Abstract
Relapsing polychondritis is a multisystem autoimmune disease involving cartilage destruction but no known causative antigen. HLA-DQ8 has been associated with various autoimmune diseases in humans. To study the role of DQ8 in autoimmune diseases, we have generated transgenic mice expressing DQ8 (DQA1*0301, DQB1*0302) in a NOD background lacking endogenous class II molecules (Aβo). Upon immunization with type II collagen (CII), 85% of NOD.DQ8 mice develop severe experimental polychondritis, auricular chondritis, and polyarthritis, with clinical and histological similarities to relapsing polychondritis (RP) in humans. CII-immunized mice mount a T cell response and produce Ab’s to type IX collagen (CIX) and self-CII. Transgene-negative littermates do not develop any serological and clinical manifestations following immunization. B10.DQ8 transgenic mice develop polyarthritis and Ab’s to CII only. The susceptibility to auricular chondritis in NOD.DQ8 mice can be attributed to response to CIX. A higher number of activated cells, CD4+CD44hiCD62Llo, and lower regulatory cells CD4+CD152+CD25+ were observed in NOD.DQ8 mice compared with B10.DQ8 mice. The NOD.DQ8 mice provide a model of RP with a high disease incidence and multiple organ involvement to investigate putative autoantigen and regulatory cells involved in disease pathogenesis. An experimental model restricted by the human class II molecule will be valuable when studying the role of various collagens in immunologic and pathologic responses in human RP.
Introduction
Relapsing polychondritis (RP) is an autoimmune disease of unknown etiology. It is characterized by recurrent episodes of inflammation resulting in destruction of cartilaginous tissues, especially the pinnae, nose, and tracheobranchial cartilage. Over 90% of patients develop auricular chondritis over the course of the disease, the most common clinical manifestation of RP (1–3). Auricular chondritis is characterized by bilateral pain, erythema, and inflammation of the cartilaginous tissue of the outer ear accompanied by a mononuclear infiltrate (4). Multiple recurrent episodes of this inflammation result in the destruction of cartilage, which, in turn, is replaced with fibrous tissue leading to disfigurement of the pinna (5), often referred to as the “cauliflower ear.” Polyarthritis is another common clinical feature that is displayed by more than 50% of the RP patients (1, 3, 6, 7). Other manifestations include nasal chondritis and ocular and renal involvement (2, 3, 8). The most critical manifestation of RP is the involvement of laryngotracheal cartilage, which is associated with high mortality in RP (3).
There is no apparent difference in susceptibility between males and females (3). No known correlation exists between predisposition to RP and the presence of specific HLA class I alleles (9). Predisposition to RP has been reported to be significantly associated with the presence of HLA-DR4, a class II allele known to be associated with rheumatoid arthritis (RA), although no predominance of DR4 subtypes has been observed (1, 10). Recently, T cells directed against type II collagen (CII) with specificity to peptide 261-273 were identified in a patient with RP (11). Cell-mediated immunity and anti-CII–specific Ab’s have been observed in both RA and RP patients (12–14). In addition to CII, there is evidence of T cell responses and Ab’s to type IX (CIX) and type XI (CXI) collagen in RP patients (15, 16).
Models of RP in susceptible strains of rats following immunization with CII have been reported (17–19). A mouse model of autoimmune ear disease required both immunization with CII and trauma to the ear to induce inflammatory response (20). Some strains of rats and mice have been shown to develop only the respiratory features of RP, as seen by immunizing animals with cartilage matrix protein (21). Recently, mice expressing both DQA1*0103/DQB1*0601 (DQ6) and DQA1*0301/DQB1*0302 (DQ8) molecules were shown to develop experimental polychondritis, exhibiting both polyarthritis and auricular chondritis (22). Auricular chondritis, however, was seen in only 25% of the immunized animals.
In this study, we describe DQ8 transgenic mice lacking endogenous class II molecules on congenic NOD and B10 backgrounds. NOD/C5+.DQ8.Aβo (NOD.DQ8; see below) mice developed auricular chondritis and polyarthritis with involvement of trachea and respiratory tract following immunization with chick CII. The clinical features were accompanied with strong anti-CII-specific and anti-CIX-specific Ab’s, thus indicating a cross-reactive response to CIX could lead to auricular chondritis. On the other hand, B10.DQ8 mice developed only polyarthritis and anti-CII-specific Ab’s. The NOD.DQ8 mice develop auricular chondritis with CIX, thus providing a novel model in the study of the role of various collagens in immunologic and pathologic responses in RP because disease occurs in these mice with a high incidence and involves multiple organs.
Methods
Mice.
The DQ8 transgene was microinjected into (CBA/JX B10.M) F2 and backcrossed onto a B10.M (H-2f/f) background. The B10.M.DQ8 mice were mated with class II–deficient mice (Aβo) to generate Aβo.DQ8 mice, which were then mated with NOD mice. The F1 generation was intercrossed and transgene-positive mice were backcrossed to NOD for ten generations to create DQ8 (Ag7–/–) mice with a NOD background, NOD/C5+.DQ8.Aβo, referred to as NOD.DQ8 here. For the B10 congenic background, B10 mice were mated with Aβo mice and backcrossed to B10 for ten generations. Congenic B10.Aβo mice were then mated with B10.M. DQ8 mice and intercrossed to obtain DQ8-positive B10 mice without endogenous class II molecules.
All mice were bred in a pathogen-free facility and maintained in a clean conventional colony in Immunogenetics Mouse Colony at the Mayo Clinic (Rochester, Minnesota, USA). All experiments were performed with the approval of Institutional Animal Care and Use Committee. Experimental mice represented both sexes and were 8–12 weeks old when immunized with collagen. Negative littermates and NOD mice were studied as controls.
Ab’s.
The expression of various cell surface markers was analyzed by flow cytometry using FACS IV (BD Biosciences, San Jose, California, USA) using specific Ab’s. Ab’s used for the expression of T cell receptor Vβ chain molecules were mAb MR9-4 (anti-Vβ5.1), MR9-8 (anti-Vβ5.1.2), 44-22-1 (anti-Vβ6), F23.1 (anti-Vβ8.1.2.3), KJ-16 (anti-Vβ8.1.2), F23.2 (anti-Vβ8.2), and KT11 (anti-Vβ11). Anti-CD3, -CD4, -CD8, -CD44, -CD25, -CD62L, and -B220 conjugated with FITC or phycoerythrin (PE) (BD Biosciences PharMingen, San Diego, California, USA) were used according to the manufacturer’s protocol on peripheral blood leukocytes (PBLs) or splenic cells of naive and immunized mice. Other Ab’s used were IVD12 (anti-DQ), K24-199 (anti-Ag7), and HB163 (anti-Ab).
Induction and evaluation of disease.
Pure native chick CII was obtained by multiple-step purification described previously (17). Transgenic mice and negative littermates were immunized with chick CII as described for the collagen-induced arthritis (CIA) protocol (23). The arthritic severity of mice was evaluated for each paw with a grading system using numbers from 0 to 3 (24). The mean arthritic score was determined using arthritic animals only. Bovine CIX (containing both low molecular weight and high molecular weight fragments of pepsin-digested CIX; Chondrex Inc., Redmond, Washington, USA) was used to induce disease using the CIA protocol (23).
Auricular chondritis was monitored from the day ears showed redness and swelling, indicating inflammation. All mice were regularly checked for any other clinical manifestations.
Histopathology.
Mice were sacrificed following the onset of auricular chondritis and arthritis. Outer ears were removed surgically, embedded in paraffin, sectioned, and stained with H&E and toluidine blue. Paws from these mice were decalcified and fixed, and sections were stained with H&E. The epiglottis, lungs, trachea, kidney, and pancreas were also removed from some diseased mice, sectioned, and assessed for histopathologic changes after H&E staining.
In addition, chondritic ears were fixed in OTC embedding medium (Sakura Finetek, Torrance, California, USA). Cryostat sections were prepared, fixed, and stained with biotinylated anti-mouse Ab’s specific for CD4, CD8, B220, Mac-1, and CD69, according to the manufacturer’s instructions (BD Biosciences PharMingen). Sections were stained with avidin-HRP to visualize cells expressing these cell surface markers. Deposition of collagen in various organs was visualized by staining frozen sections with Accustain trichrome stain (Sigma-Aldrich, St. Louis, Missouri, USA).
Anti-collagen Ab’s.
Mice were bled on day 35 after immunization, and the levels of anti-mouse CII and anti-chick CII in serum were determined using a standard ELISA technique, as described previously (25).
Anti-CIX Ab’s were tested in sera of chick CII- and CIX-primed mice by ELISA using recombinant type IX collagen (a generous gift from Linda Myers, University of Tennessee, Memphis, Tennessee, USA).
In vitro T cell proliferation.
Transgenic mice, negative littermates, and NOD mice were immunized with 200 μg of various collagens (CII and bovine CIX and CXI collagen; Chondrex Inc.) emulsified 1:1 with CFA (Difco Laboratories, Detroit, Michigan, USA). The data are presented as the stimulation index. In vitro T cell proliferation was carried out as described previously (23). For inhibition experiments, culture supernatant containing 5 μg each of specific mAb was added to the cells challenged in vitro with CII at 50 μg/ml.
Cytokines.
Cytokines were measured in supernatants collected after 48 hours from cultured splenic cells as described above. Capture ELISA was done for measuring cytokines IFN-γ, IL-2, IL-6, IL-4, IL-10, IL-18, TNF-α, and TGF-β using kits (BD Biosciences PharMingen).
Autoantibodies (anti-nuclear Ab’s) and rheumatoid factor.
Hep-2 cell line slides (BioRad Laboratories, Hercules, California, USA) were used as substrates for anti-nuclear Ab detection as described previously (26). Rheumatoid factor (RF) was measured by ELISA as previously described (26).
The C5 gene and hemolytic assay for C5.
Genomic DNA was isolated from tails of NOD.DQ8, NOD, and B10.DQ8 mice and amplified by PCR using specific C5 primers designed to amplify the genomic fragment of both NOD and B10 genomes (27). C5 levels in sera of mice were determined by a standard hemolytic assay employing sheep erythrocyte suspension sensitized with rabbit IgM-anti–sheep erythrocyte Ab using a kit (Diamedix Corp., Miami, Florida, USA) as described (28).
Statistical analysis.
The difference in incidence of arthritis between groups was analyzed using x2 test with a Yates’ correction. Ab levels and mean scores for arthritic mice were compared using the Student t test.
Results
All transgenic mice showed a similar expression of DQ8, with 45–55% of the PBLs staining positively for DQ8. Expression of H2A molecules was also studied to confirm their absence. The percentage of CD3-, CD4-, CD8-, and B220-positive cells was similar in both transgenic strains. A similar profile of Vβ T cell receptor was observed in both transgenic mice (data not shown).
NOD.DQ8 mice develop polychondritis.
Eighty-five percent of the chick CII-immunized NOD.DQ8 mice developed bilateral auricular chondritis with features typical of human RP (Figure 1), while B10.DQ8 mice, NOD mice, and negative littermates did not show any clinical or histologic involvement of the outer ear. The incidence of chondritis was equal between male and female mice (78% and 90%, respectively) and occurred with mean day of onset of 44.3 ± 4.4. Twenty-five percent of mice exhibiting chondritis died between day 50 and 100 (mean day of death, 72 ± 21).
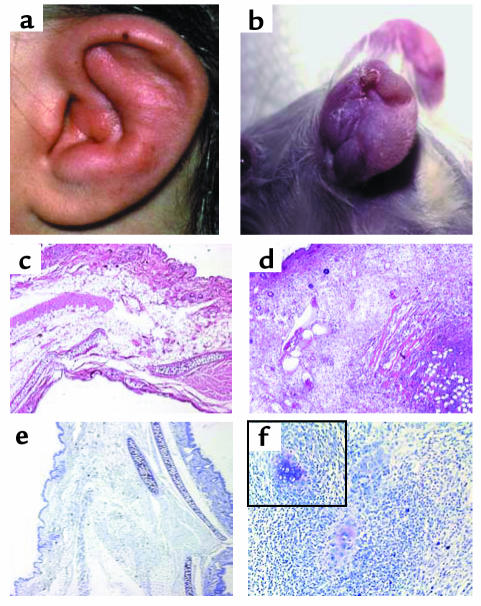
Figure 1.
Swelling and edema with a shrunken ear as observed in a human ear (a) and an affected mouse (b) with auricular chondritis. H&E-stained section of a normal (c) and a chondritic ear (d) of NOD.DQ8 transgenic mice showing massive infiltration of cells in the chondritic ear. Toludine blue staining of a normal ear (e) and a chondritic ear (f) showing destruction of cartilage in the latter, with residual cartilage seen among infiltrates as shown within the boxed area (magnification ×200). Micrographs are ×50 magnification.
The onset of chondritis was marked with swelling and erythema of outer ears, progressing eventually to destruction of the cartilaginous tissue resulting in cauliflower ear. Histopathology of the chondritic ear revealed that normal cartilage was destroyed, but fragments of cartilage were observed by toluidine staining (Figure 1f). A massive infiltration of mononuclear cells (Figure 1d), consisting of CD4+, CD8+, CD69+, B220+, and CD11b+ cells, was seen during the first 2 weeks of inflammation (Figure 2). Eight weeks after onset, most of the infiltrating cells were B220+, CD4+, and CD11b+, with B cells as aggregates resembling follicles.
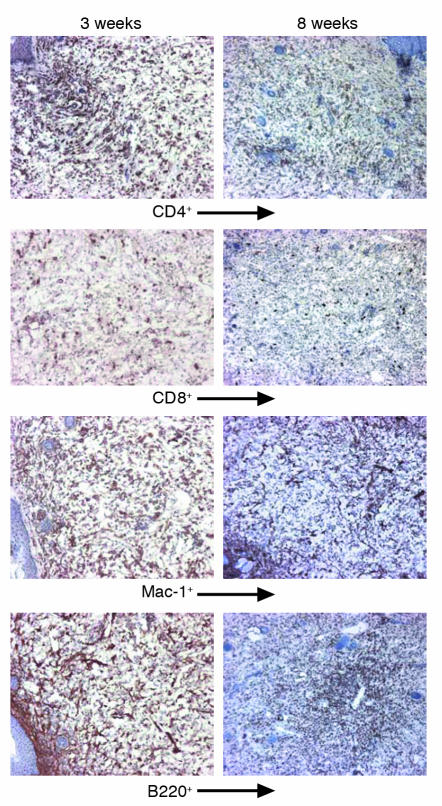
Figure 2.
Characterization of the infiltrating cells in chondritic ear of NOD.DQ8 mice at 3 weeks and 8 weeks after onset of disease. At 3 weeks, CD4+, B220+, and Mac-1+ cells were seen in abundance distributed throughout the section. Eight weeks after onset of chondritis, infiltrating CD4+, CD8+, and Mac-1+ cells were reduced. B cells were present in aggregates, giving an appearance of follicles.
Transgenic mice develop polyarthritis.
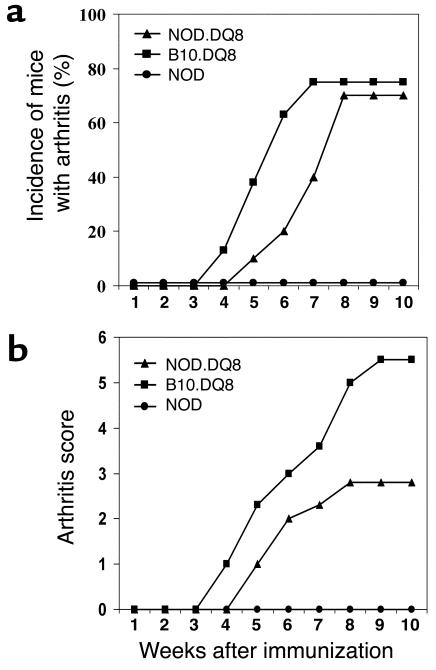
Incidence of polyarthritis was comparable in both strains (NOD.DQ8: 70%; B10.DQ8: 75%) (Figure 3a). A significantly milder disease (P < 0.01) with delayed onset was observed in NOD.DQ8 mice than in B10.DQ8 mice, however (mean day of onset 46 ± 4 in NOD.DQ8 mice and 39 ± 7 in B10.DQ8 mice) (Figure 3b). Histologic analysis of the paws showed inflammation demonstrated by infiltration of mononuclear cells, pannus formation, and destruction of cartilage as reported previously in DQ8 transgenic mice (Figure 4a). Transgene-negative littermates from both strains were resistant to CIA.
Figure 3.
(a) Incidence and onset of arthritis after immunization with CII showed a delayed onset of arthritis (P < 0.01) with similar incidence in NOD.DQ8 mice compared with B10.DQ8 mice. (b) NOD.DQ8 mice developed milder arthritis compared with B10.DQ8 mice (P < 0.01).
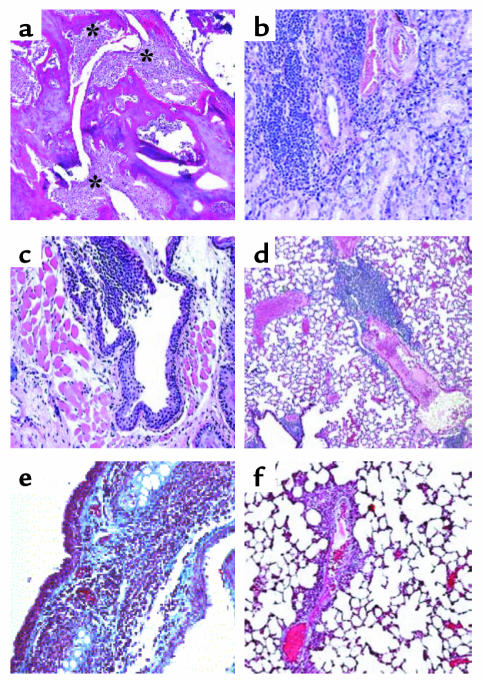
Figure 4.
Sections from various organs of NOD.DQ8 mice stained with H&E. (a) Representative inflammatory arthritis of the knee from a NOD.DQ8 mouse. Significant mononuclear infiltration with cartilage destruction and pannus formation is seen (*). (b) Salivary glands showing mononuclear infiltrate suggestive of sialadentitis. (c) Infiltration with mononuclear cells in the trachea. (d) A section of lung shows perivascular infiltration in lungs. Accustain trichrome staining of frozen sections of trachea (e) and lungs (f). Only tracheas showed deposition of collagen, as seen by the blue color. Micrographs a–d are at ×100 magnification; e and f are at ×200 magnification.
C5 activity in transgenic mice.
The C5 gene as determined by PCR was present in both DQ8 transgenic strains and B10 mice, while in NOD mice it showed deletion of 2 bp, as expected. Levels of C5 in sera of these mice showed both DQ8 transgenic and B10 strain to be C5 proficient, while NOD mice were C5 deficient (data not shown). All transgene-positive NOD.DQ8 mice were C5 proficient.
NOD.DQ8 mice share similarities with human RP in involvement of organs.
To investigate if organs other than ears and joints reported to be involved in RP patients were also affected in NOD.DQ8 mice, histology of lungs, tracheas, kidneys, pancreata, and salivary glands was done. Histopathology of these organs showed infiltration of mononuclear cells in the trachea and the perivascular region of lungs, with collagen deposition in the trachea (Figure 4). Salivary glands showed infiltration of cells, which is known to be a feature of sialadenitis. Pancreata did not show any signs of insulitis, although some peri-insulitis was seen; none of the mice developed diabetes (data not shown). No pathology was observed in kidneys, epiglottises, and hearts (data not shown). B10.DQ8 mice did not show pathology in any of these organs (data not shown).
NOD.DQ8 mice mount in vitro response to CIX and CXI.
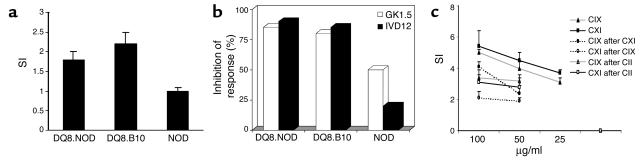
Both NOD.DQ8 and B10.DQ8 mice mounted a similar CII-specific response in vitro (Figure 5a). The in vitro response was mediated by CD4+ cells and restricted by the DQ8 molecule as observed by an inhibition experiment (Figure 5b). NOD.DQ8 mice mounted a strong dose-dependent response to both bovine CIX and CXI when immunized with specific collagen and CII (Figure 5c). Lymph node cells from CXI-primed mice also mounted a strong in vitro response to CIX. In contrast, mice immunized with CIX did not respond to CXI in vitro. There was no cross-reactive response to CII when immunized with either CIX or CXI. B10.DQ8, negative littermates, and NOD mice did not give any response to CIX and CXI when immunized with either collagen type or after immunization with CII (data not shown).
Figure 5.
(a) In vitro response of lymph node cells (LNCs) to chick CII (50 μg/ml) from primed mice in transgenic and control mice. The results shown are mean ± SD of three experiments. SDs were lower than 10% in all experiments. (b) Inhibition of response was measured from LNCs cultured as above in the presence of specific Ab’s, DQ (IVD12) or mCD4 (GK1.5). The results are shown as the percentage of inhibition of response by a specific Ab. (c) NOD.DQ8 mice primed with CIX and CXI mount a dose-dependent response to a specific collagen. Cross-reactive response to CIX was observed in CII-immunized mice. The data is mean ± SD of all experiments. SI, stimulation index.
CII-immunized NOD.DQ8 mice make Ab’s to CIX.
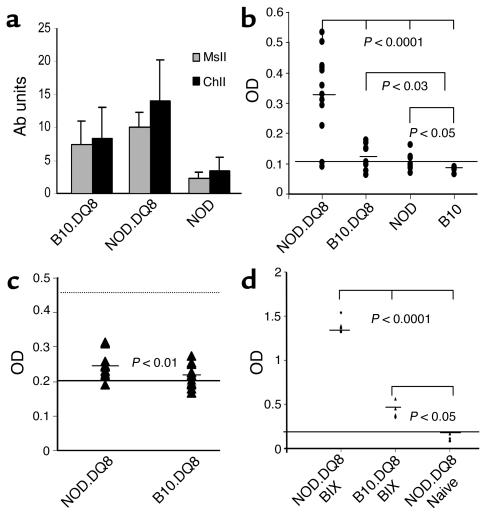
Both transgenic mice developed high levels of anti-ChII and anti-MsII IgG Ab’s following immunization with CII; NOD.DQ8 mice had significantly higher levels than B10.DQ8 mice (P < 0.05 and P < 0.001, respectively) (Figure 6a). Immunization with chick CII also induced anti-mouse CII Ab’s, which were significantly higher in NOD.DQ8 mice compared with B10.DQ8 mice (P < 0.001). NOD mice immunized with CII developed very low levels of anti-chick and anti-mouse CII Ab’s. Since collagen tissue in the outer ear has CIX as an important structural component, we measured Ab’s to recombinant collagen also. Interestingly, NOD.DQ8 mice produced significantly high levels of anti-CIX Ab’s as shown by high OD compared with B10.DQ8 (P < 0.0007). B10 and NOD mice were similar to the negative control, Aβo (Figure 6b).
Figure 6.
NOD.DQ8 mice produce higher levels of Ab’s to self, MsII, and ChII than B10.DQ8 mice (anti-MsII: P < 0.001; anti-ChII: P < 0.05). Bars depict mean ± SD (units per milliliter) of anti-collagen Ab’s. NOD mice produced very low levels of anti-CII and anti-MsII Ab’s compared with transgenic mice (P < 0.001). (b) Only NOD.DQ8 mice produced significant levels of anti-CIX Ab’s following immunization with CII. Aβo mice were used as negative controls and are depicted as cut-off line. (c) IgM-RF was produced at higher levels in NOD.DQ8 compared with B10.DQ8 mice (P < 0.01). Sera from MRL/lpr and B6 mice were used as positive (dotted line) and negative (solid line) controls, respectively. Horizontal bar for each strain indicates mean values of IgM-RF. (d) CIX-primed NOD.DQ8 mice produce significantly high amounts of anti-CIX Ab’s compared with B10.DQ8 mice. Aβo mice, which were used as negative controls, are depicted as cut-off line. BIX, bovine CIX.
CIX-immunized NOD.DQ8 mice develop auricular chondritis.
Both transgenic strains and negative littermates were immunized with bovine CIX. NOD.DQ8 mice produced higher levels of anti-CIX Ab’s compared with B10.DQ8 mice (Figure 6c; P < 0.0001), and all NOD.DQ8 mice (seven of seven) developed auricular chondritis while no pathology was observed in B10.DQ8 mice. Adoptive transfer of cells (106) from the spleen, lymph nodes, and FAC-sorted CD8 and CD4 cells from NOD.DQ8 mice with chondritis to naive B10.DQ8 mice did not result in induction of disease. Transfer of cells (106) isolated from chondritic ear did lead to swelling in both ears of one of the three NOD.DQ8 mice studied.
Autoantibodies.
Antinuclear Ab’s were not present in either of the transgenic strains or in their transgene-negative littermates (data not shown). Higher levels of IgM-RF were detected in NOD.DQ8 compared with B10.DQ8 mice (P < 0.01) (Figure 6c). Only 20% of NOD.DQ8 and 10% of B10.DQ8 mice produced low but detectable levels of IgG-RF (data not shown).
NOD.DQ8 mice have more activated cells but lower numbers of CD8+CD25hi cells.
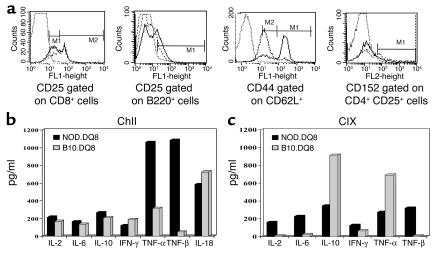
We studied various markers for activated and regulatory cells in splenic cells of immunized mice. Naive NOD.DQ8 mice had significantly lower numbers of CD25+ cells compared with B10.DQ8 mice (16 ± 2.6 and 37 ± 2.6, respectively; P < 0.01), although after immunization, CD25+ cells were fewer in B10.DQ8 mice (14% in NOD.DQ8 and 9% in B10.DQ8 mice). Differences in CD25+ cells between the two strains were reflected in CD3–CD25+ cells; 22% of B220+ cells expressed CD25 in NOD.DQ8 mice compared with 8% in B10.DQ8 mice (Figure 7). The profile of the CD4+CD25+ population was similar in both strains, although in NOD.DQ8 mice fewer cells expressed CD152 compared with B10.DQ8 mice (2% and 11%, respectively). Only 20% of CD8+ cells expressed CD25hi in NOD.DQ8 mice compared with 32% in B10.DQ8 mice. The total number of CD44 cells were lower in NOD.DQ8 mice compared with B10.DQ8 mice (62% and 85%, respectively); however, higher numbers of activated cells, CD44hiCD62Llo, were observed in NOD.DQ8 mice compared with B10.DQ8 mice (63% and 10%, respectively; data not shown).
Figure 7.
(a) Cell surface markers on splenic cells as determined by FACS after labeling them with specific mAb’s in B10.DQ8 mice (broken line) and NOD.DQ8 mice (solid line). NOD.DQ8 mice had a lower number of CD8+CD25hi, CD152+CD4+CD25+, and a higher number of B220+CD25+ cells than B10.DQ8 mice. The data shown were obtained from spleen cells pooled from two mice per strain and represent at least two separate assays. (b) NOD.DQ8 mice produce high levels of TGF-β. Cytokines produced by splenic cells isolated from primed mice and cultured in vitro in the presence of CII and (c) CIX. For each experiment, cells were pooled from two mice per strain.
NOD.DQ8 mice produce high levels of TGF-β.
Both transgenic strains produced similar levels of IL-2, IL-6, IL-10, IL-18, IFN-γ, and TNF-α when challenged in vitro with CII (Figure 7b). The most noticeable difference between the two strains was in the production of TGF-β. NOD.DQ8 mice produced very high levels of TGF-β, while in B10.DQ8 mice minimal levels could be detected by ELISA. On the other hand, B10.DQ8 mice primed with CIX produced high levels of TNF-α and IL-10 with low levels of IFN-γ in vitro, while NOD.DQ8 mice produced IL-2, IL-6, IL-10, IFN-γ, TNF-α, and TGF-β (Figure 7c).
Discussion
We have described in this study an experimental model of relapsing polychondritis, which shares clinical and histologic features with human relapsing polychondritis. Transgenic mice expressing DQ8 in the NOD background, but lacking endogenous class II molecules, develop auricular chondritis and polyarthritis with high levels of anti-CIX and anti-CII Ab’s following immunization with chick CII. Conversely, CII-immunized B10.DQ8 mice do not develop auricular chondritis and make smaller amounts of Ab’s. Transgene-negative littermates and NOD mice did not develop any disease demonstrating that DQ8 is necessary for development of disease in this model. Although CII is a putative autoantigen for both RP and RA, cross-reactive response to other collagens expressed in the outer ear, such as CIX and CXI, appears to be different in the two strains. NOD.DQ8 mice mounted a strong response to both CIX and CXI collagen, while in B10.DQ8 mice only a very mild response to CXI (data not shown) is observed. The α3 chain of CXI and the α1 chain of CII share similarities in amino acid composition, and both have been shown to be arthritogenic in animals (29, 30). Immunoreactive peptides of CIX have been identified in synovial fluid of RA patients (31). CD8+ clones generated from CII-immunized mice were shown to cross-react with CIX, thus suggesting an epitope shared between the two collagens (32). Both of our transgenic strains express DQ8; however, only NOD.DQ8 mice respond to CIX, suggesting that in NOD.DQ8 mice immunization with CII leads to stimulation of another subset of autoreactive T cells that might home to the ear, causing destruction. This was confirmed by our data of auricular chondritis in NOD.DQ8 mice when immunized with CIX. A recent study showed that recombinant CIX (rCIX) does not induce arthritis (33). Our data with DQ8 transgenic mice support the study and further suggest that anti-CIX Ab’s and response to CIX are important in pathogenesis of auricular chondritis.
The cytokine data in this study agree with the above observations. Both transgenic strains produced high levels of IL-18, a proinflammatory cytokine suggested in pathogenesis of RA (34). Both strains produced similar amounts of cytokines when challenged in vitro with CII, except TGF-β, which is only produced by NOD.DQ8 mice. In CIX-primed mice, however, more Th1 cytokines are produced by NOD.DQ8 mice than B10.DQ8 mice, suggesting that autoreactive T cells specific for CIX following CII immunization produce predominantly Th1 cytokines. Our results with TGF-β are surprising in light of the studies demonstrating a protective role of this growth factor in CIA and experimental autoimmune encephalomyelitis (35, 36). TGF-β has been shown to promote bone destruction in CIA and RA patients, which is related to increase in expression of proinflammatory cytokines and metalloproteinases (37–38). Recently, using TGF-β transgenic mice, it was shown that TGF-β does not prevent development of autoreactive T cells (39). TGF-β is a pleiotropic cytokine and has been associated with T cell homeostasis and autoimmunity (40–41). Increased production of TGF-β has been shown to promote effector expansion through inhibition of T cell apoptosis, to produce cytokines including IL-1 and TNF-α, and increase expression of integrin receptors. TGF-β produced by B and plasma cells has been shown to form immune complexes leading to tissue injury. It will be interesting to see if development of auricular chondritis is the result of one or many effects of TGF-β. One possible explanation for the increased levels of TGF-β could be an attempt to counter the inflammation. Role of TGF-β in the pathology of polychondritis is being studied.
Polychondritis observed in NOD.DQ8 mice shares similarities with RP. As seen in RP, the first clinical feature developing in most of these mice is auricular chondritis, characterized by bilateral erythema and swelling of the outer ears, with massive infiltration of mononuclear cells. More CD4+ cells are seen at the initiation of disease, suggesting that presentation of an epitope to CD4 T cells might be important for pathogenesis. Later in the course of disease, B cells appear as aggregates resembling lymphoid follicles. Polyarthritis characterized by massive infiltration of mononuclear cells and pannus formation follows after auricular chondritis in around 65–70% of mice. Perivascular infiltration of lungs and trachea and collagen deposition in trachea was observed in some mice. Our data show that these mice not only develop auricular chondritis, but some of them also develop other histopathological manifestations seen in relapsing polychondritis patients. While auricular chondritis is not a remitting/relapsing phenomenon in these mice, the histopathologic manifestations are similar to those seen in RP. The cartilaginous tissue of the ears is destroyed and shrunken, leading to a cauliflower appearance, as is characteristic of RP patients. Thus, this model has an advantage over previous models in having a higher incidence of auricular chondritis and also other associated manifestations, representing a true experimental model for relapsing polychondritis.
To address the differences seen in clinical and immunological features of both strains, we studied the regulatory and activated cells in both. NOD.DQ8 mice have a higher number of cells that express activation markers, CD25+CD44hiCD62Llo compared with B10.DQ8 mice. In NOD mice, CD4+CD62Lhi cells have been shown to be regulatory cells (42). CD4+CD25+ cells have been shown to be regulatory cells in many autoimmune diseases by in vitro, in vivo, and depletion studies (reviewed in ref. 43). The regulatory CD25+ cells have been shown to express CTLA-4 (44). Our data show that NOD.DQ8 mice have a greater number of CD25+ cells, but they are not regulatory cells. The profile of CD4+CD25+ cells is similar in both strains, while CD8+CD25hi cells are decreased in NOD.DQ8 mice, which supports our previous notion that CD8 cells might behave as regulatory cells in CIA (26) and further attributes it to this subset. Increased number of B220+CD25+ cells in NOD.DQ8 mice indicate a higher number of activated B cells, which might explain increased levels of autoantibodies in them. Adoptive transfer of CD4 and CD8 cells sorted from lymph node and spleen cells did not transfer disease, suggesting the need for both T cell response and autoantibodies in the development of polychondritis.
Deficiency in C5 in NOD mice has been shown to play a role in resistance to CIA (27, 45). Holmdahl et al. (45) found in an F2 cross of (B10.Q × NOD.Q) mice that the strongest arthritis quantitative trait loci (QTL) that maps the C5 gene was of B10 origin. Here, however, we find that NOD.DQ8 mice also develop auricular chondritis — an autoimmune disease phenotype not characteristic of NOD.Q mice. Since NOD.DQ8 mice also develop arthritis, C5 activity was determined in them. NOD.DQ8 mice were positive for C5 activity, suggesting they may have derived this gene from B10. One explanation could be the insertion of the DQ8 gene in chromosome 2, where the C5 gene maps. The second possibility is the exchange of this gene segment during backcrossing to produce the NOD.DQ8.Aβo mice. We tested the backcrosses made to NOD mice, and all DQ8-positive mice carried functional C5, thus suggesting the DQ8 transgene is inserted near C5.
In conclusion, presence of DQ8 results in CIA in both NOD and B10 background mice. NOD.DQ8 mice, however, produce higher levels of autoantibodies and develop auricular chondritis. Furthermore, immunization with CIX leads to RP, suggesting a role for CIX in this disease. NOD mice do not develop disease upon immunization with CII or CIX, even though IAg7 has structural similarity with DQ8 molecule. This may occur because of C5 deficiency, or IAg7 may not be able to present an arthritogenic epitope. Thus, the susceptibility to RP is not solely dependent on the expression of DQ8 but requires C5 sufficiency and additional non-MHC genes of NOD origin.
Acknowledgments
This study was supported by grants from Arthritis Foundation’s Investigator Award to Veena Taneja and NIH grants AI-14764 and AR-30752 to C.S. David. We are indebted to J. Strominger for the DQ8α and β cosmids and to C. Benoist and D. Mathis for the class II–deficient (Aβo) mice.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: relapsing polychondritis (RP); rheumatoid arthritis (RA); type II collagen (CII); type IX collagen (CIX); type XI collagen (CXI); DQA1*0103/DQB1*0601 (DQ6); DQA1*0301/DQB1*0302 (DQ8); class II–deficient mice (Aβo); NOD/C5+.DQ8.Aβo (NOD.DQ8); phycoerythrin (PE); peripheral blood leukocyte (PBL); collagen-induced arthritis (CIA); rheumatoid factor (RF).
References
- 1.Zeuner M, Straub RH, Albert ED, Scholmerich J, Lang B. Relapsing polychondritis: clinical and immunogenetic analysis of 62 patients. J. Rheumatol. 1997;24:96–101. [PubMed] [Google Scholar]
- 2.McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: perspective study of 23 patients and a review of literature. Medicine. 1976;55:193–215. [PubMed] [Google Scholar]
- 3.Issak BL, Liesegang TJ, Michet CJ. Ocular and systemic findings in relapsing polychondritis. Opthalmology. 1986;93:681–689. doi: 10.1016/s0161-6420(86)33695-9. [DOI] [PubMed] [Google Scholar]
- 4.Homma S, Matsumoto T, Abe H, Fukuda Y, Suzuki M. Relapsing polychondritis. Pathological and immunological findings in an autopsy case. Acta Pathol. Jpn. 1984;34:1137–1146. doi: 10.1111/j.1440-1827.1984.tb07641.x. [DOI] [PubMed] [Google Scholar]
- 5.Damiani JM, Levine HL. Relapsing polychondritis: report of ten cases. Laryngoscope. 1979;89:929–946. [PubMed] [Google Scholar]
- 6.Gunaydin I, Daikeler T, Jacki S, Mohren M. Articular involvement in patients with relapsing polychondritis. Rheumatol. Int. 1998;18:93–96. doi: 10.1007/s002960050064. [DOI] [PubMed] [Google Scholar]
- 7.Luthra, H.S. 1993. Relapsing polychondritis. In Rheumatology. J.H. Klippel and P.A. Dieppe, editors. Mosby. London, United Kingdom. 1–4.
- 8.Chang-Miller A, et al. Renal involvement in relapsing polychondritis. Medicine. 1987;66:202–217. doi: 10.1097/00005792-198705000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Luthra HS, McKenna CH, Terasaki PI. Lack of association of HLA-A and B locus antigens with relapsing polychondritis. Tissue Antigens. 1981;17:442–443. doi: 10.1111/j.1399-0039.1981.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 10.Lang B, et al. Susceptibility to relapsing polychondritis is associated with HLA-DR4. Arthritis Rheum. 1993;36:660–664. doi: 10.1002/art.1780360513. [DOI] [PubMed] [Google Scholar]
- 11.Buckner JH, Landeghen MV, Kwok KW, Tsarknaridis L. Identification of type II collagen peptide 261-273-specific T cell clones in a patient with relapsing polychondritis. Arthritis Rheum. 2002;46:238–244. doi: 10.1002/1529-0131(200201)46:1<238::AID-ART10030>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Foidart JM, et al. Antibodies to type II collagen in relapsing polychondritis. N. Eng. J. Med. 1978;299:1203–1207. doi: 10.1056/NEJM197811302992202. [DOI] [PubMed] [Google Scholar]
- 13.Terato K, et al. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990;33:1493–1500. doi: 10.1002/art.1780331006. [DOI] [PubMed] [Google Scholar]
- 14.Herman JH, Dennis MV. Immunopathologic studies in relapsing polychondritis. J. Clin. Invest. 1973;52:549–558. doi: 10.1172/JCI107215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CL, et al. Autoantibodies to cartilage collagens in relapsing polychondritis. Arch. Dermatol. Res. 1993;285:245–249. doi: 10.1007/BF00371591. [DOI] [PubMed] [Google Scholar]
- 16.Alsalameh S, et al. Preferential cellular and humoral immune reactivities to native and denatured collagen types IX and XI in a patient with fatal relapsing polychondritis. J. Rheumatol. 1993;20:1419–1424. [PubMed] [Google Scholar]
- 17.Griffiths MM, Eichwald EJ, Martin JH, Smith CB, DeWitt CW. Immunogenetic control of experimental type II collagen induced arthritis. Arthritis Rheum. 1981;24:781–789. doi: 10.1002/art.1780240605. [DOI] [PubMed] [Google Scholar]
- 18.Cremer MA, Pitcock JA, Stuart JM, Kang AH, Townes AS. Auricular chondritis in rats. An experimental model of relapsing polychondritis induced with type II collagen. J. Exp. Med. 1981;154:535–540. doi: 10.1084/jem.154.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCune WJ, Schiller AL, Dynesius-Trentham AD, Trentham DE. Type II collagen-induced auricular chondritis. Arthritis Rheum. 1982;25:266–273. doi: 10.1002/art.1780250304. [DOI] [PubMed] [Google Scholar]
- 20.Yoo TJ, et al. Epitope specificity and T cell receptor usage in type II collagen induced autoimmune ear disease. Cell Immunol. 1994;157:249–262. doi: 10.1006/cimm.1994.1220. [DOI] [PubMed] [Google Scholar]
- 21.Hansson AS, Heingard D, Holmdahl R. A new animal model for relapsing polychondritis, induced by cartilage matrix protein (matrilin-1) J. Clin. Invest. 1999;104:589–598. doi: 10.1172/JCI5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley DS, Das P, Griffiths MM, Luthra HS, David CS. HLA-DQ6/8 double transgenic mice develop auricular chondritis following type II collagen immunization: a model for human relapsing polychondritis. J. Immunol. 1998;161:5046–5053. [PubMed] [Google Scholar]
- 23.Taneja V, et al. Expression of the H2-E molecule mediates protection to collagen-induced arthritis in HLA-DQ8 transgenic mice: role of cytokines. Int. Immunol. 1997;9:1213–1218. doi: 10.1093/intimm/9.8.1213. [DOI] [PubMed] [Google Scholar]
- 24.Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J. Exp. Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths MM, et al. Collagen-induced arthritis and TCRs in SWR and B10.Q mice expressing an Ek alpha transgene. J. Immunol. 1994;153:2758–2768. [PubMed] [Google Scholar]
- 26.Taneja V, et al. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8 transgenic mice: implications for rheumatoid arthritis. J. Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- 27.Ji H, et al. Genetic influences on the end-stage effector phase of arthritis. J. Exp. Med. 2001;194:321–330. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinder CS, et al. Blockade of C5a and C5-9 generation inhibits leukocyte and platelet activation during extracorporeal circulation. J. Clin. Invest. 1995;96:1564–1572. doi: 10.1172/JCI118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cremer MA, et al. Evidence that the α3(XI) chain of type XI collagen and the α1(II) chain of type II collagen share arthritogenic determinants and induce arthritis in DBA/1 mice. J. Immunol. 1991;146:4130–4137. [PubMed] [Google Scholar]
- 30.Boissier MC, Chiocchia G, Ronziere MC, Herbage D, Fournier C. Arthritogenicity of minor cartilage collagens (types IX and XI) in mice. Arthritis Rheum. 1990;33:1–8. doi: 10.1002/art.1780330101. [DOI] [PubMed] [Google Scholar]
- 31.Wotton SF, Dieppe PA, Duance VC. Type IX collagen immunoreactive peptides in synovial fluids from arthritis patients. Rheumatology. 1999;38:338–345. doi: 10.1093/rheumatology/38.4.338. [DOI] [PubMed] [Google Scholar]
- 32.Chioccia G, Boissier MC, Ronziere MC, Harbage D, Fournier C. T cell regulation of collagen-induced arthritis in mice. I. Isolation of type II collagen-reactive T cell hybridomas with specific cytotoxic function. J. Immunol. 1990;145:519–525. [PubMed] [Google Scholar]
- 33.Myers LK, et al. Immunogenicity of recombinant type IX collagen in murine collagen-induced arthritis. Arthritis Rheum. 2002;46:1086–1093. doi: 10.1002/art.10163. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, et al. Mature form of interleukin 18 is expressed in rheumatoid arthritis synovial tissue and contributes to interferon-gamma production by synovial T cells. J. Rheumatol. 2001;28:1779–1787. [PubMed] [Google Scholar]
- 35.Thorbecke GJ, et al. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns LD, Flanders KC, Ranges GE, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta. J. Immunol. 1991;147:1792–1796. [PubMed] [Google Scholar]
- 37.Van Beuningen HM, Glansbeek HL, van der Kraan PM, van der Berg WB. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-β injections. Osteoarthritis Cartilage. 2000;8:25–28. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 38.Cheon H, et al. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGF-β1 synovial fibroblasts from rheumatoid arthritis and normal individual. Clin. Exp. Immunol. 2002;127:547–552. doi: 10.1046/j.1365-2249.2002.01785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grewal IS, et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J. Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 40.Braley-Mullen H, Chen K, Wei Y, Yu S. Role of TGFbeta in development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J. Immunol. 2001;167:7111–7118. doi: 10.4049/jimmunol.167.12.7111. [DOI] [PubMed] [Google Scholar]
- 41.Gorelik L, Flavell RA. Transforming growth factor-beta in T cell biology. Nat. Rev. Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 42.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in non-obese diabetic mice. J. Immunol. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 43.Majoly KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 44.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–400. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 45.Johansson ACM, et al. Genetic control of collagen-induced arthritis in a cross with NOD and C57BL/10 mice is dependent on gene regions encoding complement factor 5 and FcgRIIb and is not associated with loci controlling diabetes. Eur. J. Immunol. 2001;31:1847–1856. doi: 10.1002/1521-4141(200106)31:6<1847::aid-immu1847>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]