Abstract
There are two crystallographically independent organic molecules in the asymmetric unit of the title compound, C12H12Cl2N2O2·0.5H2O. The benzodiazepine ring adopts a distorted boat conformation in both molecules. The crystal packing is controlled by N—H⋯O, C—H⋯O and O—H⋯O intra- and intermolecular hydrogen bonds. A graph-set motif of R 3 3(14) dimer formation by a combination of N—H⋯O, O—H⋯O and C—H⋯O hydrogen bonds stabilizes the molecules and extends along a axis.
Related literature
For the anticonvulsant activity of benzodiazepine, see: MacDonald (2002 ▶). For their hypnotic effect, see: Gringauz (1999 ▶). For their use in the treatment of gastrointestinal and central nervous system disorders, see: Rahbaek et al. (1999 ▶). For other therapeutic applications, see: Albright et al. (1998 ▶); Lee et al. (1999 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For puckering and asymmetry parameters, see: Cremer & Pople (1975 ▶); Nardelli (1983 ▶). For details of the preparation of the title compound, see: Venkatraj et al. (2008 ▶).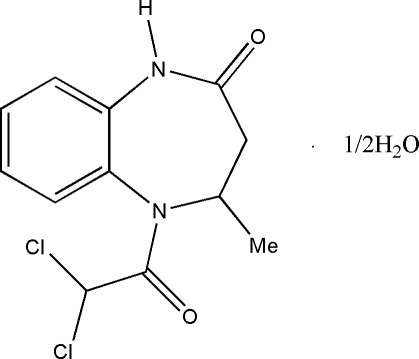
Experimental
Crystal data
C12H12Cl2N2O2·0.5H2O
M r = 592.29
Monoclinic,
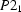
a = 8.5470 (3) Å
b = 18.0837 (6) Å
c = 8.8697 (3) Å
β = 95.405 (2)°
V = 1364.82 (8) Å3
Z = 2
Mo Kα radiation
μ = 0.48 mm−1
T = 293 K
0.26 × 0.24 × 0.22 mm
Data collection
Bruker Kappa APEXII area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2001 ▶) T min = 0.884, T max = 0.901
14191 measured reflections
5599 independent reflections
4873 reflections with I > 2σ(I)
R int = 0.022
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.110
S = 1.04
5599 reflections
352 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.46 e Å−3
Δρmin = −0.62 e Å−3
Absolute structure: Flack (1983 ▶), 2698 Friedel pairs
Flack parameter: 0.06 (6)
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809036940/bt5048sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809036940/bt5048Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1A—H1A⋯O3 | 0.87 (4) | 2.08 (4) | 2.927 (4) | 164 (3) |
| O3—H2W⋯O1Bi | 0.80 (4) | 2.02 (4) | 2.815 (4) | 173 (4) |
| C8A—H8A⋯O2Aii | 0.93 | 2.51 | 3.268 (4) | 139 |
| C10B—H10B⋯O2Biii | 0.93 | 2.39 | 3.179 (4) | 143 |
Symmetry codes: (i) 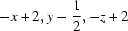 ; (ii)
; (ii) 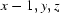 ; (iii)
; (iii) 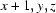 .
.
Acknowledgments
KR thanks Dr Babu Varghese, SAIF, IIT-Madras, India, for his help with the data collection and the management of Kandaswami Kandar’s College, Velur, Namakkal, India, for their encouragement to pursue the programme.
supplementary crystallographic information
Comment
The anticonvulsant activity of benzodiazepines has been utilized clinically in patients to treat specific seizure types or conditions, i.e., akinetic, myoclonic, absence variant seizures as well as to help terminate status epilepticusor serial seizures (MacDonald, 2002). Benzodiazepines are used for the purpose of hypnotic effects, owing to their less toxic and less severe withdrawal effects when compared with barbiturates (Gringauz, 1999). Benzodiazepines from aspergillus include asperlicin, which is used for treatment of gastrointestinal and central nervous system (CNS) disorders (Rahbaek et al.,1999). The other therapeutic applications (Lee et al., 1999) of benzodiazepines include vasopressin antagonists (Albright et al., 1998). In view of these importance and to ascertain the molecular conformation, crystallographic study of the title compound has been carried out.
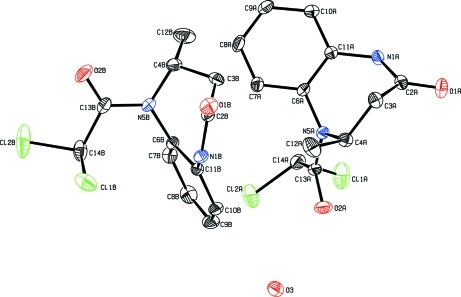
The ORTEP diagram of the title compound is shown in Fig.1. There are two crystallographically independent molecules in the asymmetric unit. The benzodiazepine rings in the two molecules adopt a distorted boat conformation. The puckering parameters (Cremer & Pople, 1975) and the asymmetry parameters (Nardelli, 1983) for the ring in molecule A are: q2 = 0.959 (3) Å, q3 = 0.150 (3) Å, φ2 = 136.1 (2)°, φ3 = 359.8 (1)° and Δ2(C4A)= 8.1 (3)°; for the ring in molecule B are: q2 = 0.962 (3) Å, q3 = 0.168 (3) Å, φ2 = 141.4 (2)°, φ3 = 5.3 (1)° and Δ2(C4B)= 3.4 (3)°. The sum of the bond angles at N1A(359.0°), N1B(359.2), N5A(358.8) and N5B(359.9°) of the benzodiazepine rings in both the molecules are in accordance with sp2 hybridization.
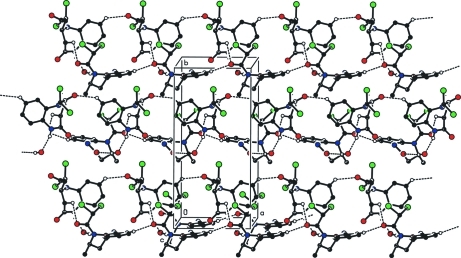
The crystal packing is controlled by N—H···O, C—H···O and O—H···O types of intermolecular interactions in addition to van der Waals forces. The water molecule connects the molecules A and B through N1A—H1A···O3 and O3—H2W···O1B hydrogen bonds. Thus the combination of N1A—H1A···O3, O3—H2W···O1B and C3A—H3A···O2B hydrogen bonds form a graph set motif of R33(14) dimer (Bernstein et al., 1995) which stabilize the molecules. Atom C8A at (x, y, z) donates a proton to O2A (x - 1, y, z), which forms a C7 one dimensional chain running along a–axis. The intermolecular hydrogen bond C10B—H10B···O2B also connects the molecule into an another C7 chain running along b–axis (Fig. 2).
Experimental
To a solution of tetrahydro-4-methyl-1,5-benzodiazepin-2-one (0.88 g) in anhydrous benzene (50 ml) was added triethylamine (2.8 ml) and dichloroacetyl chloride (1.90 ml). The contents were allowed to reflux on a water bath for 6hrs. The reaction mixture was washed with sodium bicarbonate solution (10%), water and dried. Evaporation of the solvent results a crude mass and further crystallization from ethanol gives colorless crystals (Venkatraj et al., 2008).
Refinement
The Nitrogen and Oxygen H atoms were refined and the other H atoms positioned geometrically (C—H=0.93–0.98 Å) and allowed to ride on their parent atoms, with 1.5Ueq(C) for methyl H and 1.2 Ueq(C) for other H atoms.
Figures
Fig. 1.
Perspective view of the molecule showing the thermal ellipsoids are drawn at 30% probability level.
Fig. 2.
The crystal packing of the molecules viewed down c–axis. H atoms not involved in hydrogen bonding have been omitted for clarity.
Crystal data
| C12H12Cl2N2O2·0.5H2O | F(000) = 612 |
| Mr = 592.29 | Dx = 1.441 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 4629 reflections |
| a = 8.5470 (3) Å | θ = 2.3–26.5° |
| b = 18.0837 (6) Å | µ = 0.48 mm−1 |
| c = 8.8697 (3) Å | T = 293 K |
| β = 95.405 (2)° | Block, colourless |
| V = 1364.82 (8) Å3 | 0.26 × 0.24 × 0.22 mm |
| Z = 2 |
Data collection
| Bruker Kappa APEXII area-detector diffractometer | 5599 independent reflections |
| Radiation source: fine-focus sealed tube | 4873 reflections with I > 2σ(I) |
| graphite | Rint = 0.022 |
| ω and φ scans | θmax = 26.5°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2001) | h = −10→10 |
| Tmin = 0.884, Tmax = 0.901 | k = −22→22 |
| 14191 measured reflections | l = −11→10 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.110 | w = 1/[σ2(Fo2) + (0.0438P)2 + 0.7028P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.006 |
| 5599 reflections | Δρmax = 0.46 e Å−3 |
| 352 parameters | Δρmin = −0.62 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983),2698 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.06 (6) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1A | 1.62028 (16) | −0.29670 (7) | 1.77856 (13) | 0.0941 (4) | |
| Cl1B | 1.04826 (15) | 0.13662 (6) | 0.96558 (15) | 0.0906 (4) | |
| Cl2A | 1.48485 (16) | −0.15064 (6) | 1.74118 (13) | 0.0858 (3) | |
| Cl2B | 0.8172 (2) | 0.15716 (9) | 1.17529 (14) | 0.1300 (7) | |
| O1A | 1.5519 (3) | −0.46993 (14) | 1.2925 (3) | 0.0594 (6) | |
| O1B | 1.0719 (3) | −0.07368 (15) | 0.6539 (2) | 0.0573 (6) | |
| O2A | 1.6504 (3) | −0.20942 (14) | 1.4809 (3) | 0.0635 (7) | |
| O2B | 0.7429 (3) | 0.02505 (17) | 1.0049 (4) | 0.0852 (9) | |
| O3 | 1.2224 (4) | −0.56267 (15) | 1.5161 (4) | 0.0677 (7) | |
| H1W | 1.279 (6) | −0.601 (3) | 1.502 (6) | 0.108 (19)* | |
| H2W | 1.140 (5) | −0.570 (2) | 1.467 (4) | 0.059 (11)* | |
| N1A | 1.3405 (3) | −0.42583 (13) | 1.3919 (3) | 0.0403 (5) | |
| H1A | 1.325 (4) | −0.469 (2) | 1.432 (4) | 0.057 (10)* | |
| N1B | 1.2097 (3) | −0.03383 (14) | 0.8658 (3) | 0.0399 (5) | |
| H1B | 1.261 (3) | −0.0095 (17) | 0.811 (3) | 0.031 (7)* | |
| C2A | 1.4518 (3) | −0.42199 (16) | 1.2939 (3) | 0.0420 (6) | |
| C2B | 1.1026 (3) | −0.07798 (16) | 0.7907 (3) | 0.0410 (6) | |
| C3A | 1.4398 (4) | −0.35821 (17) | 1.1848 (3) | 0.0462 (7) | |
| H3A | 1.3314 | −0.3539 | 1.1423 | 0.055* | |
| H3B | 1.5028 | −0.3694 | 1.1023 | 0.055* | |
| C3B | 1.0275 (4) | −0.13513 (16) | 0.8847 (3) | 0.0465 (7) | |
| H3C | 1.1089 | −0.1594 | 0.9505 | 0.056* | |
| H3D | 0.9773 | −0.1723 | 0.8178 | 0.056* | |
| C4A | 1.4920 (3) | −0.28414 (16) | 1.2521 (3) | 0.0441 (7) | |
| H4A | 1.6071 | −0.2832 | 1.2637 | 0.053* | |
| C4B | 0.9057 (4) | −0.10270 (17) | 0.9813 (4) | 0.0490 (7) | |
| H4B | 0.8130 | −0.0886 | 0.9140 | 0.059* | |
| N5A | 1.4357 (2) | −0.27668 (12) | 1.4044 (2) | 0.0368 (5) | |
| N5B | 0.9699 (3) | −0.03578 (14) | 1.0580 (2) | 0.0403 (5) | |
| C6A | 1.2763 (3) | −0.29648 (16) | 1.4220 (3) | 0.0355 (5) | |
| C6B | 1.1307 (3) | −0.03545 (15) | 1.1206 (3) | 0.0363 (6) | |
| C7A | 1.1664 (3) | −0.24204 (17) | 1.4443 (3) | 0.0457 (7) | |
| H7A | 1.1961 | −0.1926 | 1.4462 | 0.055* | |
| C7B | 1.1690 (4) | −0.03548 (18) | 1.2758 (3) | 0.0485 (7) | |
| H7B | 1.0906 | −0.0415 | 1.3407 | 0.058* | |
| C8A | 1.0148 (3) | −0.2607 (2) | 1.4635 (4) | 0.0548 (8) | |
| H8A | 0.9424 | −0.2242 | 1.4820 | 0.066* | |
| C8B | 1.3225 (4) | −0.0267 (2) | 1.3344 (4) | 0.0587 (9) | |
| H8B | 1.3482 | −0.0259 | 1.4386 | 0.070* | |
| C9A | 0.9695 (3) | −0.3334 (2) | 1.4554 (4) | 0.0530 (8) | |
| H9A | 0.8658 | −0.3458 | 1.4675 | 0.064* | |
| C9B | 1.4383 (4) | −0.01891 (19) | 1.2369 (4) | 0.0571 (9) | |
| H9B | 1.5418 | −0.0109 | 1.2758 | 0.069* | |
| C10A | 1.0749 (3) | −0.38800 (17) | 1.4298 (4) | 0.0467 (7) | |
| H10A | 1.0422 | −0.4370 | 1.4237 | 0.056* | |
| C10B | 1.4017 (3) | −0.02294 (17) | 1.0826 (4) | 0.0461 (7) | |
| H10B | 1.4812 | −0.0194 | 1.0182 | 0.055* | |
| C11A | 1.2304 (3) | −0.37036 (15) | 1.4130 (3) | 0.0353 (5) | |
| C11B | 1.2482 (3) | −0.03216 (15) | 1.0231 (3) | 0.0363 (5) | |
| C12A | 1.4358 (6) | −0.2213 (2) | 1.1517 (4) | 0.0707 (11) | |
| H12A | 1.3230 | −0.2210 | 1.1396 | 0.106* | |
| H12B | 1.4751 | −0.2271 | 1.0544 | 0.106* | |
| H12C | 1.4733 | −0.1755 | 1.1964 | 0.106* | |
| C12B | 0.8546 (5) | −0.1577 (3) | 1.0958 (5) | 0.0806 (12) | |
| H12D | 0.9429 | −0.1705 | 1.1660 | 0.121* | |
| H12E | 0.8147 | −0.2015 | 1.0443 | 0.121* | |
| H12F | 0.7738 | −0.1361 | 1.1498 | 0.121* | |
| C13A | 1.5282 (3) | −0.23943 (16) | 1.5082 (3) | 0.0425 (6) | |
| C13B | 0.8785 (3) | 0.02480 (19) | 1.0567 (3) | 0.0483 (7) | |
| C14A | 1.4827 (4) | −0.2412 (2) | 1.6705 (3) | 0.0516 (7) | |
| H14A | 1.3773 | −0.2624 | 1.6720 | 0.062* | |
| C14B | 0.9587 (5) | 0.0969 (2) | 1.1152 (4) | 0.0651 (10) | |
| H14B | 1.0379 | 0.0860 | 1.1995 | 0.078* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1A | 0.1134 (9) | 0.0939 (8) | 0.0740 (7) | 0.0444 (7) | 0.0047 (6) | 0.0100 (6) |
| Cl1B | 0.1093 (9) | 0.0570 (6) | 0.1045 (8) | −0.0225 (6) | 0.0047 (7) | 0.0035 (6) |
| Cl2A | 0.1158 (9) | 0.0691 (6) | 0.0740 (6) | 0.0202 (6) | 0.0169 (6) | −0.0282 (5) |
| Cl2B | 0.1609 (13) | 0.1493 (13) | 0.0748 (7) | 0.1052 (12) | −0.0141 (7) | −0.0412 (8) |
| O1A | 0.0640 (14) | 0.0558 (13) | 0.0609 (13) | 0.0228 (12) | 0.0193 (11) | 0.0018 (11) |
| O1B | 0.0562 (13) | 0.0768 (16) | 0.0377 (11) | −0.0032 (12) | −0.0020 (9) | −0.0029 (11) |
| O2A | 0.0445 (12) | 0.0707 (16) | 0.0778 (16) | −0.0228 (11) | 0.0190 (11) | −0.0222 (13) |
| O2B | 0.0332 (12) | 0.087 (2) | 0.133 (3) | 0.0137 (13) | −0.0012 (14) | 0.0200 (19) |
| O3 | 0.0578 (16) | 0.0485 (14) | 0.093 (2) | 0.0005 (13) | −0.0113 (15) | 0.0087 (13) |
| N1A | 0.0465 (13) | 0.0319 (13) | 0.0435 (13) | 0.0045 (10) | 0.0100 (10) | 0.0037 (10) |
| N1B | 0.0367 (12) | 0.0480 (13) | 0.0360 (12) | −0.0030 (11) | 0.0087 (10) | 0.0039 (11) |
| C2A | 0.0474 (16) | 0.0378 (15) | 0.0408 (14) | 0.0031 (13) | 0.0035 (12) | −0.0043 (12) |
| C2B | 0.0388 (14) | 0.0439 (16) | 0.0400 (15) | 0.0031 (12) | 0.0026 (11) | −0.0056 (12) |
| C3A | 0.0575 (18) | 0.0471 (16) | 0.0348 (13) | 0.0020 (14) | 0.0083 (12) | −0.0023 (12) |
| C3B | 0.0504 (17) | 0.0371 (15) | 0.0511 (16) | −0.0102 (13) | −0.0010 (13) | −0.0056 (12) |
| C4A | 0.0461 (16) | 0.0472 (17) | 0.0404 (14) | −0.0062 (13) | 0.0108 (12) | −0.0014 (13) |
| C4B | 0.0382 (15) | 0.0495 (18) | 0.0588 (19) | −0.0087 (13) | 0.0016 (13) | 0.0007 (14) |
| N5A | 0.0328 (11) | 0.0392 (12) | 0.0394 (11) | −0.0022 (9) | 0.0082 (9) | −0.0032 (9) |
| N5B | 0.0282 (11) | 0.0521 (14) | 0.0409 (12) | 0.0018 (10) | 0.0047 (9) | 0.0010 (11) |
| C6A | 0.0277 (12) | 0.0417 (14) | 0.0367 (13) | 0.0022 (11) | 0.0010 (10) | 0.0018 (11) |
| C6B | 0.0334 (13) | 0.0375 (13) | 0.0372 (13) | 0.0031 (11) | −0.0004 (10) | 0.0018 (11) |
| C7A | 0.0420 (15) | 0.0402 (16) | 0.0552 (17) | 0.0070 (12) | 0.0057 (13) | 0.0037 (13) |
| C7B | 0.0540 (17) | 0.0523 (17) | 0.0390 (14) | 0.0062 (14) | 0.0031 (12) | 0.0047 (13) |
| C8A | 0.0372 (16) | 0.057 (2) | 0.071 (2) | 0.0182 (14) | 0.0091 (14) | 0.0025 (16) |
| C8B | 0.071 (2) | 0.060 (2) | 0.0419 (16) | 0.0053 (18) | −0.0129 (15) | −0.0005 (15) |
| C9A | 0.0292 (15) | 0.065 (2) | 0.0648 (19) | −0.0016 (14) | 0.0056 (13) | 0.0010 (17) |
| C9B | 0.0434 (17) | 0.057 (2) | 0.066 (2) | −0.0052 (15) | −0.0182 (15) | 0.0001 (16) |
| C10A | 0.0376 (15) | 0.0460 (17) | 0.0563 (17) | −0.0072 (12) | 0.0031 (13) | −0.0004 (13) |
| C10B | 0.0328 (13) | 0.0472 (17) | 0.0568 (17) | −0.0013 (12) | −0.0033 (12) | 0.0023 (14) |
| C11A | 0.0356 (13) | 0.0376 (14) | 0.0326 (12) | −0.0005 (11) | 0.0032 (10) | −0.0005 (11) |
| C11B | 0.0342 (13) | 0.0347 (13) | 0.0393 (13) | 0.0007 (11) | 0.0009 (10) | 0.0015 (11) |
| C12A | 0.104 (3) | 0.049 (2) | 0.061 (2) | −0.0015 (19) | 0.019 (2) | 0.0172 (17) |
| C12B | 0.083 (3) | 0.075 (3) | 0.087 (3) | −0.036 (2) | 0.026 (2) | 0.004 (2) |
| C13A | 0.0333 (14) | 0.0402 (15) | 0.0551 (16) | −0.0060 (12) | 0.0107 (12) | −0.0084 (13) |
| C13B | 0.0353 (15) | 0.0581 (19) | 0.0525 (17) | 0.0096 (14) | 0.0086 (13) | 0.0103 (15) |
| C14A | 0.0420 (15) | 0.064 (2) | 0.0482 (16) | 0.0011 (14) | 0.0015 (12) | −0.0146 (15) |
| C14B | 0.073 (2) | 0.064 (2) | 0.0539 (19) | 0.0303 (18) | −0.0134 (17) | −0.0112 (16) |
Geometric parameters (Å, °)
| Cl1A—C14A | 1.759 (3) | N5B—C13B | 1.345 (4) |
| Cl1B—C14B | 1.748 (4) | N5B—C6B | 1.432 (3) |
| Cl2A—C14A | 1.754 (3) | C6A—C7A | 1.388 (4) |
| Cl2B—C14B | 1.748 (4) | C6A—C11A | 1.393 (4) |
| O1A—C2A | 1.219 (4) | C6B—C7B | 1.385 (4) |
| O1B—C2B | 1.220 (3) | C6B—C11B | 1.387 (4) |
| O2A—C13A | 1.221 (3) | C7A—C8A | 1.365 (4) |
| O2B—C13B | 1.206 (4) | C7A—H7A | 0.9300 |
| O3—H1W | 0.86 (6) | C7B—C8B | 1.374 (5) |
| O3—H2W | 0.80 (4) | C7B—H7B | 0.9300 |
| N1A—C2A | 1.349 (4) | C8A—C9A | 1.370 (5) |
| N1A—C11A | 1.400 (4) | C8A—H8A | 0.9300 |
| N1A—H1A | 0.87 (4) | C8B—C9B | 1.381 (5) |
| N1B—C2B | 1.343 (4) | C8B—H8B | 0.9300 |
| N1B—C11B | 1.403 (3) | C9A—C10A | 1.370 (4) |
| N1B—H1B | 0.81 (3) | C9A—H9A | 0.9300 |
| C2A—C3A | 1.503 (4) | C9B—C10B | 1.376 (5) |
| C2B—C3B | 1.508 (4) | C9B—H9B | 0.9300 |
| C3A—C4A | 1.516 (4) | C10A—C11A | 1.389 (4) |
| C3A—H3A | 0.9700 | C10A—H10A | 0.9300 |
| C3A—H3B | 0.9700 | C10B—C11B | 1.378 (4) |
| C3B—C4B | 1.527 (5) | C10B—H10B | 0.9300 |
| C3B—H3C | 0.9700 | C12A—H12A | 0.9600 |
| C3B—H3D | 0.9700 | C12A—H12B | 0.9600 |
| C4A—N5A | 1.482 (3) | C12A—H12C | 0.9600 |
| C4A—C12A | 1.495 (5) | C12B—H12D | 0.9600 |
| C4A—H4A | 0.9800 | C12B—H12E | 0.9600 |
| C4B—N5B | 1.469 (4) | C12B—H12F | 0.9600 |
| C4B—C12B | 1.515 (5) | C13A—C14A | 1.526 (4) |
| C4B—H4B | 0.9800 | C13B—C14B | 1.540 (5) |
| N5A—C13A | 1.338 (4) | C14A—H14A | 0.9800 |
| N5A—C6A | 1.431 (3) | C14B—H14B | 0.9800 |
| H1W—O3—H2W | 106 (5) | C7A—C8A—C9A | 119.9 (3) |
| C2A—N1A—C11A | 125.0 (2) | C7A—C8A—H8A | 120.1 |
| C2A—N1A—H1A | 117 (2) | C9A—C8A—H8A | 120.1 |
| C11A—N1A—H1A | 117 (2) | C7B—C8B—C9B | 119.4 (3) |
| C2B—N1B—C11B | 126.2 (3) | C7B—C8B—H8B | 120.3 |
| C2B—N1B—H1B | 114 (2) | C9B—C8B—H8B | 120.3 |
| C11B—N1B—H1B | 119 (2) | C10A—C9A—C8A | 120.9 (3) |
| O1A—C2A—N1A | 120.5 (3) | C10A—C9A—H9A | 119.6 |
| O1A—C2A—C3A | 123.0 (3) | C8A—C9A—H9A | 119.6 |
| N1A—C2A—C3A | 116.4 (3) | C10B—C9B—C8B | 120.5 (3) |
| O1B—C2B—N1B | 121.9 (3) | C10B—C9B—H9B | 119.7 |
| O1B—C2B—C3B | 122.0 (3) | C8B—C9B—H9B | 119.7 |
| N1B—C2B—C3B | 116.1 (2) | C9A—C10A—C11A | 120.2 (3) |
| C2A—C3A—C4A | 115.1 (2) | C9A—C10A—H10A | 119.9 |
| C2A—C3A—H3A | 108.5 | C11A—C10A—H10A | 119.9 |
| C4A—C3A—H3A | 108.5 | C9B—C10B—C11B | 120.4 (3) |
| C2A—C3A—H3B | 108.5 | C9B—C10B—H10B | 119.8 |
| C4A—C3A—H3B | 108.5 | C11B—C10B—H10B | 119.8 |
| H3A—C3A—H3B | 107.5 | C10A—C11A—C6A | 118.8 (2) |
| C2B—C3B—C4B | 113.3 (2) | C10A—C11A—N1A | 120.8 (3) |
| C2B—C3B—H3C | 108.9 | C6A—C11A—N1A | 120.4 (2) |
| C4B—C3B—H3C | 108.9 | C10B—C11B—C6B | 119.0 (3) |
| C2B—C3B—H3D | 108.9 | C10B—C11B—N1B | 120.6 (3) |
| C4B—C3B—H3D | 108.9 | C6B—C11B—N1B | 120.3 (2) |
| H3C—C3B—H3D | 107.7 | C4A—C12A—H12A | 109.5 |
| N5A—C4A—C12A | 111.1 (3) | C4A—C12A—H12B | 109.5 |
| N5A—C4A—C3A | 109.3 (2) | H12A—C12A—H12B | 109.5 |
| C12A—C4A—C3A | 111.8 (3) | C4A—C12A—H12C | 109.5 |
| N5A—C4A—H4A | 108.2 | H12A—C12A—H12C | 109.5 |
| C12A—C4A—H4A | 108.2 | H12B—C12A—H12C | 109.5 |
| C3A—C4A—H4A | 108.2 | C4B—C12B—H12D | 109.5 |
| N5B—C4B—C12B | 110.4 (3) | C4B—C12B—H12E | 109.5 |
| N5B—C4B—C3B | 109.4 (2) | H12D—C12B—H12E | 109.5 |
| C12B—C4B—C3B | 112.3 (3) | C4B—C12B—H12F | 109.5 |
| N5B—C4B—H4B | 108.2 | H12D—C12B—H12F | 109.5 |
| C12B—C4B—H4B | 108.2 | H12E—C12B—H12F | 109.5 |
| C3B—C4B—H4B | 108.2 | O2A—C13A—N5A | 123.3 (3) |
| C13A—N5A—C6A | 123.9 (2) | O2A—C13A—C14A | 119.7 (3) |
| C13A—N5A—C4A | 116.8 (2) | N5A—C13A—C14A | 116.9 (2) |
| C6A—N5A—C4A | 118.1 (2) | O2B—C13B—N5B | 122.9 (3) |
| C13B—N5B—C6B | 122.4 (3) | O2B—C13B—C14B | 120.4 (3) |
| C13B—N5B—C4B | 118.4 (2) | N5B—C13B—C14B | 116.5 (3) |
| C6B—N5B—C4B | 119.1 (2) | C13A—C14A—Cl2A | 108.8 (2) |
| C7A—C6A—C11A | 119.8 (2) | C13A—C14A—Cl1A | 108.0 (2) |
| C7A—C6A—N5A | 120.2 (3) | Cl2A—C14A—Cl1A | 110.73 (17) |
| C11A—C6A—N5A | 120.0 (2) | C13A—C14A—H14A | 109.7 |
| C7B—C6B—C11B | 120.2 (2) | Cl2A—C14A—H14A | 109.7 |
| C7B—C6B—N5B | 120.9 (3) | Cl1A—C14A—H14A | 109.7 |
| C11B—C6B—N5B | 118.8 (2) | C13B—C14B—Cl2B | 109.4 (3) |
| C8A—C7A—C6A | 120.4 (3) | C13B—C14B—Cl1B | 107.7 (2) |
| C8A—C7A—H7A | 119.8 | Cl2B—C14B—Cl1B | 109.9 (2) |
| C6A—C7A—H7A | 119.8 | C13B—C14B—H14B | 109.9 |
| C8B—C7B—C6B | 120.2 (3) | Cl2B—C14B—H14B | 109.9 |
| C8B—C7B—H7B | 119.9 | Cl1B—C14B—H14B | 109.9 |
| C6B—C7B—H7B | 119.9 | ||
| C11A—N1A—C2A—O1A | 173.3 (3) | C7B—C8B—C9B—C10B | −2.6 (5) |
| C11A—N1A—C2A—C3A | −9.3 (4) | C8A—C9A—C10A—C11A | 0.6 (5) |
| C11B—N1B—C2B—O1B | 177.0 (3) | C8B—C9B—C10B—C11B | 2.3 (5) |
| C11B—N1B—C2B—C3B | −5.4 (4) | C9A—C10A—C11A—C6A | −0.2 (4) |
| O1A—C2A—C3A—C4A | −106.8 (3) | C9A—C10A—C11A—N1A | 177.8 (3) |
| N1A—C2A—C3A—C4A | 75.9 (3) | C7A—C6A—C11A—C10A | −1.4 (4) |
| O1B—C2B—C3B—C4B | −107.2 (3) | N5A—C6A—C11A—C10A | −179.7 (2) |
| N1B—C2B—C3B—C4B | 75.2 (3) | C7A—C6A—C11A—N1A | −179.4 (2) |
| C2A—C3A—C4A—N5A | −40.6 (4) | N5A—C6A—C11A—N1A | 2.2 (4) |
| C2A—C3A—C4A—C12A | −164.0 (3) | C2A—N1A—C11A—C10A | 138.6 (3) |
| C2B—C3B—C4B—N5B | −46.2 (3) | C2A—N1A—C11A—C6A | −43.4 (4) |
| C2B—C3B—C4B—C12B | −169.2 (3) | C9B—C10B—C11B—C6B | 1.6 (4) |
| C12A—C4A—N5A—C13A | −90.1 (3) | C9B—C10B—C11B—N1B | 177.9 (3) |
| C3A—C4A—N5A—C13A | 146.1 (3) | C7B—C6B—C11B—C10B | −5.2 (4) |
| C12A—C4A—N5A—C6A | 78.1 (3) | N5B—C6B—C11B—C10B | 172.3 (3) |
| C3A—C4A—N5A—C6A | −45.7 (3) | C7B—C6B—C11B—N1B | 178.5 (3) |
| C12B—C4B—N5B—C13B | −102.0 (3) | N5B—C6B—C11B—N1B | −3.9 (4) |
| C3B—C4B—N5B—C13B | 134.0 (3) | C2B—N1B—C11B—C10B | 141.2 (3) |
| C12B—C4B—N5B—C6B | 83.0 (3) | C2B—N1B—C11B—C6B | −42.6 (4) |
| C3B—C4B—N5B—C6B | −41.1 (3) | C6A—N5A—C13A—O2A | −164.1 (3) |
| C13A—N5A—C6A—C7A | 58.9 (4) | C4A—N5A—C13A—O2A | 3.3 (4) |
| C4A—N5A—C6A—C7A | −108.3 (3) | C6A—N5A—C13A—C14A | 21.3 (4) |
| C13A—N5A—C6A—C11A | −122.8 (3) | C4A—N5A—C13A—C14A | −171.3 (3) |
| C4A—N5A—C6A—C11A | 70.0 (3) | C6B—N5B—C13B—O2B | −179.7 (3) |
| C13B—N5B—C6B—C7B | 75.4 (4) | C4B—N5B—C13B—O2B | 5.4 (4) |
| C4B—N5B—C6B—C7B | −109.8 (3) | C6B—N5B—C13B—C14B | 4.5 (4) |
| C13B—N5B—C6B—C11B | −102.1 (3) | C4B—N5B—C13B—C14B | −170.4 (3) |
| C4B—N5B—C6B—C11B | 72.7 (3) | O2A—C13A—C14A—Cl2A | 53.9 (3) |
| C11A—C6A—C7A—C8A | 2.6 (4) | N5A—C13A—C14A—Cl2A | −131.2 (2) |
| N5A—C6A—C7A—C8A | −179.0 (3) | O2A—C13A—C14A—Cl1A | −66.3 (3) |
| C11B—C6B—C7B—C8B | 4.9 (5) | N5A—C13A—C14A—Cl1A | 108.5 (3) |
| N5B—C6B—C7B—C8B | −172.6 (3) | O2B—C13B—C14B—Cl2B | 27.4 (4) |
| C6A—C7A—C8A—C9A | −2.3 (5) | N5B—C13B—C14B—Cl2B | −156.7 (2) |
| C6B—C7B—C8B—C9B | −1.0 (5) | O2B—C13B—C14B—Cl1B | −92.0 (3) |
| C7A—C8A—C9A—C10A | 0.7 (5) | N5B—C13B—C14B—Cl1B | 84.0 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C4A—H4A···O2A | 0.98 | 2.34 | 2.694 (4) | 100 |
| C4B—H4B···O2B | 0.98 | 2.31 | 2.715 (4) | 104 |
| N1A—H1A···O3 | 0.87 (4) | 2.08 (4) | 2.927 (4) | 164 (3) |
| O3—H2W···O1Bi | 0.80 (4) | 2.02 (4) | 2.815 (4) | 173 (4) |
| C3A—H3A···O2Bi | 0.97 | 2.60 | 3.038 (4) | 108 |
| C8A—H8A···O2Aii | 0.93 | 2.51 | 3.268 (4) | 139 |
| C10B—H10B···O2Biii | 0.93 | 2.39 | 3.179 (4) | 143 |
Symmetry codes: (i) −x+2, y−1/2, −z+2; (ii) x−1, y, z; (iii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5048).
References
- Albright, J. D., Feich, M. F., Santos, E. G. D., Dusza, J. P., Sum, F.-W., Venkatesan, A. M., Coupet, J., Chan, P. S., Ru, X., Mazandarani, H. & Bailey, T. (1998). J. Med. Chem.41, 2442–2444. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N. L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc. Madison, Wisconsin, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Gringauz, A. (1999). Introduction to Medicinal Chemistry, pp. 578–580. New York: Wiley-VCH.
- Lee, J., Gauthier, D. & Rivero, R. A. (1999). J. Org. Chem, 64, 3060–3064. [DOI] [PubMed]
- MacDonald, R. L. (2002). Benzodiazepines Mechanisms of Action. In Antiepileptic Drugs, 5th ed., edited by R. H. Levy, R. H. Mattson, B. S. Meldrum & E. Perucca, pp. 179–186. Philadelphia: Lippincott Williams and Wilkins.
- Nardelli, M. (1983). Acta Cryst. C39, 1141–1142.
- Rahbaek, L., Breinholt, J., Frisvad, J. C. & Christophersen, C. (1999). J. Org. Chem.64, 1689–1692. [DOI] [PubMed]
- Sheldrick, G. M. (2001). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst D65, 148–155. [DOI] [PMC free article] [PubMed]
- Venkatraj, M., Ponnuswamy, S. & Jeyaraman, R. (2008). Indian J. Chem. Sect. B, 47, 129–135.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809036940/bt5048sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809036940/bt5048Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report