | 0.7725 (3) | 0.0415 (7) | |
| C4B | 0.1434 (3) | 0.61849 (10) | 0.8969 (3) | 0.0440 (7) | |
| H4B | 0.1590 | 0.6537 | 0.9162 | 0.053* | |
| C5B | 0.2488 (3) | 0.58354 (10) | 0.9954 (3) | 0.0420 (6) | |
| C6B | 0.2297 (3) | 0.53046 (10) | 0.9714 (3) | 0.0418 (6) | |
| H6B | 0.2988 | 0.5069 | 1.0375 | 0.050* | |
| C7B | 0.1032 (3) | 0.51477 (9) | 0.8449 (3) | 0.0403 (6) | |
| C8B | 0.0538 (3) | 0.46110 (10) | 0.7869 (3) | 0.0522 (7) | |
| H8B1 | 0.1386 | 0.4442 | 0.7477 | 0.063* | |
| H8B2 | 0.0302 | 0.4406 | 0.8750 | 0.063* | |
| C9B | −0.0723 (4) | 0.68778 (10) | 0.7035 (4) | 0.0620 (8) | |
| H9B1 | −0.0863 | 0.6948 | 0.8117 | 0.093* | |
| H9B2 | −0.1527 | 0.7062 | 0.6232 | 0.093* | |
| H9B3 | 0.0335 | 0.6988 | 0.6973 | 0.093* | |
| C10B | 0.4778 (3) | 0.57130 (11) | 1.2222 (3) | 0.0589 (8) | |
| H10D | 0.4192 | 0.5485 | 1.2769 | 0.088* | |
| H10E | 0.5531 | 0.5913 | 1.3028 | 0.088* | |
| H10F | 0.5356 | 0.5514 | 1.1587 | 0.088* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0734 (15) | 0.0519 (13) | 0.0597 (13) | 0.0139 (11) | 0.0105 (11) | 0.0112 (10) |
| O2A | 0.0663 (14) | 0.0782 (15) | 0.0517 (13) | 0.0101 (12) | 0.0029 (11) | 0.0066 (11) |
| O3A | 0.0585 (13) | 0.0487 (13) | 0.0580 (12) | 0.0039 (10) | 0.0028 (10) | −0.0025 (10) |
| O4A | 0.0558 (12) | 0.0596 (13) | 0.0618 (13) | 0.0065 (10) | −0.0066 (11) | −0.0011 (10) |
| C1A | 0.055 (2) | 0.059 (2) | 0.0442 (18) | 0.0101 (16) | 0.0194 (16) | 0.0078 (15) |
| C2A | 0.0409 (16) | 0.0434 (16) | 0.0435 (16) | 0.0030 (13) | 0.0157 (13) | 0.0015 (13) |
| C3A | 0.0446 (17) | 0.0492 (18) | 0.0479 (18) | −0.0023 (14) | 0.0129 (14) | −0.0027 (14) |
| C4A | 0.0491 (17) | 0.0413 (16) | 0.0515 (17) | 0.0026 (13) | 0.0132 (15) | −0.0009 (13) |
| C5A | 0.0420 (17) | 0.0535 (19) | 0.0498 (17) | 0.0078 (14) | 0.0095 (14) | 0.0018 (14) |
| C6A | 0.0468 (18) | 0.0459 (17) | 0.0530 (18) | −0.0022 (13) | 0.0120 (15) | −0.0059 (13) |
| C7A | 0.0444 (17) | 0.0441 (17) | 0.0520 (17) | 0.0035 (13) | 0.0215 (14) | 0.0037 (14) |
| C8A | 0.064 (2) | 0.0491 (18) | 0.0544 (18) | 0.0077 (14) | 0.0233 (16) | 0.0054 (14) |
| C9A | 0.069 (2) | 0.0492 (19) | 0.071 (2) | −0.0037 (15) | 0.0088 (17) | −0.0008 (15) |
| C10A | 0.068 (2) | 0.078 (2) | 0.069 (2) | 0.0022 (18) | −0.0115 (18) | −0.0118 (18) |
| O1B | 0.0522 (13) | 0.0547 (13) | 0.0659 (13) | −0.0078 (10) | 0.0111 (10) | −0.0179 (10) |
| O2B | 0.0443 (12) | 0.0910 (16) | 0.0590 (13) | −0.0001 (12) | −0.0020 (10) | −0.0168 (11) |
| O3B | 0.0514 (12) | 0.0544 (13) | 0.0538 (12) | 0.0078 (10) | 0.0018 (9) | 0.0017 (10) |
| O4B | 0.0504 (12) | 0.0478 (11) | 0.0521 (12) | 0.0024 (9) | −0.0089 (10) | −0.0010 (9) |
| C1B | 0.0381 (18) | 0.067 (2) | 0.0520 (19) | −0.0063 (15) | 0.0115 (15) | −0.0141 (15) |
| C2B | 0.0382 (16) | 0.0581 (18) | 0.0409 (16) | −0.0021 (14) | 0.0117 (13) | −0.0038 (14) |
| C3B | 0.0381 (16) | 0.0455 (17) | 0.0410 (16) | 0.0036 (13) | 0.0098 (13) | 0.0033 (13) |
| C4B | 0.0428 (16) | 0.0402 (16) | 0.0474 (16) | −0.0020 (13) | 0.0080 (14) | 0.0000 (13) |
| C5B | 0.0386 (16) | 0.0485 (18) | 0.0387 (15) | −0.0018 (13) | 0.0090 (13) | −0.0029 (13) |
| C6B | 0.0381 (16) | 0.0438 (16) | 0.0437 (16) | 0.0047 (12) | 0.0107 (13) | 0.0030 (12) |
| C7B | 0.0417 (16) | 0.0397 (16) | 0.0431 (15) | 0.0003 (13) | 0.0175 (13) | −0.0005 (13) |
| C8B | 0.0486 (18) | 0.0493 (18) | 0.0581 (18) | −0.0027 (14) | 0.0118 (14) | −0.0050 (14) |
| C9B | 0.064 (2) | 0.050 (2) | 0.068 (2) | 0.0100 (15) | 0.0074 (16) | 0.0063 (15) |
| C10B | 0.0473 (19) | 0.062 (2) | 0.0578 (19) | 0.0068 (15) | −0.0056 (15) | −0.0028 (15) |
Geometric parameters (Å, °)
| O1A—C1A | 1.373 (3) | O1B—C1B | 1.376 (3) |
| O1A—C8A | 1.437 (3) | O1B—C8B | 1.434 (3) |
| O2A—C1A | 1.200 (3) | O2B—C1B | 1.202 (3) |
| O3A—C3A | 1.346 (3) | O3B—C3B | 1.357 (3) |
| O3A—C9A | 1.440 (3) | O3B—C9B | 1.422 (3) |
| O4A—C5A | 1.355 (3) | O4B—C5B | 1.354 (3) |
| O4A—C10A | 1.428 (3) | O4B—C10B | 1.434 (3) |
| C1A—C2A | 1.458 (4) | C1B—C2B | 1.463 (4) |
| C2A—C7A | 1.377 (3) | C2B—C7B | 1.372 (3) |
| C2A—C3A | 1.410 (4) | C2B—C3B | 1.400 (4) |
| C3A—C4A | 1.376 (3) | C3B—C4B | 1.368 (3) |
| C4A—C5A | 1.408 (4) | C4B—C5B | 1.393 (3) |
| C4A—H4A | 0.9300 | C4B—H4B | 0.9300 |
| C5A—C6A | 1.371 (4) | C5B—C6B | 1.392 (4) |
| C6A—C7A | 1.374 (3) | C6B—C7B | 1.373 (3) |
| C6A—H6A | 0.9300 | C6B—H6B | 0.9300 |
| C7A—C8A | 1.495 (3) | C7B—C8B | 1.497 (3) |
| C8A—H8A1 | 0.9700 | C8B—H8B1 | 0.9700 |
| C8A—H8A2 | 0.9700 | C8B—H8B2 | 0.9700 |
| C9A—H9A1 | 0.9599 | C9B—H9B1 | 0.9599 |
| C9A—H9A2 | 0.9599 | C9B—H9B2 | 0.9599 |
| C9A—H9A3 | 0.9599 | C9B—H9B3 | 0.9599 |
| C10A—H10A | 0.9599 | C10B—H10D | 0.9599 |
| C10A—H10B | 0.9599 | C10B—H10E | 0.9599 |
| C10A—H10C | 0.9599 | C10B—H10F | 0.9599 |
| C1A—O1A—C8A | 110.8 (2) | C1B—O1B—C8B | 110.8 (2) |
| C3A—O3A—C9A | 116.7 (2) | C3B—O3B—C9B | 117.3 (2) |
| C5A—O4A—C10A | 117.5 (2) | C5B—O4B—C10B | 117.7 (2) |
| O2A—C1A—O1A | 120.2 (3) | O2B—C1B—O1B | 120.0 (3) |
| O2A—C1A—C2A | 132.1 (3) | O2B—C1B—C2B | 132.7 (3) |
| O1A—C1A—C2A | 107.7 (2) | O1B—C1B—C2B | 107.3 (2) |
| C7A—C2A—C3A | 119.4 (2) | C7B—C2B—C3B | 120.2 (2) |
| C7A—C2A—C1A | 108.8 (2) | C7B—C2B—C1B | 109.1 (3) |
| C3A—C2A—C1A | 131.8 (3) | C3B—C2B—C1B | 130.6 (3) |
| O3A—C3A—C4A | 125.1 (3) | O3B—C3B—C4B | 124.3 (2) |
| O3A—C3A—C2A | 116.7 (2) | O3B—C3B—C2B | 118.0 (2) |
| C4A—C3A—C2A | 118.2 (2) | C4B—C3B—C2B | 117.7 (2) |
| C3A—C4A—C5A | 120.4 (3) | C3B—C4B—C5B | 121.3 (2) |
| C3A—C4A—H4A | 119.8 | C3B—C4B—H4B | 119.4 |
| C5A—C4A—H4A | 119.8 | C5B—C4B—H4B | 119.4 |
| O4A—C5A—C6A | 124.8 (3) | O4B—C5B—C6B | 123.7 (2) |
| O4A—C5A—C4A | 113.5 (2) | O4B—C5B—C4B | 114.9 (2) |
| C6A—C5A—C4A | 121.6 (3) | C6B—C5B—C4B | 121.3 (2) |
| C5A—C6A—C7A | 117.1 (2) | C7B—C6B—C5B | 116.4 (2) |
| C5A—C6A—H6A | 121.5 | C7B—C6B—H6B | 121.8 |
| C7A—C6A—H6A | 121.5 | C5B—C6B—H6B | 121.8 |
| C6A—C7A—C2A | 123.3 (2) | C2B—C7B—C6B | 123.1 (2) |
| C6A—C7A—C8A | 128.5 (3) | C2B—C7B—C8B | 107.9 (2) |
| C2A—C7A—C8A | 108.2 (2) | C6B—C7B—C8B | 129.0 (2) |
| O1A—C8A—C7A | 104.5 (2) | O1B—C8B—C7B | 104.8 (2) |
| O1A—C8A—H8A1 | 110.9 | O1B—C8B—H8B1 | 110.8 |
| C7A—C8A—H8A1 | 110.9 | C7B—C8B—H8B1 | 110.8 |
| O1A—C8A—H8A2 | 110.9 | O1B—C8B—H8B2 | 110.8 |
| C7A—C8A—H8A2 | 110.9 | C7B—C8B—H8B2 | 110.8 |
| H8A1—C8A—H8A2 | 108.9 | H8B1—C8B—H8B2 | 108.9 |
| O3A—C9A—H9A1 | 109.5 | O3B—C9B—H9B1 | 109.5 |
| O3A—C9A—H9A2 | 109.5 | O3B—C9B—H9B2 | 109.5 |
| H9A1—C9A—H9A2 | 109.5 | H9B1—C9B—H9B2 | 109.5 |
| O3A—C9A—H9A3 | 109.5 | O3B—C9B—H9B3 | 109.5 |
| H9A1—C9A—H9A3 | 109.5 | H9B1—C9B—H9B3 | 109.5 |
| H9A2—C9A—H9A3 | 109.5 | H9B2—C9B—H9B3 | 109.5 |
| O4A—C10A—H10A | 109.5 | O4B—C10B—H10D | 109.5 |
| O4A—C10A—H10B | 109.5 | O4B—C10B—H10E | 109.5 |
| H10A—C10A—H10B | 109.5 | H10D—C10B—H10E | 109.5 |
| O4A—C10A—H10C | 109.5 | O4B—C10B—H10F | 109.5 |
| H10A—C10A—H10C | 109.5 | H10D—C10B—H10F | 109.5 |
| H10B—C10A—H10C | 109.5 | H10E—C10B—H10F | 109.5 |
| C8A—O1A—C1A—O2A | −179.8 (2) | C8B—O1B—C1B—O2B | 179.5 (2) |
| C8A—O1A—C1A—C2A | −0.5 (3) | C8B—O1B—C1B—C2B | −1.5 (3) |
| O2A—C1A—C2A—C7A | 178.2 (3) | O2B—C1B—C2B—C7B | 178.6 (3) |
| O1A—C1A—C2A—C7A | −1.0 (3) | O1B—C1B—C2B—C7B | −0.3 (3) |
| O2A—C1A—C2A—C3A | −0.6 (5) | O2B—C1B—C2B—C3B | 0.5 (5) |
| O1A—C1A—C2A—C3A | −179.8 (3) | O1B—C1B—C2B—C3B | −178.5 (2) |
| C9A—O3A—C3A—C4A | 6.2 (4) | C9B—O3B—C3B—C4B | −3.4 (4) |
| C9A—O3A—C3A—C2A | −172.6 (2) | C9B—O3B—C3B—C2B | 177.8 (2) |
| C7A—C2A—C3A—O3A | 177.0 (2) | C7B—C2B—C3B—O3B | 180.0 (2) |
| C1A—C2A—C3A—O3A | −4.3 (4) | C1B—C2B—C3B—O3B | −2.0 (4) |
| C7A—C2A—C3A—C4A | −1.9 (4) | C7B—C2B—C3B—C4B | 1.1 (4) |
| C1A—C2A—C3A—C4A | 176.8 (3) | C1B—C2B—C3B—C4B | 179.1 (2) |
| O3A—C3A—C4A—C5A | −178.2 (2) | O3B—C3B—C4B—C5B | −179.5 (2) |
| C2A—C3A—C4A—C5A | 0.6 (4) | C2B—C3B—C4B—C5B | −0.7 (4) |
| C10A—O4A—C5A—C6A | 0.5 (4) | C10B—O4B—C5B—C6B | 0.2 (4) |
| C10A—O4A—C5A—C4A | −179.9 (2) | C10B—O4B—C5B—C4B | 179.1 (2) |
| C3A—C4A—C5A—O4A | −178.9 (2) | C3B—C4B—C5B—O4B | −179.2 (2) |
| C3A—C4A—C5A—C6A | 0.7 (4) | C3B—C4B—C5B—C6B | −0.2 (4) |
| O4A—C5A—C6A—C7A | 179.0 (2) | O4B—C5B—C6B—C7B | 179.6 (2) |
| C4A—C5A—C6A—C7A | −0.6 (4) | C4B—C5B—C6B—C7B | 0.8 (4) |
| C5A—C6A—C7A—C2A | −0.8 (4) | C3B—C2B—C7B—C6B | −0.6 (4) |
| C5A—C6A—C7A—C8A | −179.5 (3) | C1B—C2B—C7B—C6B | −179.0 (2) |
| C3A—C2A—C7A—C6A | 2.1 (4) | C3B—C2B—C7B—C8B | −179.8 (2) |
| C1A—C2A—C7A—C6A | −176.9 (2) | C1B—C2B—C7B—C8B | 1.8 (3) |
| C3A—C2A—C7A—C8A | −178.9 (2) | C5B—C6B—C7B—C2B | −0.4 (4) |
| C1A—C2A—C7A—C8A | 2.1 (3) | C5B—C6B—C7B—C8B | 178.7 (2) |
| C1A—O1A—C8A—C7A | 1.7 (3) | C1B—O1B—C8B—C7B | 2.5 (3) |
| C6A—C7A—C8A—O1A | 176.6 (2) | C2B—C7B—C8B—O1B | −2.6 (3) |
| C2A—C7A—C8A—O1A | −2.3 (3) | C6B—C7B—C8B—O1B | 178.2 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6A—H6A···O1Bi | 0.93 | 2.51 | 3.397 (3) | 161 |
| C8A—H8A1···O2Bii | 0.97 | 2.53 | 3.337 (3) | 140 |
| C6B—H6B···O1Aiii | 0.93 | 2.44 | 3.325 (3) | 159 |
Symmetry codes: (i) x+1, y, z; (ii) −x, −y+1, −z+1; (iii) x, y, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WM2246).
References
- Bruker (2000). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Dang, Q., Brown, B. S., Poelje, P. D., Colby, T. J. & Erion, M. D. (1999). Bioorg. Med. Chem. Lett.9, 1505–1510. [DOI] [PubMed]
- Fürstner, A., Thiel, O. R., Kindler, N. & Bartkowska, B. (2000). J. Org. Chem.65, 7990–7995. [DOI] [PubMed]
- Lee, Y., Fujiwara, Y., Ujita, K., Nagatomo, M., Ohata, H. & Shimizu, I. (2001). Bull. Chem. Soc. Jpn, 74, 1437–1443.
- Orito, K., Miyazawa, M. & Suginome, H. (1995). Tetrahedron, 51, 2489–2496.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Talapatra, B. & Monoj, K. R. (1980). Indian J. Chem. Sect. B, 19, 927–929.
- Zuo, L., Yao, S. Y. & Duan, W. H. (2008). Chin. J. Org. Chem.28, 1982–1985.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809031183/wm2246sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809031183/wm2246Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report