Abstract
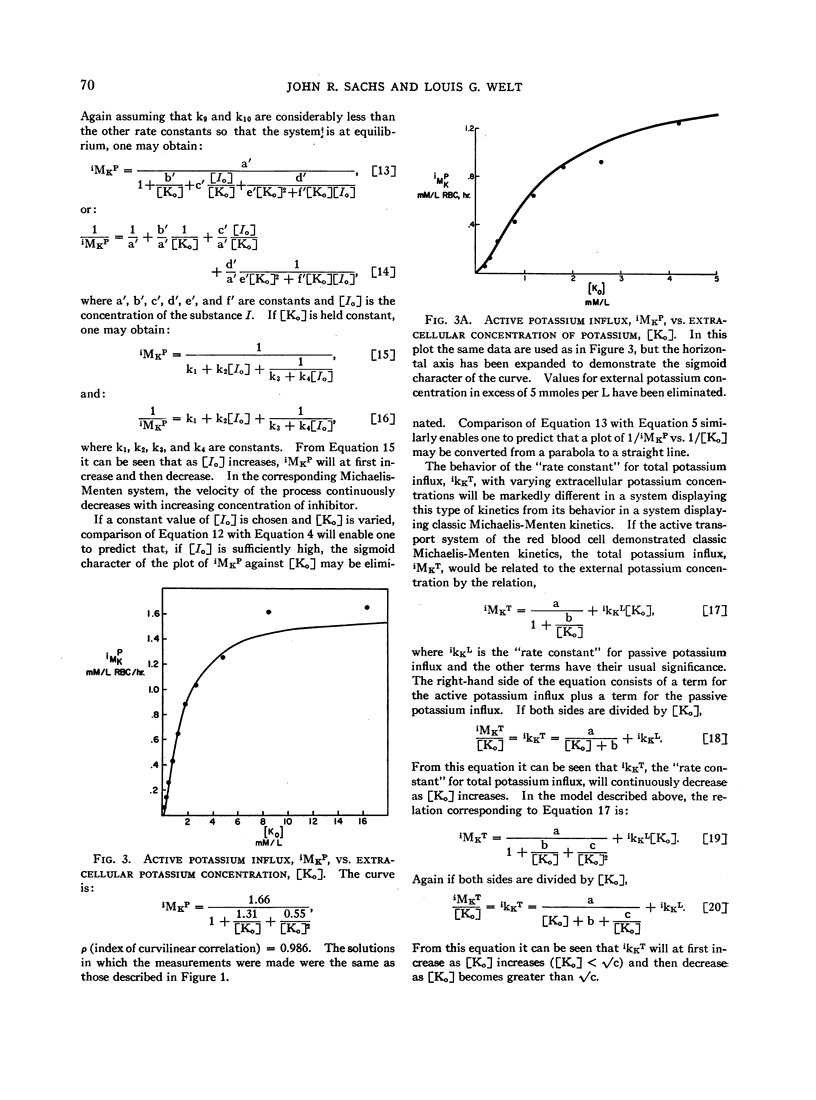
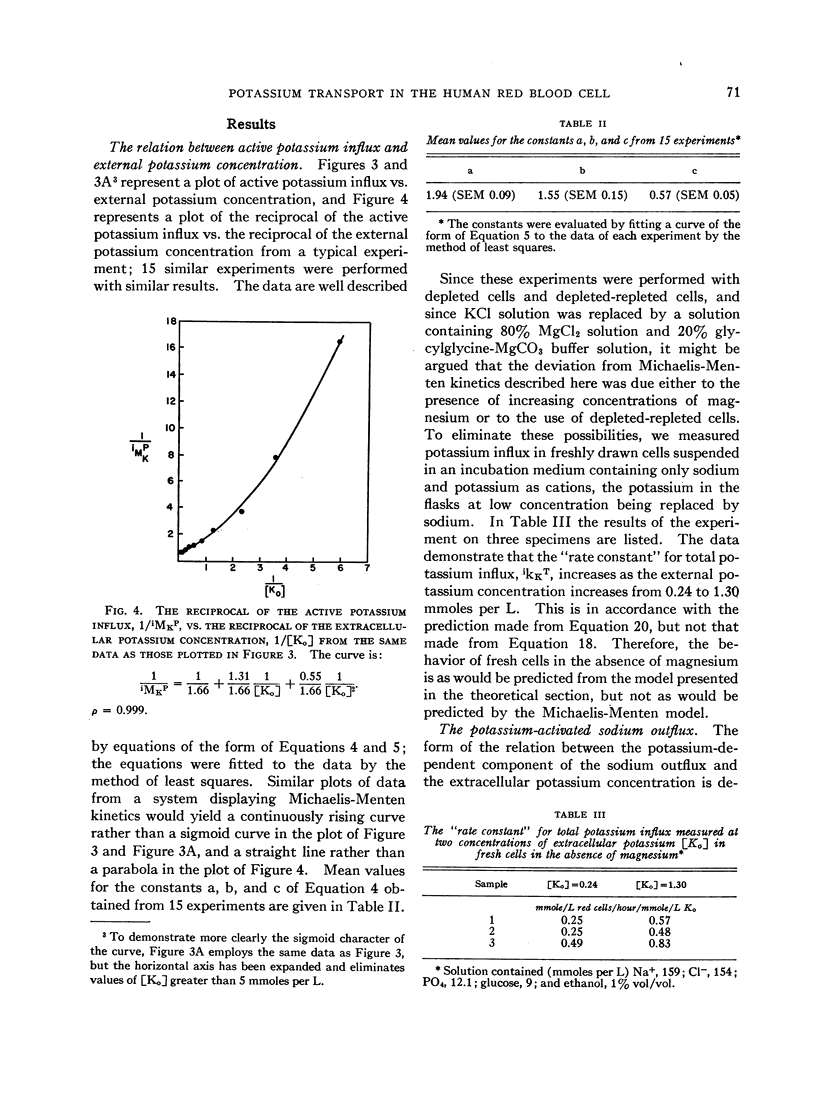
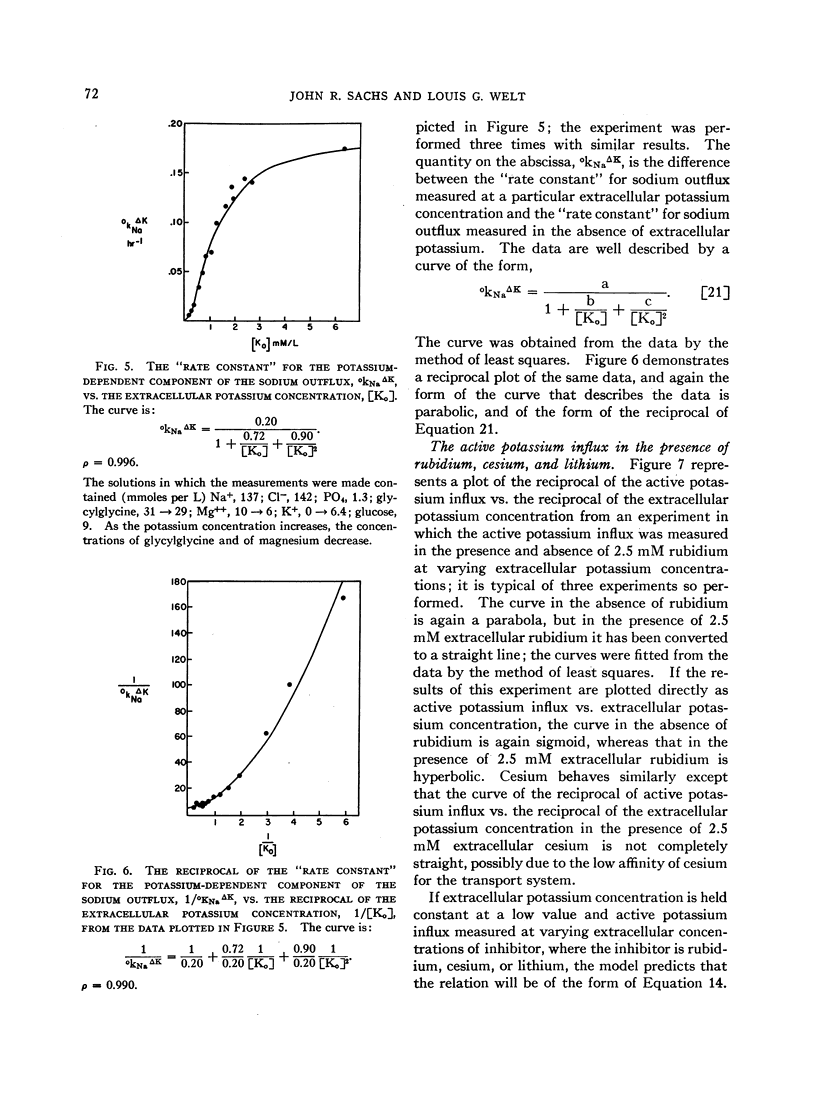
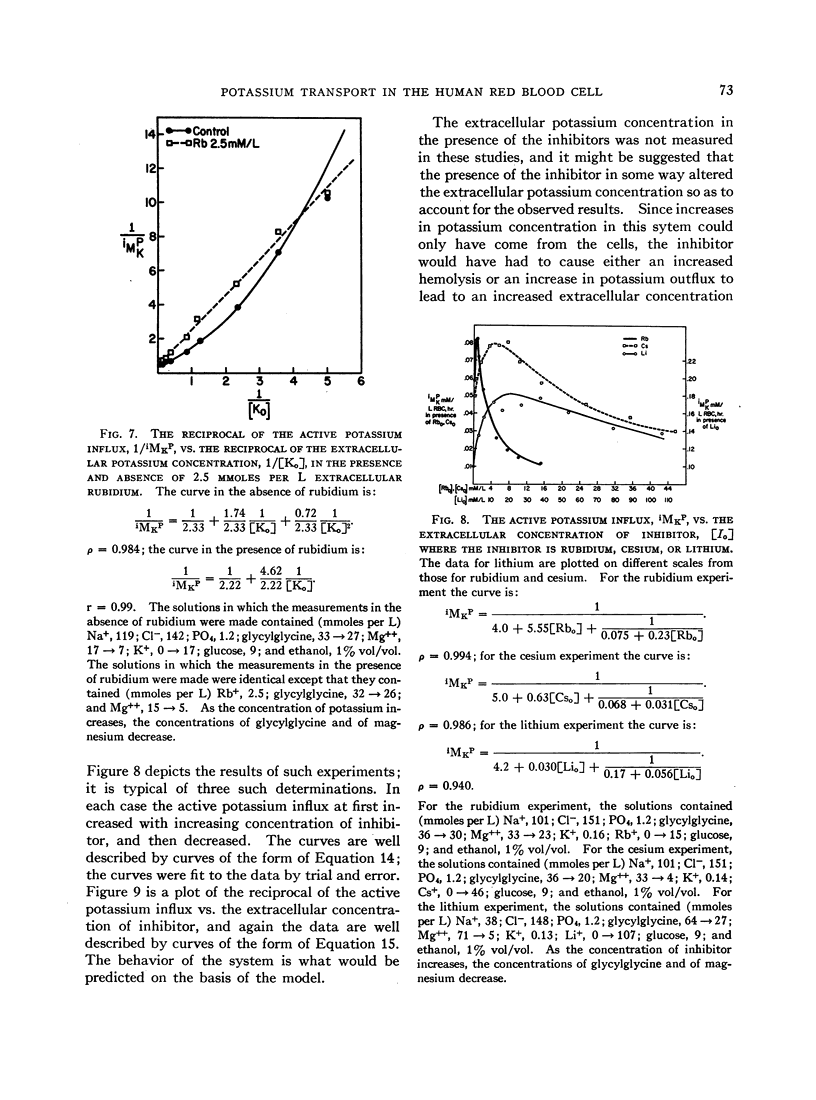
The relation between the active potassium influx in the human red blood cell and the extracellular potassium concentration does not appear to be consistent with the Michaelis-Menten model, but is adequately described by a model in which two potassium ions are required simultaneously at some site or sites in the transport mechanism before transport occurs. The same type of relation appears to exist between that portion of the sodium outflux that requires the presence of extracellular potassium and the extracellular potassium concentration. Rubidium, cesium, and lithium, which are apparently transported by the same system that transports potassium, stimulate the potassium influx when both potassium and the second ion are present at low concentrations, as is predicted by the two-site model.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FLYNN F., MAIZELS M. Cation control in human erythrocytes. J Physiol. 1949 Dec;110(3-4):301–318. doi: 10.1113/jphysiol.1949.sp004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., YODA A. EFFECTS OF ADENOSINE TRIPHOSPHATE, POTASSIUM, AND STROPHANTHIDIN ON THE INHIBITION OF A OUABAIN-SENSITIVE ADENOSINE TRIPHOSPHATASE BY DIISOPROPYLFLUOROPHOSPHATE. Biochim Biophys Acta. 1965 Mar 8;97:594–595. doi: 10.1016/0304-4165(65)90175-3. [DOI] [PubMed] [Google Scholar]
- JACQUEZ J. A. Carrier-amino acid stoichiometry in amino acid transport in Ehrlich ascites cells. Biochim Biophys Acta. 1963 Apr;71:15–33. doi: 10.1016/0006-3002(63)90981-8. [DOI] [PubMed] [Google Scholar]
- JACQUEZ J. A. THE KINETICS OF CARRIER-MEDIATED TRANSPORT: STATIONARY-STATE APPROXIMATIONS. Biochim Biophys Acta. 1964 Mar 30;79:318–328. [PubMed] [Google Scholar]
- KAHN J. B., Jr The entry of rubidium into human erythrocytes. J Pharmacol Exp Ther. 1962 May;136:197–204. [PubMed] [Google Scholar]
- KEYNES R. D. SOME FURTHER OBSERVATIONS ON THE SODIUM EFFLUX IN FROG MUSCLE. J Physiol. 1965 May;178:305–325. doi: 10.1113/jphysiol.1965.sp007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVE W. D., BURCH G. E. A comparison of potassium 42, rubidium 86, and cesium 134 as tracers of potassium in the study of cation metabolism of human erythrocytes in vitro. J Lab Clin Med. 1953 Mar;41(3):351–362. [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., FRUMENTO A. S. The concentration dependence of sodium efflux from muscle. J Gen Physiol. 1963 Mar;46:629–654. doi: 10.1085/jgp.46.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaghey P. D., Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962 Aug;162(3):485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNISHI T. Extraction of actin- and myosin-like proteins from erythrocyte membrane. J Biochem. 1962 Oct;52:307–308. doi: 10.1093/oxfordjournals.jbchem.a127620. [DOI] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SHAW T. I. Potassium movements in washed erythrocytes. J Physiol. 1955 Sep 28;129(3):464–475. doi: 10.1113/jphysiol.1955.sp005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOMON A. K. The permeability of the human erythrocyte to sodium and potassium. J Gen Physiol. 1952 May;36(1):57–110. doi: 10.1085/jgp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]


