Abstract
Neurofibromas are benign tumors comprised primarily of Schwann cells and fibroblasts. Mast cell infiltration is a well-known phenomenon; however, their role in tumor pathogenesis has been enigmatic. In an elegant set of experiments using cells derived from a murine model of neurofibromatosis 1 (NF1), Yang et al. dissect the molecular pathways involved in mast cell migration to neurofibromin-deficient Schwann cells. These results set the stage for rational development of therapeutics that could influence the multicellular microenvironment of neurofibromas to inhibit the development and/or progression of these tumors in human NF
In an Original article series published through the March of Dimes Birth Defects Foundation in 1981, Vic Riccardi outlined his “NF cellular interaction hypothesis,” implicating the mast cell as a major player neurofibroma formation (1). He was attempting to explain the variable expression of neurofibromatosis 1 (NF1) by cellular interactions and the finding of high numbers of mast cells in neurofibromas when he posited that “the mast cell now is seen not as a secondary arrival in a developing neurofibroma but as an inciting factor contributing in a primary, direct fashion to tumor development.” It has taken over 20 years, but in this issue of the JCI, Yang et al. (2) clearly set the record straight by identifying the molecular mechanisms underlying mast cell infiltration of neurofibromas. The authors demonstrated that the inciting factor for mast cell migration is Kit ligand and that it is hypersecreted from Nf1–/– Schwann cell populations. Nevertheless, that Riccardi hypothesized paracrine events involving the mast cell as a major player in neurofibroma formation is a testament to his intuition regarding the pathogenesis of this complex genetic condition.
Yang and colleagues were intrigued by the seminal observations of Zhu et al. (3) of neurofibroma formation in mice expressing the conditional deletion of Nf1, whereby double inactivation of the gene Nf1–/– in Schwann cells could only induce neurofibroma formation in the context of a heterozygous Nf1+/– genetic background. This supports the paracrine model for neurofibromas; other cells in these mixed-cell tumors must be haploinsufficient for neurofibromin to support proliferation. Yang et al. (2) have taken these observations a step further by dissecting the cell types and the molecular mechanisms by which the tumor microenvironment contributes to neurofibroma formation. Through a set of elegant experiments, they have delineated a complex biological system and provided a model demonstrating not just who the major players are in this process, but how those players interact in concert to develop neurofibromas (Figure 1).
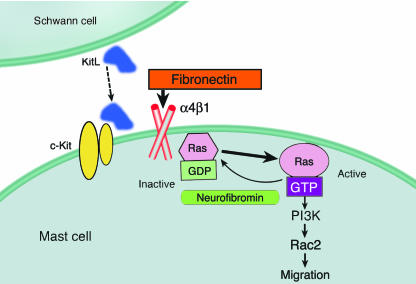
Figure 1.
Illustration depicting the molecular pathways involved in mast cell recruitment to neurofibromas. A neurofibromin-deficient Schwann cell (Nf1–/–) secretes five times the normal Kit ligand, which serves as a chemoattractant for mast cells expressing c-Kit. Mast cell migration is mediated by the Ras/PI3K/Rac2 signal transduction pathway, which is enhanced in Nf1+/– cells. Although not shown, endothelial cells also play a role in mast cell migration through the interaction of the endothelial cell VCAM-1 receptor and α4β1 integrin of the mast cell. Heterozygous inactivation of Nf1 promotes rapid mast cell haptotaxis specifically on α4β1 integrins in response to KitL. It is known that mast cells are activated in neurofibromas and degranulate; however it is yet to be determined if and how the presence of mast cells induces tumor progression. Figure courtesy of D. Wade Clapp, Indiana University School of Medicine. KitL, Kit ligand.
Neurofibroma microenvironment
The basic components of an evolving neurofibroma are: (i) Nf1–/– Schwann cells, which act as tumorigenic instigators (4–7); (ii) Nf1+/– mast cells, which act as inducers (2); and (iii) Nf1+/– fibroblasts, Schwann cells, perineural cells, and endothelial cells, which act as the responders (8). The pathways involved in neurofibroma formation identified by Yang et al. (2) involve Kit ligand (secreted by Nf1–/– Schwann cells), c-Kit receptor (expressed by mast cells), α4β1 integrin (a mast cell surface protein), VCAM-1 (an endothelial receptor for α4β1 integrin), Ras (a substrate for neurofibromin), and the class IA-PI3K–Rac2 pathway (mast cell transduction downstream of activated Ras). The primary observation of Yang et al. is the enhanced migration of neurofibromin haploinsufficient mast cells toward the double-inactivated, Nf1–/– Schwann cells that secrete five times the normal levels of Kit ligand. According to this paradigm, in human neurofibromas, a “second hit,” or somatic mutation of the normal NF1 tumor suppressor gene allele in heterozygous Schwann cells as a random event, is the initiating event which sets the stage for mast cells to induce neurofibroma formation by paracrine/autocrine mechanisms.
Mast cells and neurofibromas
The presence of mast cells in human neurofibromas, initially reported in 1911 by Greggio (9), is well known to clinical pathologists. Additional studies have since supported the finding of mast cells in peripheral nerve trunks (10, 11), peripheral nerve tumors (12–14), and neurofibromas in neurofibromatosis 1 (15–17). The potential involvement of the c-Kit receptor and its ligand (18, 19) in the formation of neurofibromas has also been reported, but Yang et al. (2) are the first investigators to evaluate living cells of different genetic backgrounds in order to demonstrate how increased mast cells populate neurofibromas. Now that a plausible mechanism has been established for the migration of mast cells to developing neurofibromas, it is important to dissect potential mechanisms whereby mast cells can induce cells in the microenvironment to either tolerate or enhance cell proliferation. Because we know that neurofibromas, like other tumors, are complex tissues with interactions between genetically altered Schwann cells, mast cells, and other supporting coconspirators lead to cancer development (20), it now becomes important to determine the role mast cells play in the microenvironment of NF1-related peripheral nerve sheath tumors.
Mast cells in cancer
Mast cell secretions are known to be integral components of wound healing and tissue repair, even in the peripheral nervous system. In addition to their role in inflammation, mast cells provide mitogens for fibroblasts, endothelial cells, and nerve cells to enhance tissue remodeling, and they are also now being recognized as potential epigenetic contributors to cancer (21). It will be important to establish the functions of the mast cell after it has migrated to the Kit ligand–secreting Schwann cells. In addition to secreting mitogenic and angiogenic substances, including basic FGF, VEGF, histamine, heparin, prostaglandins, leukotrienes, and proteolytic enzymes, mast cells may also have novel cell-to-cell interactions (22) or other unknown functions that could induce neurofibromas. Thus, the development of medical treatment targeted to mast cell function may be a rational approach to decreasing neurofibroma formation in NF1. In a pilot study, Riccardi used ketotifen as a stabilizer of mast cells to demonstrate less small-vessel bleeding in surgical excisions of neurofibromas (23). He followed up this study with a controlled multiphase trial of ketotifen in 27 patients, assessing pain and itching associated with neurofibromas, and showed a response (24) that was reviewed as encouraging, but not definitely beneficial (25). Given that specific paracrine pathways have yet to be defined in the microenvironment of NF1-related neurofibromas, Yang et al. (2) appropriately point out that therapeutic agents that target the migration of mast cells (i.e., inhibition of c-kit activity and adhesion to α4β1 integrin) may hold promise in the early treatment and/or prevention of neurofibromas.
Mast cells: missing in action
There is one caveat to bear in mind in regard to the role Nf1+/– mast cells may play as they nestle into their new-found microenvironment. With respect to the role of inflammation in cancer and the recognition that mast cells are one of the inflammatory cell types involved in protumor actions (26), it should be acknowledged that neurofibromas are benign tumors, especially the dermal neurofibromas, which never progress to malignancy. It is also important to recognize that the neurofibromas that develop in the mouse models are not dermal neurofibromas, but are more like human plexiform neurofibromas. These tumors often progress to malignant peripheral nerve sheath tumors (MPNSTs); however, unlike in other tumor model systems where inflammatory cells are in excess in tumor microenvironments, there are relatively few mast cells in MPNSTs (ref. 27; H. Zhou, personal communication). Is it possible that an influx of mast cells, drawn by a small cohort of malignant Nf1–/– Schwann cells, induces fibroblasts to synthesize the plethora of extracellular matrix seen in dermal neurofibromas, which could then serve as a “glue” to corral the deviant Schwann cells? By this reasoning, only when mast cells are lost, as seen in the MPNSTs, would there be diminution of the anti-cancer effects of mast cells in maintaining the benign nature of neurofibromas. Thus, until the paracrine/autocrine paradigm is deciphered in neurofibroma formation, it behooves the NF1 research community to continue working toward the identification of mast cell signals (inducers) that specify responses from the microenvironment before implementing clinical trials using agents that inhibit mast cell migration toward the tumorigenic Schwann cell. To this end, Yang and colleagues (2) will likely make more outstanding contributions both to the NF1 research field and to the work of those who are dissecting the roles of inflammation and the microenvironment in cancer.
Footnotes
See the related article beginning on page 1851.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: neurofibromatosis 1 (NF1); malignant peripheral nerve sheath tumor (MPNST).
References
- 1.Riccardi, V.M. 1981. Cutaneous manifestation of neurofibromatosis: Cellular interaction, pigmentation, and mast cells. In Birth defects: original article series. Volume 17. R. Blandau, N. Paul, and F. Dickman, editors. Alan R. Lis Inc. New York, New York, USA. 129–145. [PubMed]
- 2.Yang F-C, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/– mast cells. J. Clin. Invest. 2003;112:1851–1861. doi:10.1172/JCI200319195. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluwe L, Friedrich R, Mautner VF. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer. 1999;24:283–285. doi: 10.1002/(sici)1098-2264(199903)24:3<283::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Serra E, et al. Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different Schwann cell subpopulations. Hum. Mol. Genet. 2000;12:3055–3064. doi: 10.1093/hmg/9.20.3055. [DOI] [PubMed] [Google Scholar]
- 6.Rutkowski JL, Wu K, Gutmann DH, Boyer PJ, Legius E. Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1. Hum. Mol. Genet. 2000;12:1059–1066. doi: 10.1093/hmg/9.7.1059. [DOI] [PubMed] [Google Scholar]
- 7.Muir D, Neubauer D, Lim IT, Yachnis AT, Wallace MR. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am. J. Pathol. 2001;158:501–513. doi: 10.1016/S0002-9440(10)63992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fialkow PJ, Sagebiel RW, Gartler SM, Rimoin DL. Multiple cell origin of hereditary neurofibromas. N. Engl. J. Med. 1971;284:298–300. doi: 10.1056/NEJM197102112840604. [DOI] [PubMed] [Google Scholar]
- 9.Greggio H. Les cellules granuleuses (Mastzellen) dans les tissus normaux et dans certaines maladies chirurgicales. Arch. Med. Exp. 1911;23:323–375. [Google Scholar]
- 10.Gamble HJ, Goldby S. Mast cells in peripheral nerve trunks. Nature. 1961;189:766–767. doi: 10.1038/189766a0. [DOI] [PubMed] [Google Scholar]
- 11.Olsson Y. Mast cells in human peripheral nerve. Acta Neurol. Scand. 1971;47:357–368. doi: 10.1111/j.1600-0404.1971.tb07490.x. [DOI] [PubMed] [Google Scholar]
- 12.Baroni C. On the relationship of mast cells to various soft tissue tumours. Br. J. Cancer. 1964;18:686–690. doi: 10.1038/bjc.1964.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pineda A. Mast cells – their presence and ultrastructural characteristics in peripheral nerve tumors. Arch. Neurol. 1965;13:372–382. doi: 10.1001/archneur.1965.00470040038006. [DOI] [PubMed] [Google Scholar]
- 14.Isaacson P. Mast cells in benign nerve sheath tumours. J. Pathol. 1976;119:193–196. doi: 10.1002/path.1711190402. [DOI] [PubMed] [Google Scholar]
- 15.Giorno R, Lieber J, Claman HN. Ultrastructural evidence for mast cell activation in a case of neurofibromatosis. Neurofibromatosis. 1989;2:35–41. [PubMed] [Google Scholar]
- 16.Carr NJ, Warren AY. Mast cell numbers in melanocytic naevi and cutaneous neurofibromas. J. Clin. Pathol. 1993;46:86–87. doi: 10.1136/jcp.46.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurnberger M, Moll I. Semiquantitative aspects of mast cells in normal skin and in neurofibromas of neurofibromatosis types 1 and 5. Dermatology. 1994;188:296–299. doi: 10.1159/000247170. [DOI] [PubMed] [Google Scholar]
- 18.Hirota S, et al. Possible involvement of c-kit receptor and its ligand in increase of mast cells in neurofibroma tissues. Arch. Pathol. Lab. Med. 1993;117:996–999. [PubMed] [Google Scholar]
- 19.Bedache A, Muja N, De Vries GH. Expression of Kit in neurofibromain-deficient human Schwann cells: role in Schwann cell hyperplasia associated with type 1 neurofibromatosis. Oncogene. 1998;17:795–800. doi: 10.1038/sj.onc.1201978. [DOI] [PubMed] [Google Scholar]
- 20.Hannahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 21.Coussens LM, Werb Z. Inflammatory cells and cancer: think different. J. Exp. Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg G, Burnstock G. A novel cell-to-cell interaction between mast cells and other cell types. Exp. Cell. Res. 1983;147:1–13. doi: 10.1016/0014-4827(83)90265-3. [DOI] [PubMed] [Google Scholar]
- 23.Riccardi VM. Mast-cell stabilization to decrease neurofibroma growth. Arch. Dermatol. 1987;123:1011–1016. [PubMed] [Google Scholar]
- 24.Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch. Dermatol. 1993;129:577–581. [PubMed] [Google Scholar]
- 25.Meyer LJ. Drug therapy for neurofibromatosis? Arch. Dermatol. 1993;129:625–626. [PubMed] [Google Scholar]
- 26.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MD, Kamso-Pratt J, Federspiel CF, Whetsell WO. Mast cell and lymphoreticular infiltrates in neurofibromas. Comparison with nerve sheath tumors. Arch. Pathol. Lab. Med. 1989;113:1263–1270. [PubMed] [Google Scholar]