Abstract
The title compound, C10H7Cl3O, obtained as a major byproduct from a classical Schmidt reaction. The cyclohexyl ring is distorted from a classical chair conformation, as observed for monocyclic analogues, presumably due to conjugation of the planar annulated benzo ring and the ketone group (r.m.s. deviation 0.024 Å). There are no significant intermolecular interactions.
Related literature
For the Schmidt reaction, see: Schmidt (1923 ▶). Lactams and their derived amidines are common structural moieties in a variety of phamaceutical agents (Fylaktakidou et al., 2008 ▶), and are common in antipsychotics (Capuano et al., 2002 ▶, 2008 ▶). For the conformation of the cyclohexyl ring in monocyclic analogues, see: Lectard et al. (1973 ▶); Lichanot et al. (1974 ▶).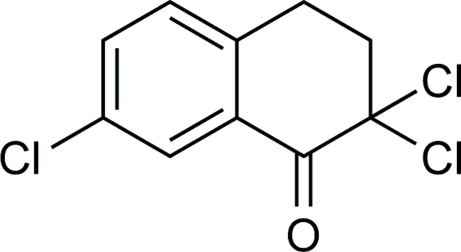
Experimental
Crystal data
C10H7Cl3O
M r = 249.51
Monoclinic,
a = 8.5233 (1) Å
b = 8.0182 (2) Å
c = 14.8698 (3) Å
β = 102.561 (1)°
V = 991.90 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.88 mm−1
T = 123 K
0.28 × 0.10 × 0.10 mm
Data collection
Nonius Kappa CCD diffractometer
Absorption correction: none
9399 measured reflections
2275 independent reflections
1859 reflections with I > 2σ(I)
R int = 0.064
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.100
S = 1.06
2275 reflections
127 parameters
H-atom parameters constrained
Δρmax = 0.53 e Å−3
Δρmin = −0.34 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: DENZO–SMN (Otwinowski & Minor, 1997 ▶); data reduction: DENZO–SMN; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: CIFTAB (Sheldrick, 1997 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809032772/hg2549sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809032772/hg2549Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We acknowledge support from Monash University and the Monash Institue of Pharmaceutical Sciences (Clayton and Parkville campuses) for funding this work.
supplementary crystallographic information
Comment
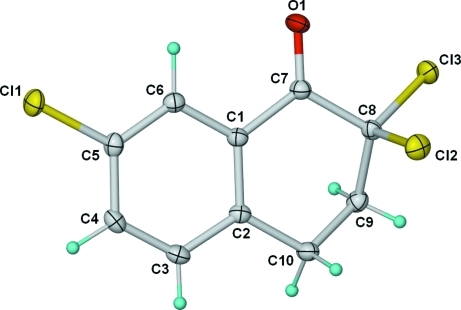
The reaction between hydrazoic acid and carbonyl compounds in the presence of strong acid is known as the Schmidt reaction (Schmidt, 1923) and provides a method for conversion of cyclic ketones to lactams. Lactams as well as their derived amidines are common structural moieties in a variety of phamaceutical agents (Fylaktakidou, et al. 2008), but are specifically of interest to our group as they are common in antipsychotics (Capuano, et al. 2002, 2008). In the current study, reaction of 7-chloro-1-tetralone with sodium azide and hydrochloric acid gave the desired alkyl migration lactam, 8-chloro-2,3,4,5-tetrahydro-1 H-2-benzazepin-1-one, but also a significant amount of the title compound. The solid state structure shows a typical bicyclic ketone framework with two fused six-membered rings and a gem-dichloro substituent in the 2 position. The cyclohexyl ring is distorted from a classical chair conformation, as observed for monocyclic analogues (Lectard, et al., 1973, Lichanot, et al., 1974), presumably due to conjugation of the planar annulated benzo ring and the ketone group (RMS deviation 0.024Å). There are no significant intermolecular interactions.
Experimental
Sodium azide (1.30 g, 20.0 mmol) was added to a stirred solution of 7-chloro-3,4-dihydronaphthalen-1(2H)-one (1.00 g, 5.54 mmol) in concentrated HCl maintained at 0 °C. After warming to room temperature and stirring overnight, the mixture was poured into water and neutralized with K2CO3. The crude product mixture was extracted with CH2Cl2 and purified by flash chromatography (silica; ethyl acetate). The fractions containing the title compound were evaporated and the residue was recrystallized from CHCl3/hexane yielding beige prismatic crystals. (m.p. 435–436 K). 1H NMR (300 MHz, CDCl3δ, p.p.m.): 8.12 (d, 1H, J = 2.5 Hz, H8), 7.52 (dd, 1H, J = 8.0, 2.5 Hz, H6), 7.23 (d, 1H, J = 8.0 Hz, H5), 3.18 (t, 2H, J = 6.0 Hz, H4), 2.95 (t, 2H, J = 6.0 Hz, H3). 13C NMR (75 MHz, CDCl3δ, p.p.m.): 183.0, 140.4, 134.6, 133.9, 130.3, 129.8, 129.4, 85.7, 43.0, 27.0. m/z (EI, 70 ev): 254 (1%, M+[37Cl]3), 252 (7, M+[35Cl][37Cl]2, 250 (24, M+[35Cl]2[37Cl]), 248 (26, M+[35Cl]3), 213 (20), 152 (100), 124 (36), 89 (19). Calcd. for C10H7Cl3O: C 48.1, H 2.8, Cl 42.6; found C 48.1, H 2.9, Cl 42.6%.
Refinement
All H atoms for the primary molecules were initially located in the difference Fourier map but were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances in the range 0.95–1.00 Å and Uiso(H) = 1.2–1.5 Ueq(C).
Figures
Fig. 1.
Molecular diagram of the title compound. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C10H7Cl3O | F(000) = 504 |
| Mr = 249.51 | Dx = 1.671 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 9399 reflections |
| a = 8.5233 (1) Å | θ = 2.8–27.5° |
| b = 8.0182 (2) Å | µ = 0.88 mm−1 |
| c = 14.8698 (3) Å | T = 123 K |
| β = 102.561 (1)° | Prism, colourless |
| V = 991.90 (3) Å3 | 0.28 × 0.10 × 0.10 mm |
| Z = 4 |
Data collection
| Nonius Kappa CCD diffractometer | 1859 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.064 |
| graphite | θmax = 27.5°, θmin = 2.8° |
| φ and ω scans | h = −11→11 |
| 9399 measured reflections | k = −10→10 |
| 2275 independent reflections | l = −19→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.100 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0478P)2 + 0.6127P] where P = (Fo2 + 2Fc2)/3 |
| 2275 reflections | (Δ/σ)max = 0.001 |
| 127 parameters | Δρmax = 0.53 e Å−3 |
| 0 restraints | Δρmin = −0.34 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | −0.11870 (6) | 0.58915 (7) | 0.26554 (4) | 0.02493 (16) | |
| Cl2 | 0.48515 (6) | 1.11552 (6) | 0.63056 (4) | 0.02410 (15) | |
| Cl3 | 0.25735 (6) | 0.91201 (7) | 0.70159 (4) | 0.02542 (16) | |
| O1 | 0.20373 (18) | 1.05238 (18) | 0.48477 (11) | 0.0256 (4) | |
| C1 | 0.2039 (2) | 0.7561 (2) | 0.48441 (13) | 0.0167 (4) | |
| C2 | 0.2751 (2) | 0.6089 (2) | 0.52503 (14) | 0.0165 (4) | |
| C3 | 0.2196 (2) | 0.4562 (3) | 0.48443 (14) | 0.0199 (4) | |
| H3 | 0.2659 | 0.3555 | 0.5116 | 0.024* | |
| C4 | 0.0986 (2) | 0.4492 (3) | 0.40537 (14) | 0.0200 (4) | |
| H4 | 0.0616 | 0.3449 | 0.3786 | 0.024* | |
| C5 | 0.0320 (2) | 0.5981 (3) | 0.36575 (14) | 0.0187 (4) | |
| C6 | 0.0818 (2) | 0.7514 (2) | 0.40393 (13) | 0.0180 (4) | |
| H6 | 0.0346 | 0.8514 | 0.3763 | 0.022* | |
| C7 | 0.2542 (2) | 0.9236 (2) | 0.52247 (14) | 0.0182 (4) | |
| C8 | 0.3773 (2) | 0.9241 (2) | 0.61578 (14) | 0.0173 (4) | |
| C9 | 0.4939 (2) | 0.7787 (3) | 0.62603 (14) | 0.0200 (4) | |
| H9A | 0.5645 | 0.7810 | 0.6884 | 0.024* | |
| H9B | 0.5627 | 0.7904 | 0.5806 | 0.024* | |
| C10 | 0.4060 (2) | 0.6127 (2) | 0.61131 (14) | 0.0198 (4) | |
| H10A | 0.4845 | 0.5231 | 0.6079 | 0.024* | |
| H10B | 0.3583 | 0.5894 | 0.6650 | 0.024* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0203 (3) | 0.0295 (3) | 0.0223 (3) | −0.0046 (2) | −0.0012 (2) | −0.0031 (2) |
| Cl2 | 0.0264 (3) | 0.0186 (3) | 0.0248 (3) | −0.0065 (2) | 0.0002 (2) | −0.0013 (2) |
| Cl3 | 0.0253 (3) | 0.0289 (3) | 0.0240 (3) | 0.0017 (2) | 0.0097 (2) | 0.0008 (2) |
| O1 | 0.0284 (8) | 0.0142 (7) | 0.0295 (8) | 0.0003 (6) | −0.0037 (7) | 0.0008 (6) |
| C1 | 0.0165 (9) | 0.0157 (10) | 0.0181 (9) | −0.0002 (7) | 0.0043 (8) | 0.0014 (8) |
| C2 | 0.0156 (9) | 0.0158 (10) | 0.0188 (9) | 0.0010 (7) | 0.0054 (7) | 0.0017 (8) |
| C3 | 0.0206 (10) | 0.0152 (10) | 0.0250 (10) | 0.0018 (8) | 0.0070 (8) | 0.0012 (8) |
| C4 | 0.0207 (10) | 0.0168 (9) | 0.0240 (10) | −0.0026 (8) | 0.0083 (8) | −0.0040 (8) |
| C5 | 0.0157 (9) | 0.0225 (11) | 0.0178 (9) | −0.0038 (8) | 0.0035 (8) | −0.0020 (8) |
| C6 | 0.0176 (9) | 0.0176 (10) | 0.0190 (10) | 0.0012 (8) | 0.0043 (8) | 0.0017 (8) |
| C7 | 0.0173 (9) | 0.0169 (10) | 0.0198 (10) | 0.0005 (8) | 0.0029 (8) | 0.0006 (8) |
| C8 | 0.0190 (9) | 0.0148 (9) | 0.0186 (9) | −0.0027 (8) | 0.0050 (8) | 0.0006 (8) |
| C9 | 0.0174 (9) | 0.0214 (10) | 0.0203 (10) | −0.0007 (8) | 0.0021 (8) | 0.0012 (8) |
| C10 | 0.0208 (10) | 0.0157 (10) | 0.0215 (10) | 0.0012 (8) | 0.0018 (8) | 0.0027 (8) |
Geometric parameters (Å, °)
| Cl1—C5 | 1.744 (2) | C4—C5 | 1.396 (3) |
| Cl2—C8 | 1.778 (2) | C4—H4 | 0.9500 |
| Cl3—C8 | 1.803 (2) | C5—C6 | 1.382 (3) |
| O1—C7 | 1.208 (2) | C6—H6 | 0.9500 |
| C1—C2 | 1.402 (3) | C7—C8 | 1.547 (3) |
| C1—C6 | 1.405 (3) | C8—C9 | 1.518 (3) |
| C1—C7 | 1.484 (3) | C9—C10 | 1.520 (3) |
| C2—C3 | 1.401 (3) | C9—H9A | 0.9900 |
| C2—C10 | 1.506 (3) | C9—H9B | 0.9900 |
| C3—C4 | 1.386 (3) | C10—H10A | 0.9900 |
| C3—H3 | 0.9500 | C10—H10B | 0.9900 |
| C2—C1—C6 | 120.99 (18) | C1—C7—C8 | 115.33 (16) |
| C2—C1—C7 | 122.35 (17) | C9—C8—C7 | 112.93 (16) |
| C6—C1—C7 | 116.65 (17) | C9—C8—Cl2 | 109.90 (14) |
| C3—C2—C1 | 118.42 (18) | C7—C8—Cl2 | 110.16 (13) |
| C3—C2—C10 | 120.16 (17) | C9—C8—Cl3 | 110.33 (14) |
| C1—C2—C10 | 121.41 (17) | C7—C8—Cl3 | 104.88 (13) |
| C4—C3—C2 | 121.35 (19) | Cl2—C8—Cl3 | 108.46 (11) |
| C4—C3—H3 | 119.3 | C8—C9—C10 | 111.50 (16) |
| C2—C3—H3 | 119.3 | C8—C9—H9A | 109.3 |
| C3—C4—C5 | 118.84 (19) | C10—C9—H9A | 109.3 |
| C3—C4—H4 | 120.6 | C8—C9—H9B | 109.3 |
| C5—C4—H4 | 120.6 | C10—C9—H9B | 109.3 |
| C6—C5—C4 | 121.79 (19) | H9A—C9—H9B | 108.0 |
| C6—C5—Cl1 | 119.40 (16) | C2—C10—C9 | 112.99 (16) |
| C4—C5—Cl1 | 118.80 (15) | C2—C10—H10A | 109.0 |
| C5—C6—C1 | 118.60 (18) | C9—C10—H10A | 109.0 |
| C5—C6—H6 | 120.7 | C2—C10—H10B | 109.0 |
| C1—C6—H6 | 120.7 | C9—C10—H10B | 109.0 |
| O1—C7—C1 | 123.49 (19) | H10A—C10—H10B | 107.8 |
| O1—C7—C8 | 121.17 (18) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2549).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Capuano, B., Crosby, I. T. & Lloyd, E. J. (2002). Curr. Med. Chem.9, 521–548. [DOI] [PubMed]
- Capuano, B., Crosby, I. T., Lloyd, E. J., Podloucka, A. & Taylor, D. A. (2008). Aust. J. Chem.61, 930–940.
- Fylaktakidou, K. C., Hadjipavlou-Litina, D. J., Litinas, K. E., Varella, E. A. & Nicolaides, D. N. (2008). Curr. Pharm. Des.14, 1001–1047. [DOI] [PubMed]
- Lectard, A. J., Petrissans, J. & Hauw, C. (1973). Cryst. Struct. Commun.2, 1–4.
- Lichanot, A., J. Petrissans, J., Hauw, C. & Gaultier, J. (1974). Cryst. Struct. Commun.3, 223–225.
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Schmidt, K. F. (1923). Angew. Chem.36, 511–524.
- Sheldrick, G. M. (1997). CIFTAB University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809032772/hg2549sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809032772/hg2549Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report