Abstract
In the title compound, C21H23N3O, the dihedral angles formed by the pyrazolone ring with two phenyl rings are 10.38 (8) and 76.94 (6)°. The sec-butylamino group is disordered over two positions, with refined site-occupancy factors of 0.730 (4) and 0.270 (4). The compound could potentially be ligand stabilized in the solid state in a keto–enamine tautomeric form. The amine functionality is involved in an intramolecular N—H⋯O hydrogen bond, while weak intermolecular C—H⋯O and C—H⋯N hydrogen bonds participate in the formation of the crystal structure.
Related literature
For the antibacterial, biological and analgesic activity of metal complexes of 1-phenyl-3-methyl-4-benzoylpyrazolon-5-one, see: Li et al. (1997 ▶); Liu et al. (1980 ▶); Zhou et al. (1999 ▶).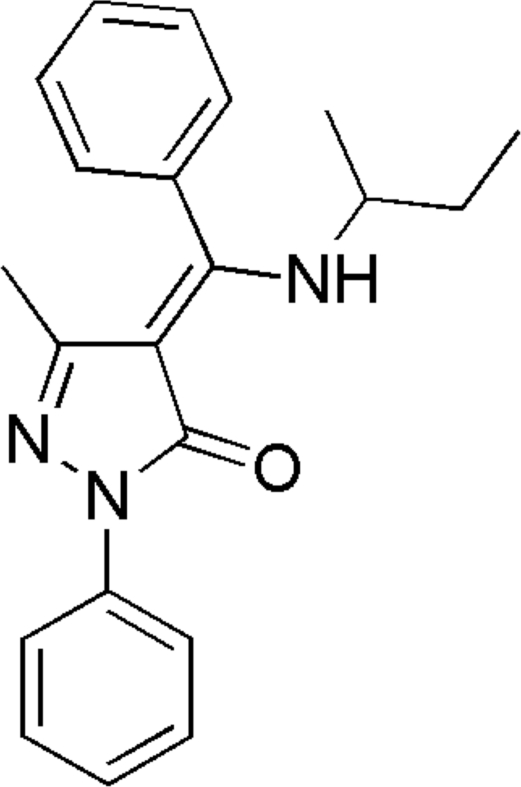
Experimental
Crystal data
C21H23N3O
M r = 333.42
Triclinic,

a = 9.3631 (19) Å
b = 10.077 (2) Å
c = 10.687 (2) Å
α = 107.07 (3)°
β = 100.30 (3)°
γ = 100.14 (3)°
V = 920.0 (4) Å3
Z = 2
Mo Kα radiation
μ = 0.08 mm−1
T = 113 K
0.20 × 0.18 × 0.16 mm
Data collection
Rigaku Saturn CCD area-detector diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2005 ▶) T min = 0.985, T max = 0.988
8309 measured reflections
4296 independent reflections
2944 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.135
S = 1.08
4296 reflections
272 parameters
16 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.32 e Å−3
Δρmin = −0.21 e Å−3
Data collection: CrystalClear (Rigaku, 2005 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680902950X/bh2238sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680902950X/bh2238Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Enhanced figure: interactive version of Fig. 1
Table 1. Selected bond lengths (Å).
| O1—C7 | 1.2529 (17) |
| C7—C8 | 1.4382 (19) |
| C8—C11 | 1.402 (2) |
| C11—N3′ | 1.311 (5) |
| C11—N3 | 1.359 (2) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3′—H3′⋯O1 | 0.904 (10) | 1.99 (4) | 2.705 (6) | 135 (5) |
| N3—H3⋯O1 | 0.902 (10) | 1.933 (15) | 2.699 (2) | 141.6 (18) |
| C16—H16A⋯O1i | 0.95 | 2.53 | 3.2743 (19) | 135 |
| C13—H13A⋯N2ii | 0.95 | 2.60 | 3.537 (2) | 167 |
Symmetry codes: (i) 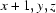 ; (ii)
; (ii)  .
.
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (grant No. 20772066).
supplementary crystallographic information
Comment
1-Phenyl-3-methyl-4-benzoylpyrazolon-5-one (HPMBP), an effective β-diketonate, is widely used and well known for its extractive ability. In recent years, HPMBP and its metal complexes have also been found to have good antibacterial and biological properties. Its metal complexes have analgesic activity (Liu et al., 1980; Li et al., 1997; Zhou et al., 1999). In order to develop new medicines, we have synthesized the title compound, (I), and its structure is reported here.
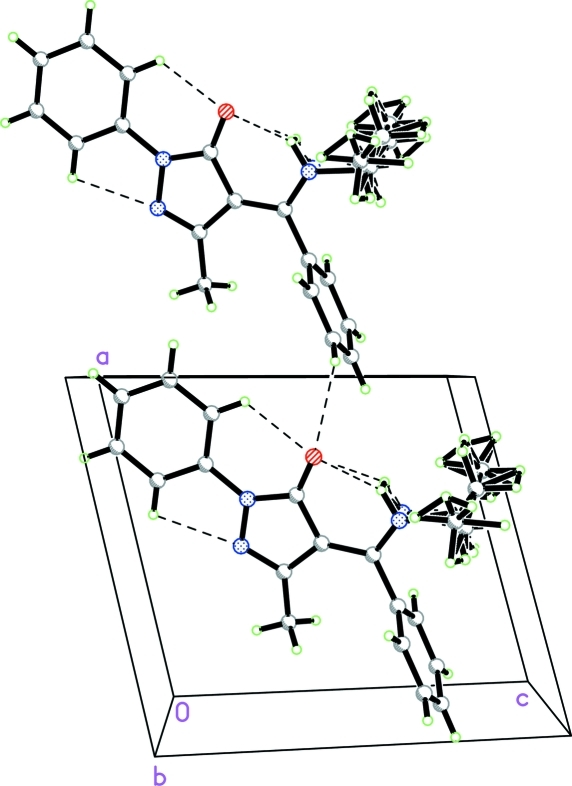
The structure of (I) is shown in Fig. 1. The dihedral angles formed by the pyrazolone ring with the two phenyl rings C1···C6 and C12···C17 are 10.38 (8) and 76.94 (6)°, respectively. The O atom of the 3-methyl-1-phenylpyrazol-5-one moiety and the N atom of the sec-butylamino group are available for coordination with metals. The pyrazole ring is planar and atoms O1, C7, C8, C11 and N3 (or N3') are almost coplanar, the largest deviation being 0.0323 (13) Å [or 0.201 (3) Å] for atom C11. The dihedral angle between this mean plane and the pyrazoline ring of PMBP is 3.00 (11)° [or 12.10 (18)°]. The bond lengths within this part of the molecule (Table 1) lie between classical single- and double-bond lengths, indicating extensive conjugation. A strong intramolecular N3—H3···O1 hydrogen bond (Table 2) is observed, leading to a keto-enamine form. The molecule is further stabilized by C—H···O and C—H···N intramolecular hydrogen bonds (Table 2), while the crystal structure includes C—H···O and C—H···N intermolecular hydrogen bonds (Table 2 and Fig. 2).
Experimental
Compound (I) was synthesized by refluxing a mixture of 1-phenyl-3- methyl-4-benzoylpyrazol-5-one (10 mmol) and sec-butylamine (10 mmol) in ethanol (80 ml) over a steam bath for about 4 h. Excess solvent was removed by evaporation and the solution was cooled to room temperature. After 2 days a yellow solid was obtained and this was dried in air. The product was recrystallized from ethanol, to afford yellow crystals of (I) suitable for X-ray analysis.
Refinement
The sec-butylamino group shows positional disorder. At the final stage of the refinement, the occupancy factors of two possible sites, N3/C18/C19/C20/C21 and N3'/C18'/C19'/C20'/C21', converged to 0.730 (4) and 0.270 (4), respectively. The geometry of this disordered group was regularized using 16 restraints. C-bonded H atoms were positioned geometrically, with C—H = 0.95–1.00 Å and amine H atoms (H3 and H3') were found in a difference map. Amine H atoms were refined freely, while C-bonded H atoms were included in the final cycles of refinement using a riding model, with Uiso(H) = 1.2Ueq(CH2 and CH) or 1.5Ueq(CH3).
Figures
Fig. 1.
View of the title compound, with displacement ellipsoids drawn at the 30% probability level.
Fig. 2.
Intermolecular hydrogen bonds (dashed line) in the structure of (I).
Crystal data
| C21H23N3O | Z = 2 |
| Mr = 333.42 | F(000) = 356 |
| Triclinic, P1 | Dx = 1.204 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.3631 (19) Å | Cell parameters from 2809 reflections |
| b = 10.077 (2) Å | θ = 2.2–27.9° |
| c = 10.687 (2) Å | µ = 0.08 mm−1 |
| α = 107.07 (3)° | T = 113 K |
| β = 100.30 (3)° | Block, yellow |
| γ = 100.14 (3)° | 0.20 × 0.18 × 0.16 mm |
| V = 920.0 (4) Å3 |
Data collection
| Rigaku Saturn CCD area-detector diffractometer | 4296 independent reflections |
| Radiation source: rotating anode | 2944 reflections with I > 2σ(I) |
| confocal | Rint = 0.026 |
| Detector resolution: 7.31 pixels mm-1 | θmax = 27.9°, θmin = 2.2° |
| ω and φ scans | h = −12→12 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2005) | k = −11→13 |
| Tmin = 0.985, Tmax = 0.988 | l = −14→13 |
| 8309 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.047 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.135 | w = 1/[σ2(Fo2) + (0.0708P)2 + 0.0483P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max = 0.005 |
| 4296 reflections | Δρmax = 0.32 e Å−3 |
| 272 parameters | Δρmin = −0.21 e Å−3 |
| 16 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.155 (17) |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.22643 (10) | 0.11615 (12) | 0.45573 (9) | 0.0366 (3) | |
| N1 | 0.35369 (12) | 0.31219 (12) | 0.64961 (11) | 0.0281 (3) | |
| N2 | 0.49444 (13) | 0.40916 (13) | 0.69807 (12) | 0.0337 (3) | |
| C1 | 0.25255 (16) | 0.31996 (14) | 0.73383 (13) | 0.0273 (3) | |
| C2 | 0.10503 (16) | 0.24104 (16) | 0.68465 (15) | 0.0331 (3) | |
| H2A | 0.0705 | 0.1794 | 0.5934 | 0.040* | |
| C3 | 0.00826 (17) | 0.25261 (16) | 0.76926 (16) | 0.0377 (4) | |
| H3A | −0.0926 | 0.1982 | 0.7355 | 0.045* | |
| C4 | 0.05684 (19) | 0.34266 (16) | 0.90262 (16) | 0.0385 (4) | |
| H4A | −0.0103 | 0.3510 | 0.9599 | 0.046* | |
| C5 | 0.20343 (19) | 0.41966 (16) | 0.95079 (15) | 0.0387 (4) | |
| H5A | 0.2377 | 0.4806 | 1.0423 | 0.046* | |
| C6 | 0.30202 (17) | 0.40978 (15) | 0.86797 (14) | 0.0332 (3) | |
| H6A | 0.4029 | 0.4640 | 0.9025 | 0.040* | |
| C7 | 0.33879 (15) | 0.21606 (14) | 0.52318 (13) | 0.0261 (3) | |
| C8 | 0.47946 (14) | 0.25477 (14) | 0.49077 (13) | 0.0260 (3) | |
| C9 | 0.56792 (16) | 0.37451 (14) | 0.60449 (14) | 0.0293 (3) | |
| C10 | 0.72210 (17) | 0.46179 (17) | 0.62636 (16) | 0.0402 (4) | |
| H10A | 0.7509 | 0.5366 | 0.7153 | 0.060* | |
| H10B | 0.7244 | 0.5061 | 0.5562 | 0.060* | |
| H10C | 0.7924 | 0.4000 | 0.6220 | 0.060* | |
| C11 | 0.50824 (15) | 0.17900 (16) | 0.36892 (14) | 0.0325 (4) | |
| C12 | 0.65777 (15) | 0.20895 (15) | 0.33908 (13) | 0.0282 (3) | |
| C13 | 0.70609 (16) | 0.32385 (15) | 0.29807 (14) | 0.0321 (3) | |
| H13A | 0.6424 | 0.3850 | 0.2857 | 0.039* | |
| C14 | 0.84815 (17) | 0.34901 (16) | 0.27525 (15) | 0.0359 (4) | |
| H14A | 0.8823 | 0.4282 | 0.2481 | 0.043* | |
| C15 | 0.93991 (16) | 0.25914 (16) | 0.29191 (15) | 0.0352 (4) | |
| H15A | 1.0371 | 0.2769 | 0.2764 | 0.042* | |
| C16 | 0.89140 (16) | 0.14361 (16) | 0.33096 (15) | 0.0354 (4) | |
| H16A | 0.9546 | 0.0814 | 0.3411 | 0.042* | |
| C17 | 0.75037 (16) | 0.11839 (16) | 0.35532 (15) | 0.0336 (4) | |
| H17A | 0.7170 | 0.0394 | 0.3831 | 0.040* | |
| N3 | 0.4045 (2) | 0.0595 (2) | 0.2841 (2) | 0.0280 (5) | 0.730 (4) |
| H3 | 0.3183 (15) | 0.043 (2) | 0.310 (2) | 0.033 (5)* | 0.730 (4) |
| C18 | 0.4079 (7) | −0.1799 (5) | 0.1378 (6) | 0.0449 (11) | 0.730 (4) |
| H18A | 0.5018 | −0.1793 | 0.1960 | 0.067* | 0.730 (4) |
| H18B | 0.4014 | −0.2365 | 0.0442 | 0.067* | 0.730 (4) |
| H18C | 0.3232 | −0.2222 | 0.1674 | 0.067* | 0.730 (4) |
| C19 | 0.4035 (2) | −0.0265 (2) | 0.14681 (18) | 0.0280 (6) | 0.730 (4) |
| H19 | 0.4940 | 0.0175 | 0.1222 | 0.034* | 0.730 (4) |
| C20 | 0.2651 (2) | −0.0251 (3) | 0.0494 (2) | 0.0409 (7) | 0.730 (4) |
| H20A | 0.1751 | −0.0664 | 0.0747 | 0.049* | 0.730 (4) |
| H20B | 0.2624 | −0.0866 | −0.0427 | 0.049* | 0.730 (4) |
| C21 | 0.2595 (8) | 0.1239 (7) | 0.0476 (11) | 0.0692 (17) | 0.730 (4) |
| H21A | 0.2495 | 0.1821 | 0.1354 | 0.104* | 0.730 (4) |
| H21B | 0.1735 | 0.1176 | −0.0232 | 0.104* | 0.730 (4) |
| H21C | 0.3518 | 0.1684 | 0.0295 | 0.104* | 0.730 (4) |
| N3' | 0.3875 (6) | 0.1202 (8) | 0.2699 (5) | 0.0319 (14) | 0.270 (4) |
| H3' | 0.300 (3) | 0.126 (6) | 0.294 (5) | 0.033 (5)* | 0.270 (4) |
| C18' | 0.297 (3) | 0.1380 (19) | 0.049 (3) | 0.0692 (17) | 0.270 (4) |
| H18D | 0.1966 | 0.1306 | 0.0662 | 0.104* | 0.270 (4) |
| H18E | 0.2874 | 0.0952 | −0.0480 | 0.104* | 0.270 (4) |
| H18F | 0.3502 | 0.2390 | 0.0797 | 0.104* | 0.270 (4) |
| C19' | 0.3832 (6) | 0.0589 (6) | 0.1254 (4) | 0.0332 (17) | 0.270 (4) |
| H19' | 0.4876 | 0.0723 | 0.1132 | 0.040* | 0.270 (4) |
| C20' | 0.3103 (7) | −0.0988 (6) | 0.0803 (6) | 0.0424 (19) | 0.270 (4) |
| H20C | 0.3058 | −0.1420 | −0.0168 | 0.051* | 0.270 (4) |
| H20D | 0.2063 | −0.1109 | 0.0905 | 0.051* | 0.270 (4) |
| C21' | 0.391 (2) | −0.1790 (15) | 0.1582 (19) | 0.0449 (11) | 0.270 (4) |
| H21D | 0.4973 | −0.1589 | 0.1573 | 0.067* | 0.270 (4) |
| H21E | 0.3467 | −0.2820 | 0.1160 | 0.067* | 0.270 (4) |
| H21F | 0.3820 | −0.1479 | 0.2517 | 0.067* | 0.270 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0214 (5) | 0.0490 (6) | 0.0292 (5) | −0.0004 (4) | 0.0063 (4) | 0.0034 (4) |
| N1 | 0.0238 (6) | 0.0280 (6) | 0.0298 (6) | 0.0032 (5) | 0.0074 (5) | 0.0070 (5) |
| N2 | 0.0286 (7) | 0.0283 (6) | 0.0382 (7) | 0.0003 (5) | 0.0107 (5) | 0.0047 (5) |
| C1 | 0.0295 (7) | 0.0259 (7) | 0.0307 (7) | 0.0102 (5) | 0.0121 (6) | 0.0109 (5) |
| C2 | 0.0309 (8) | 0.0336 (8) | 0.0338 (7) | 0.0073 (6) | 0.0117 (6) | 0.0078 (6) |
| C3 | 0.0328 (8) | 0.0373 (8) | 0.0440 (8) | 0.0071 (6) | 0.0174 (7) | 0.0114 (7) |
| C4 | 0.0463 (10) | 0.0359 (8) | 0.0423 (8) | 0.0144 (7) | 0.0254 (7) | 0.0149 (7) |
| C5 | 0.0504 (10) | 0.0354 (8) | 0.0321 (7) | 0.0123 (7) | 0.0169 (7) | 0.0088 (6) |
| C6 | 0.0346 (8) | 0.0325 (8) | 0.0320 (7) | 0.0077 (6) | 0.0093 (6) | 0.0096 (6) |
| C7 | 0.0231 (7) | 0.0311 (7) | 0.0248 (6) | 0.0068 (6) | 0.0051 (5) | 0.0105 (5) |
| C8 | 0.0216 (7) | 0.0303 (7) | 0.0272 (7) | 0.0065 (5) | 0.0054 (5) | 0.0113 (6) |
| C9 | 0.0267 (7) | 0.0266 (7) | 0.0339 (7) | 0.0047 (6) | 0.0079 (6) | 0.0099 (6) |
| C10 | 0.0342 (9) | 0.0356 (8) | 0.0400 (8) | −0.0042 (7) | 0.0107 (7) | 0.0031 (7) |
| C11 | 0.0228 (7) | 0.0447 (9) | 0.0275 (7) | 0.0034 (6) | 0.0054 (6) | 0.0116 (6) |
| C12 | 0.0213 (7) | 0.0356 (8) | 0.0247 (6) | 0.0028 (6) | 0.0053 (5) | 0.0082 (6) |
| C13 | 0.0306 (8) | 0.0326 (7) | 0.0357 (7) | 0.0100 (6) | 0.0108 (6) | 0.0123 (6) |
| C14 | 0.0345 (8) | 0.0323 (8) | 0.0414 (8) | 0.0028 (6) | 0.0152 (7) | 0.0127 (6) |
| C15 | 0.0206 (7) | 0.0378 (8) | 0.0409 (8) | 0.0011 (6) | 0.0092 (6) | 0.0061 (6) |
| C16 | 0.0223 (7) | 0.0378 (8) | 0.0419 (8) | 0.0082 (6) | 0.0016 (6) | 0.0105 (7) |
| C17 | 0.0274 (8) | 0.0366 (8) | 0.0353 (7) | 0.0018 (6) | 0.0023 (6) | 0.0166 (6) |
| N3 | 0.0215 (10) | 0.0311 (11) | 0.0271 (9) | 0.0031 (9) | 0.0079 (7) | 0.0040 (8) |
| C18 | 0.054 (2) | 0.0336 (9) | 0.048 (2) | 0.0125 (9) | 0.0204 (15) | 0.0082 (11) |
| C19 | 0.0251 (11) | 0.0284 (12) | 0.0262 (10) | 0.0039 (9) | 0.0080 (8) | 0.0032 (8) |
| C20 | 0.0368 (13) | 0.0511 (15) | 0.0311 (11) | 0.0160 (11) | 0.0051 (10) | 0.0071 (10) |
| C21 | 0.096 (5) | 0.0693 (18) | 0.0589 (14) | 0.044 (2) | 0.019 (3) | 0.0321 (16) |
| N3' | 0.023 (3) | 0.043 (4) | 0.028 (3) | 0.012 (3) | 0.007 (2) | 0.007 (3) |
| C18' | 0.096 (5) | 0.0693 (18) | 0.0589 (14) | 0.044 (2) | 0.019 (3) | 0.0321 (16) |
| C19' | 0.025 (3) | 0.046 (4) | 0.031 (3) | 0.006 (3) | 0.011 (2) | 0.015 (3) |
| C20' | 0.035 (4) | 0.043 (4) | 0.039 (3) | 0.000 (3) | 0.008 (3) | 0.007 (3) |
| C21' | 0.054 (2) | 0.0336 (9) | 0.048 (2) | 0.0125 (9) | 0.0204 (15) | 0.0082 (11) |
Geometric parameters (Å, °)
| O1—C7 | 1.2529 (17) | C16—C17 | 1.386 (2) |
| N1—C7 | 1.3785 (18) | C16—H16A | 0.9500 |
| N1—N2 | 1.4019 (17) | C17—H17A | 0.9500 |
| N1—C1 | 1.4150 (18) | N3—C19 | 1.466 (3) |
| N2—C9 | 1.3119 (19) | N3—H3 | 0.902 (10) |
| C1—C2 | 1.387 (2) | N3—H3' | 1.28 (4) |
| C1—C6 | 1.394 (2) | C18—C19 | 1.528 (4) |
| C2—C3 | 1.386 (2) | C18—H18A | 0.9800 |
| C2—H2A | 0.9500 | C18—H18B | 0.9800 |
| C3—C4 | 1.387 (2) | C18—H18C | 0.9800 |
| C3—H3A | 0.9500 | C19—C20 | 1.516 (3) |
| C4—C5 | 1.374 (2) | C19—H19 | 1.0000 |
| C4—H4A | 0.9500 | C20—C21 | 1.517 (5) |
| C5—C6 | 1.385 (2) | C20—H20A | 0.9900 |
| C5—H5A | 0.9500 | C20—H20B | 0.9900 |
| C6—H6A | 0.9500 | C21—H21A | 0.9800 |
| C7—C8 | 1.4382 (19) | C21—H21B | 0.9800 |
| C8—C11 | 1.402 (2) | C21—H21C | 0.9800 |
| C8—C9 | 1.430 (2) | N3'—C19' | 1.475 (6) |
| C9—C10 | 1.491 (2) | N3'—H3 | 1.149 (18) |
| C10—H10A | 0.9800 | N3'—H3' | 0.904 (10) |
| C10—H10B | 0.9800 | C18'—C19' | 1.527 (9) |
| C10—H10C | 0.9800 | C18'—H18D | 0.9800 |
| C11—N3' | 1.311 (5) | C18'—H18E | 0.9800 |
| C11—N3 | 1.359 (2) | C18'—H18F | 0.9800 |
| C11—C12 | 1.4901 (19) | C19'—C20' | 1.508 (7) |
| C12—C13 | 1.386 (2) | C19'—H19' | 1.0000 |
| C12—C17 | 1.390 (2) | C20'—C21' | 1.519 (9) |
| C13—C14 | 1.388 (2) | C20'—H20C | 0.9900 |
| C13—H13A | 0.9500 | C20'—H20D | 0.9900 |
| C14—C15 | 1.380 (2) | C21'—H21D | 0.9800 |
| C14—H14A | 0.9500 | C21'—H21E | 0.9800 |
| C15—C16 | 1.379 (2) | C21'—H21F | 0.9800 |
| C15—H15A | 0.9500 | ||
| C7—N1—N2 | 111.72 (11) | C15—C16—C17 | 119.92 (14) |
| C7—N1—C1 | 129.43 (12) | C15—C16—H16A | 120.0 |
| N2—N1—C1 | 118.78 (11) | C17—C16—H16A | 120.0 |
| C9—N2—N1 | 106.50 (11) | C16—C17—C12 | 119.82 (14) |
| C2—C1—C6 | 119.64 (14) | C16—C17—H17A | 120.1 |
| C2—C1—N1 | 121.12 (12) | C12—C17—H17A | 120.1 |
| C6—C1—N1 | 119.24 (13) | C11—N3—C19 | 128.01 (18) |
| C3—C2—C1 | 119.69 (14) | C11—N3—H3 | 114.1 (14) |
| C3—C2—H2A | 120.2 | C19—N3—H3 | 116.6 (13) |
| C1—C2—H2A | 120.2 | C11—N3—H3' | 91 (2) |
| C2—C3—C4 | 120.83 (14) | C19—N3—H3' | 116 (2) |
| C2—C3—H3A | 119.6 | H3—N3—H3' | 47 (2) |
| C4—C3—H3A | 119.6 | N3—C19—C20 | 109.15 (17) |
| C5—C4—C3 | 119.10 (15) | N3—C19—C18 | 111.0 (3) |
| C5—C4—H4A | 120.4 | C20—C19—C18 | 110.6 (3) |
| C3—C4—H4A | 120.4 | N3—C19—H19 | 108.7 |
| C4—C5—C6 | 121.02 (14) | C20—C19—H19 | 108.7 |
| C4—C5—H5A | 119.5 | C18—C19—H19 | 108.7 |
| C6—C5—H5A | 119.5 | C19—C20—C21 | 113.0 (3) |
| C5—C6—C1 | 119.71 (14) | C19—C20—H20A | 109.0 |
| C5—C6—H6A | 120.1 | C21—C20—H20A | 109.0 |
| C1—C6—H6A | 120.1 | C19—C20—H20B | 109.0 |
| O1—C7—N1 | 126.01 (13) | C21—C20—H20B | 109.0 |
| O1—C7—C8 | 129.17 (13) | H20A—C20—H20B | 107.8 |
| N1—C7—C8 | 104.80 (12) | C11—N3'—C19' | 126.0 (5) |
| C11—C8—C9 | 132.65 (13) | C11—N3'—H3 | 102.0 (11) |
| C11—C8—C7 | 121.77 (13) | C19'—N3'—H3 | 113.7 (11) |
| C9—C8—C7 | 105.58 (12) | C11—N3'—H3' | 115 (4) |
| N2—C9—C8 | 111.40 (13) | C19'—N3'—H3' | 119 (4) |
| N2—C9—C10 | 118.92 (13) | H3—N3'—H3' | 52 (4) |
| C8—C9—C10 | 129.65 (14) | C19'—C18'—H18D | 109.5 |
| C9—C10—H10A | 109.5 | C19'—C18'—H18E | 109.5 |
| C9—C10—H10B | 109.5 | H18D—C18'—H18E | 109.5 |
| H10A—C10—H10B | 109.5 | C19'—C18'—H18F | 109.5 |
| C9—C10—H10C | 109.5 | H18D—C18'—H18F | 109.5 |
| H10A—C10—H10C | 109.5 | H18E—C18'—H18F | 109.5 |
| H10B—C10—H10C | 109.5 | N3'—C19'—C20' | 107.5 (5) |
| N3'—C11—C8 | 113.3 (3) | N3'—C19'—C18' | 108.1 (14) |
| N3—C11—C8 | 118.82 (15) | C20'—C19'—C18' | 112.7 (8) |
| N3'—C11—C12 | 120.1 (3) | N3'—C19'—H19' | 109.5 |
| N3—C11—C12 | 117.86 (15) | C20'—C19'—H19' | 109.5 |
| C8—C11—C12 | 122.46 (13) | C18'—C19'—H19' | 109.5 |
| C13—C12—C17 | 120.12 (13) | C19'—C20'—C21' | 113.7 (7) |
| C13—C12—C11 | 122.04 (13) | C19'—C20'—H20C | 108.8 |
| C17—C12—C11 | 117.84 (13) | C21'—C20'—H20C | 108.8 |
| C12—C13—C14 | 119.60 (14) | C19'—C20'—H20D | 108.8 |
| C12—C13—H13A | 120.2 | C21'—C20'—H20D | 108.8 |
| C14—C13—H13A | 120.2 | H20C—C20'—H20D | 107.7 |
| C15—C14—C13 | 120.10 (14) | C20'—C21'—H21D | 109.5 |
| C15—C14—H14A | 119.9 | C20'—C21'—H21E | 109.5 |
| C13—C14—H14A | 119.9 | H21D—C21'—H21E | 109.5 |
| C16—C15—C14 | 120.43 (14) | C20'—C21'—H21F | 109.5 |
| C16—C15—H15A | 119.8 | H21D—C21'—H21F | 109.5 |
| C14—C15—H15A | 119.8 | H21E—C21'—H21F | 109.5 |
| C7—N1—N2—C9 | −0.44 (16) | C7—C8—C11—N3 | −5.0 (2) |
| C1—N1—N2—C9 | 176.80 (12) | C9—C8—C11—C12 | 4.8 (2) |
| C7—N1—C1—C2 | −12.6 (2) | C7—C8—C11—C12 | −174.22 (13) |
| N2—N1—C1—C2 | 170.69 (12) | N3'—C11—C12—C13 | 76.6 (4) |
| C7—N1—C1—C6 | 168.11 (13) | N3—C11—C12—C13 | 111.8 (2) |
| N2—N1—C1—C6 | −8.57 (19) | C8—C11—C12—C13 | −78.87 (19) |
| C6—C1—C2—C3 | 0.2 (2) | N3'—C11—C12—C17 | −104.2 (4) |
| N1—C1—C2—C3 | −179.08 (13) | N3—C11—C12—C17 | −69.0 (2) |
| C1—C2—C3—C4 | 0.3 (2) | C8—C11—C12—C17 | 100.28 (17) |
| C2—C3—C4—C5 | −0.7 (2) | C17—C12—C13—C14 | −0.9 (2) |
| C3—C4—C5—C6 | 0.8 (2) | C11—C12—C13—C14 | 178.22 (13) |
| C4—C5—C6—C1 | −0.3 (2) | C12—C13—C14—C15 | 0.7 (2) |
| C2—C1—C6—C5 | −0.1 (2) | C13—C14—C15—C16 | 0.2 (2) |
| N1—C1—C6—C5 | 179.13 (13) | C14—C15—C16—C17 | −0.8 (2) |
| N2—N1—C7—O1 | 178.94 (13) | C15—C16—C17—C12 | 0.6 (2) |
| C1—N1—C7—O1 | 2.1 (2) | C13—C12—C17—C16 | 0.3 (2) |
| N2—N1—C7—C8 | 0.35 (15) | C11—C12—C17—C16 | −178.90 (13) |
| C1—N1—C7—C8 | −176.52 (12) | N3'—C11—N3—C19 | 85.3 (6) |
| O1—C7—C8—C11 | 0.6 (2) | C8—C11—N3—C19 | 172.81 (19) |
| N1—C7—C8—C11 | 179.14 (12) | C12—C11—N3—C19 | −17.5 (3) |
| O1—C7—C8—C9 | −178.66 (14) | C11—N3—C19—C20 | −118.9 (3) |
| N1—C7—C8—C9 | −0.13 (14) | C11—N3—C19—C18 | 118.9 (4) |
| N1—N2—C9—C8 | 0.35 (16) | N3—C19—C20—C21 | 61.1 (5) |
| N1—N2—C9—C10 | 178.68 (12) | C18—C19—C20—C21 | −176.5 (5) |
| C11—C8—C9—N2 | −179.29 (15) | N3—C11—N3'—C19' | −85.2 (8) |
| C7—C8—C9—N2 | −0.14 (16) | C8—C11—N3'—C19' | 167.1 (5) |
| C11—C8—C9—C10 | 2.6 (3) | C12—C11—N3'—C19' | 9.5 (9) |
| C7—C8—C9—C10 | −178.24 (15) | C11—N3'—C19'—C20' | 116.6 (7) |
| C9—C8—C11—N3' | −152.2 (4) | C11—N3'—C19'—C18' | −121.5 (11) |
| C7—C8—C11—N3' | 28.8 (4) | N3'—C19'—C20'—C21' | −58.9 (10) |
| C9—C8—C11—N3 | 174.01 (18) | C18'—C19'—C20'—C21' | −177.9 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3'—H3'···O1 | 0.90 (1) | 1.99 (4) | 2.705 (6) | 135 (5) |
| N3—H3···O1 | 0.90 (1) | 1.93 (2) | 2.699 (2) | 142 (2) |
| C2—H2A···O1 | 0.95 | 2.29 | 2.9243 (19) | 123 |
| C6—H6A···N2 | 0.95 | 2.44 | 2.777 (2) | 101 |
| C16—H16A···O1i | 0.95 | 2.53 | 3.2743 (19) | 135 |
| C13—H13A···N2ii | 0.95 | 2.60 | 3.537 (2) | 167 |
Symmetry codes: (i) x+1, y, z; (ii) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2238).
References
- Li, J.-Z., Yu, W.-J. & Du, X.-Y. (1997). Chin. J. Appl. Chem.14, 98–100.
- Liu, J.-M., Yang, R.-D. & Ma, T.-R. (1980). Chem. J. Chin. Univ.1, 23–29.
- Rigaku (2005). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zhou, Y.-P., Yang, Zh.-Y., Yu, H.-J. & Yang, R.-D. (1999). Chin. J. Appl. Chem.16, 37–41.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680902950X/bh2238sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680902950X/bh2238Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Enhanced figure: interactive version of Fig. 1