Abstract
The title compound, {[Zn(C14H16N5O3)2]·2H2O}n or [Zn(ppa)2]·2H2O}n, where ppa = 8-ethyl-5,8-dihydro-5-oxo-2-(1-piperazinyl)-pyrido(2,3-d)-pyrimidine-6-carboxylate, was synthesized under hydrothermal conditions. The ZnII atom (site symmetry  ) exhibits a distorted trans-ZnN2O4 octahedral geometry defined by two monodentate N-bonded and two bidentate O,O-bonded ppa monoanions. The extended two-dimensional structure arising from this connectivity is a square grid and the disordered uncoordinated water molecules occupy cavities within the grid. An N—H⋯O hydrogen bond occurs.
) exhibits a distorted trans-ZnN2O4 octahedral geometry defined by two monodentate N-bonded and two bidentate O,O-bonded ppa monoanions. The extended two-dimensional structure arising from this connectivity is a square grid and the disordered uncoordinated water molecules occupy cavities within the grid. An N—H⋯O hydrogen bond occurs.
Related literature
For manganese complexes of the ppa anion, see: Huang et al. (2008 ▶). For background to the medicinal uses of pipemidic acid, see: Mizuki et al. (1996 ▶).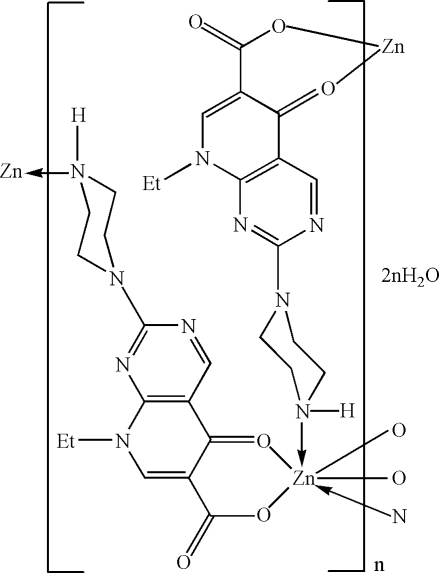
Experimental
Crystal data
[Zn(C14H16N5O3)2]·2H2O
M r = 704.05
Monoclinic,
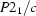
a = 6.1146 (12) Å
b = 21.424 (4) Å
c = 12.577 (3) Å
β = 101.10 (3)°
V = 1616.9 (6) Å3
Z = 2
Mo Kα radiation
μ = 0.82 mm−1
T = 295 K
0.36 × 0.28 × 0.18 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: multi-scan (CrystalStructure; Rigaku/MSC, 2002 ▶) T min = 0.756, T max = 0.866
15697 measured reflections
3684 independent reflections
2570 reflections with I > 2σ(I)
R int = 0.045
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.210
S = 1.06
3684 reflections
228 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.83 e Å−3
Δρmin = −0.83 e Å−3
Data collection: RAPID-AUTO (Rigaku, 1998 ▶); cell refinement: RAPID-AUTO; data reduction: CrystalStructure (Rigaku/MSC, 2002 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPII (Johnson, 1976 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809036939/hb5062sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809036939/hb5062Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Zn1—O1 | 2.031 (3) |
| Zn1—O3 | 2.107 (2) |
| Zn1—N5i | 2.275 (3) |
Symmetry code: (i) 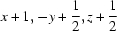 .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H5N⋯O2ii | 0.91 (5) | 2.28 (5) | 3.168 (5) | 166 (4) |
Symmetry code: (ii) 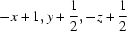 .
.
Acknowledgments
The authors thank the Innovation Science Foundation of Harbin Medical University for financial support (grant No. 060041).
supplementary crystallographic information
Comment
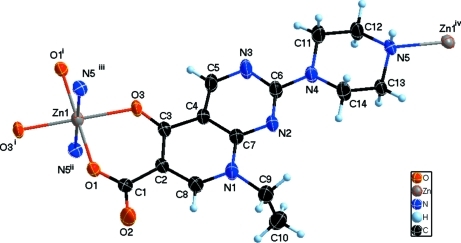
Pipemidic acid (Hppa, C14H16N5O3, 8-Ethyl-5,8-dihydro-5-oxo-2- (1-piperazinyl)-pyrido(2,3 - d)-pyrimidine-6-carboxylic acid) is member of a class of quinolones used to treat infections (Mizuki et al., 1996). The manganese complex of the ppa anion has been reported (Huang et al., 2008); the title zinc(II) complex is reported here (Fig. 1).
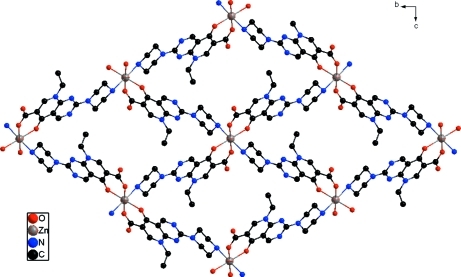
The zinc(II) atom is coordinated by four oxygen atoms and two N atoms from four ppa ligands (two monodentate-N and two O,O-bidentate) to form a square grid (Fig. 2). The disordered, uncoordinated, water molecules occupy the cavities.
Experimental
A mixture of Zn(CH3COO)2.2H2O (0.055 g, 0.25 mmol), Hppa (0.15 g, 0.5 mmol), sodium hydroxide (0.04 g, 1 mmol) and water (12 ml) was stirred for 30 min in air. The mixture was then transferred to a 23 ml Teflon-lined hydrothermal bomb. The bomb was kept at 433 K for 72 h under autogenous pressure. Upon cooling, colourless prisms of (I) were obtained from the reaction mixture.
Refinement
The carbon-bound H atoms were positioned geometrically (C—H = 0.93–0.97 Å) and were included in the refinement in the riding model approximation, with Uiso(H) = 1.2Ueq(C). The H on Nitrogen atoms were located in a difference Fourier map, and were refined with a distance restraint of N—H = 0.86 (1)Å and with Uiso(H) = 1.2Ueq(N).
The water H atoms could not be placed due to the disorder of the O atoms.
Figures
Fig. 1.
The asymmetric unit of (I) extended to show the zinc coordination sphere showing the showing 50% displacement ellipsoids (water molecule O atoms have been omitted for clarity).
Fig. 2.
A view of part of a two-dimensional polymeric sheet in (I) showing the square-grid connectivity (H atoms and water molecule O atoms omitted for clarity).
Crystal data
| [Zn(C14H16N5O3)2]·2H2O | Z = 2 |
| Mr = 704.05 | F(000) = 728 |
| Monoclinic, P21/c | Dx = 1.442 Mg m−3 |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.1146 (12) Å | µ = 0.82 mm−1 |
| b = 21.424 (4) Å | T = 295 K |
| c = 12.577 (3) Å | Prism, colorless |
| β = 101.10 (3)° | 0.36 × 0.28 × 0.18 mm |
| V = 1616.9 (6) Å3 |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 3684 independent reflections |
| Radiation source: fine-focus sealed tube | 2570 reflections with I > 2σ(I) |
| graphite | Rint = 0.045 |
| Detector resolution: 10.000 pixels mm-1 | θmax = 27.5°, θmin = 3.3° |
| ω scans | h = −7→7 |
| Absorption correction: multi-scan (CrystalStructure; Rigaku/MSC, 2002) | k = −27→25 |
| Tmin = 0.756, Tmax = 0.866 | l = −16→16 |
| 15697 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.057 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.210 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.1254P)2 + 1.7801P] where P = (Fo2 + 2Fc2)/3 |
| 3684 reflections | (Δ/σ)max < 0.001 |
| 228 parameters | Δρmax = 0.83 e Å−3 |
| 1 restraint | Δρmin = −0.83 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1W | −0.045 (3) | −0.0632 (10) | −0.0746 (11) | 0.187 (8) | 0.50 |
| O2W | 0.340 (5) | 0.0205 (10) | −0.0364 (12) | 0.251 (14) | 0.50 |
| Zn1 | 0.5000 | 0.0000 | 0.5000 | 0.0265 (2) | |
| O1 | 0.6981 (4) | −0.00325 (10) | 0.3877 (2) | 0.0271 (6) | |
| O2 | 0.8573 (7) | 0.01818 (18) | 0.2500 (3) | 0.0616 (11) | |
| O3 | 0.3495 (5) | 0.07935 (11) | 0.4179 (2) | 0.0317 (6) | |
| N1 | 0.4916 (7) | 0.17173 (17) | 0.1532 (3) | 0.0481 (10) | |
| N2 | 0.2252 (6) | 0.24690 (15) | 0.1677 (3) | 0.0386 (8) | |
| N3 | −0.0127 (6) | 0.23572 (16) | 0.2988 (3) | 0.0436 (9) | |
| N4 | −0.0227 (6) | 0.32384 (15) | 0.1907 (3) | 0.0349 (8) | |
| N5 | −0.2450 (5) | 0.43908 (14) | 0.1084 (2) | 0.0273 (7) | |
| H5N | −0.154 (8) | 0.466 (2) | 0.152 (4) | 0.065 (16)* | |
| C1 | 0.7147 (7) | 0.02891 (17) | 0.3064 (3) | 0.0316 (8) | |
| C2 | 0.5658 (7) | 0.08450 (17) | 0.2772 (3) | 0.0317 (8) | |
| C3 | 0.3947 (6) | 0.10453 (16) | 0.3346 (3) | 0.0271 (7) | |
| C4 | 0.2744 (7) | 0.15974 (16) | 0.2910 (3) | 0.0303 (8) | |
| C5 | 0.0937 (8) | 0.18359 (19) | 0.3318 (3) | 0.0398 (10) | |
| H5 | 0.0454 | 0.1610 | 0.3861 | 0.048* | |
| C6 | 0.0671 (7) | 0.26762 (18) | 0.2197 (3) | 0.0327 (8) | |
| C7 | 0.3246 (7) | 0.19340 (18) | 0.2034 (3) | 0.0360 (9) | |
| C8 | 0.6010 (8) | 0.1189 (2) | 0.1902 (4) | 0.0457 (11) | |
| H8 | 0.7096 | 0.1047 | 0.1535 | 0.055* | |
| C9 | 0.5540 (11) | 0.2051 (3) | 0.0585 (5) | 0.0665 (16) | |
| H9B | 0.5306 | 0.2496 | 0.0653 | 0.080* | |
| H9A | 0.7107 | 0.1983 | 0.0582 | 0.080* | |
| C10 | 0.4247 (16) | 0.1834 (5) | −0.0401 (7) | 0.116 (3) | |
| H10C | 0.4566 | 0.1401 | −0.0496 | 0.174* | |
| H10B | 0.4605 | 0.2071 | −0.0993 | 0.174* | |
| H10A | 0.2693 | 0.1883 | −0.0385 | 0.174* | |
| C11 | −0.1608 (8) | 0.3572 (2) | 0.2553 (4) | 0.0475 (11) | |
| H11B | −0.0673 | 0.3839 | 0.3077 | 0.057* | |
| H11A | −0.2346 | 0.3275 | 0.2947 | 0.057* | |
| C12 | −0.3356 (7) | 0.3970 (2) | 0.1813 (4) | 0.0398 (10) | |
| H12B | −0.4429 | 0.3694 | 0.1379 | 0.048* | |
| H12A | −0.4150 | 0.4218 | 0.2262 | 0.048* | |
| C13 | −0.1090 (6) | 0.40176 (17) | 0.0469 (3) | 0.0317 (8) | |
| H13A | −0.0405 | 0.4295 | 0.0018 | 0.038* | |
| H13B | −0.2057 | 0.3734 | −0.0006 | 0.038* | |
| C14 | 0.0708 (7) | 0.36460 (18) | 0.1185 (4) | 0.0369 (9) | |
| H14B | 0.1500 | 0.3397 | 0.0737 | 0.044* | |
| H14A | 0.1768 | 0.3929 | 0.1610 | 0.044* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1W | 0.209 (18) | 0.28 (2) | 0.094 (9) | −0.004 (16) | 0.091 (11) | 0.013 (12) |
| O2W | 0.47 (4) | 0.202 (17) | 0.077 (9) | 0.11 (2) | 0.049 (16) | −0.057 (12) |
| Zn1 | 0.0317 (4) | 0.0194 (3) | 0.0294 (3) | 0.0006 (2) | 0.0079 (2) | 0.0028 (2) |
| O1 | 0.0276 (13) | 0.0218 (12) | 0.0333 (14) | 0.0019 (9) | 0.0089 (10) | 0.0029 (10) |
| O2 | 0.073 (3) | 0.062 (2) | 0.060 (2) | 0.040 (2) | 0.0387 (19) | 0.0275 (18) |
| O3 | 0.0378 (15) | 0.0223 (12) | 0.0368 (14) | 0.0092 (10) | 0.0116 (11) | 0.0121 (11) |
| N1 | 0.065 (3) | 0.0398 (19) | 0.046 (2) | 0.0223 (18) | 0.0265 (19) | 0.0195 (17) |
| N2 | 0.051 (2) | 0.0282 (16) | 0.0400 (18) | 0.0135 (15) | 0.0160 (16) | 0.0124 (14) |
| N3 | 0.044 (2) | 0.0361 (18) | 0.055 (2) | 0.0166 (16) | 0.0219 (17) | 0.0231 (17) |
| N4 | 0.0390 (19) | 0.0283 (16) | 0.0415 (18) | 0.0096 (14) | 0.0177 (15) | 0.0119 (14) |
| N5 | 0.0265 (16) | 0.0219 (14) | 0.0324 (15) | 0.0064 (12) | 0.0028 (12) | 0.0034 (13) |
| C1 | 0.037 (2) | 0.0274 (18) | 0.0316 (18) | 0.0046 (15) | 0.0112 (16) | −0.0002 (16) |
| C2 | 0.038 (2) | 0.0238 (17) | 0.0354 (19) | 0.0070 (15) | 0.0110 (16) | 0.0035 (15) |
| C3 | 0.0283 (18) | 0.0219 (16) | 0.0302 (17) | 0.0007 (13) | 0.0032 (14) | 0.0013 (14) |
| C4 | 0.036 (2) | 0.0242 (17) | 0.0318 (18) | 0.0021 (15) | 0.0087 (15) | 0.0055 (15) |
| C5 | 0.048 (3) | 0.033 (2) | 0.043 (2) | 0.0088 (18) | 0.0188 (19) | 0.0174 (18) |
| C6 | 0.033 (2) | 0.0273 (18) | 0.0380 (19) | 0.0046 (15) | 0.0078 (16) | 0.0077 (16) |
| C7 | 0.044 (2) | 0.0323 (19) | 0.0347 (19) | 0.0092 (17) | 0.0137 (17) | 0.0067 (17) |
| C8 | 0.060 (3) | 0.037 (2) | 0.046 (2) | 0.018 (2) | 0.026 (2) | 0.0107 (19) |
| C9 | 0.083 (4) | 0.063 (3) | 0.062 (3) | 0.030 (3) | 0.038 (3) | 0.024 (3) |
| C10 | 0.117 (7) | 0.144 (9) | 0.090 (5) | 0.031 (6) | 0.028 (5) | 0.024 (6) |
| C11 | 0.055 (3) | 0.045 (2) | 0.047 (2) | 0.025 (2) | 0.021 (2) | 0.020 (2) |
| C12 | 0.040 (2) | 0.036 (2) | 0.047 (2) | 0.0157 (17) | 0.0179 (18) | 0.0161 (19) |
| C13 | 0.035 (2) | 0.0247 (17) | 0.0366 (19) | 0.0101 (15) | 0.0094 (16) | 0.0081 (15) |
| C14 | 0.033 (2) | 0.0293 (18) | 0.051 (2) | 0.0043 (16) | 0.0147 (17) | 0.0119 (18) |
Geometric parameters (Å, °)
| Zn1—O1 | 2.031 (3) | C2—C8 | 1.370 (6) |
| Zn1—O1i | 2.031 (3) | C2—C3 | 1.446 (5) |
| Zn1—O3i | 2.107 (2) | C3—C4 | 1.444 (5) |
| Zn1—O3 | 2.107 (2) | C4—C7 | 1.399 (5) |
| Zn1—N5ii | 2.275 (3) | C4—C5 | 1.401 (6) |
| Zn1—N5iii | 2.275 (3) | C5—H5 | 0.9300 |
| O1—C1 | 1.253 (5) | C8—H8 | 0.9300 |
| O2—C1 | 1.247 (5) | C9—C10 | 1.415 (11) |
| O3—C3 | 1.256 (4) | C9—H9B | 0.9700 |
| N1—C8 | 1.351 (5) | C9—H9A | 0.9700 |
| N1—C7 | 1.381 (5) | C10—H10C | 0.9600 |
| N1—C9 | 1.500 (6) | C10—H10B | 0.9600 |
| N2—C7 | 1.334 (5) | C10—H10A | 0.9600 |
| N2—C6 | 1.343 (5) | C11—C12 | 1.534 (5) |
| N3—C5 | 1.319 (5) | C11—H11B | 0.9700 |
| N3—C6 | 1.373 (5) | C11—H11A | 0.9700 |
| N4—C6 | 1.345 (5) | C12—H12B | 0.9700 |
| N4—C14 | 1.454 (5) | C12—H12A | 0.9700 |
| N4—C11 | 1.466 (5) | C13—C14 | 1.508 (5) |
| N5—C12 | 1.468 (5) | C13—H13A | 0.9700 |
| N5—C13 | 1.475 (5) | C13—H13B | 0.9700 |
| N5—Zn1iv | 2.275 (3) | C14—H14B | 0.9700 |
| N5—H5N | 0.900 (10) | C14—H14A | 0.9700 |
| C1—C2 | 1.501 (5) | ||
| O1—Zn1—O1i | 180.0 | N2—C6—N4 | 117.3 (3) |
| O1—Zn1—O3i | 92.90 (10) | N2—C6—N3 | 125.3 (4) |
| O1i—Zn1—O3i | 87.10 (10) | N4—C6—N3 | 117.3 (4) |
| O1—Zn1—O3 | 87.10 (10) | N2—C7—N1 | 117.6 (3) |
| O1i—Zn1—O3 | 92.90 (10) | N2—C7—C4 | 123.6 (4) |
| O3i—Zn1—O3 | 180.0 | N1—C7—C4 | 118.7 (3) |
| O1—Zn1—N5ii | 89.74 (11) | N1—C8—C2 | 125.6 (4) |
| O1i—Zn1—N5ii | 90.26 (11) | N1—C8—H8 | 117.2 |
| O3i—Zn1—N5ii | 90.86 (11) | C2—C8—H8 | 117.2 |
| O3—Zn1—N5ii | 89.14 (11) | C10—C9—N1 | 110.8 (7) |
| O1—Zn1—N5iii | 90.26 (11) | C10—C9—H9B | 109.5 |
| O1i—Zn1—N5iii | 89.74 (11) | N1—C9—H9B | 109.5 |
| O3i—Zn1—N5iii | 89.14 (11) | C10—C9—H9A | 109.5 |
| O3—Zn1—N5iii | 90.86 (11) | N1—C9—H9A | 109.5 |
| N5ii—Zn1—N5iii | 180.0 | H9B—C9—H9A | 108.1 |
| C1—O1—Zn1 | 134.5 (2) | C9—C10—H10C | 109.5 |
| C3—O3—Zn1 | 127.6 (2) | C9—C10—H10B | 109.5 |
| C8—N1—C7 | 119.0 (3) | H10C—C10—H10B | 109.5 |
| C8—N1—C9 | 119.2 (4) | C9—C10—H10A | 109.5 |
| C7—N1—C9 | 121.8 (4) | H10C—C10—H10A | 109.5 |
| C7—N2—C6 | 116.3 (3) | H10B—C10—H10A | 109.5 |
| C5—N3—C6 | 115.3 (4) | N4—C11—C12 | 110.0 (3) |
| C6—N4—C14 | 121.2 (3) | N4—C11—H11B | 109.7 |
| C6—N4—C11 | 122.4 (3) | C12—C11—H11B | 109.7 |
| C14—N4—C11 | 113.0 (3) | N4—C11—H11A | 109.7 |
| C12—N5—C13 | 108.3 (3) | C12—C11—H11A | 109.7 |
| C12—N5—Zn1iv | 115.4 (2) | H11B—C11—H11A | 108.2 |
| C13—N5—Zn1iv | 112.8 (2) | N5—C12—C11 | 114.7 (3) |
| C12—N5—H5N | 106 (4) | N5—C12—H12B | 108.6 |
| C13—N5—H5N | 108 (4) | C11—C12—H12B | 108.6 |
| Zn1iv—N5—H5N | 106 (4) | N5—C12—H12A | 108.6 |
| O2—C1—O1 | 122.6 (4) | C11—C12—H12A | 108.6 |
| O2—C1—C2 | 117.7 (3) | H12B—C12—H12A | 107.6 |
| O1—C1—C2 | 119.7 (3) | N5—C13—C14 | 113.1 (3) |
| C8—C2—C3 | 118.6 (3) | N5—C13—H13A | 109.0 |
| C8—C2—C1 | 116.2 (3) | C14—C13—H13A | 109.0 |
| C3—C2—C1 | 125.2 (3) | N5—C13—H13B | 109.0 |
| O3—C3—C4 | 119.4 (3) | C14—C13—H13B | 109.0 |
| O3—C3—C2 | 125.8 (3) | H13A—C13—H13B | 107.8 |
| C4—C3—C2 | 114.7 (3) | N4—C14—C13 | 111.2 (3) |
| C7—C4—C5 | 114.1 (3) | N4—C14—H14B | 109.4 |
| C7—C4—C3 | 123.2 (4) | C13—C14—H14B | 109.4 |
| C5—C4—C3 | 122.6 (3) | N4—C14—H14A | 109.4 |
| N3—C5—C4 | 124.7 (4) | C13—C14—H14A | 109.4 |
| N3—C5—H5 | 117.6 | H14B—C14—H14A | 108.0 |
| C4—C5—H5 | 117.6 | ||
| O1i—Zn1—O1—C1 | −50 (2) | C11—N4—C6—N2 | −167.0 (4) |
| O3i—Zn1—O1—C1 | 179.5 (4) | C14—N4—C6—N3 | 171.7 (4) |
| O3—Zn1—O1—C1 | −0.5 (4) | C11—N4—C6—N3 | 14.0 (6) |
| N5ii—Zn1—O1—C1 | 88.7 (4) | C5—N3—C6—N2 | 7.0 (7) |
| N5iii—Zn1—O1—C1 | −91.3 (4) | C5—N3—C6—N4 | −174.0 (4) |
| O1—Zn1—O3—C3 | 0.4 (3) | C6—N2—C7—N1 | −178.5 (4) |
| O1i—Zn1—O3—C3 | −179.6 (3) | C6—N2—C7—C4 | −1.0 (6) |
| O3i—Zn1—O3—C3 | 65 (100) | C8—N1—C7—N2 | 177.5 (4) |
| N5ii—Zn1—O3—C3 | −89.4 (3) | C9—N1—C7—N2 | −2.7 (7) |
| N5iii—Zn1—O3—C3 | 90.6 (3) | C8—N1—C7—C4 | −0.1 (7) |
| Zn1—O1—C1—O2 | 178.2 (3) | C9—N1—C7—C4 | 179.7 (5) |
| Zn1—O1—C1—C2 | 1.0 (6) | C5—C4—C7—N2 | 6.2 (6) |
| O2—C1—C2—C8 | −0.1 (6) | C3—C4—C7—N2 | −174.9 (4) |
| O1—C1—C2—C8 | 177.1 (4) | C5—C4—C7—N1 | −176.3 (4) |
| O2—C1—C2—C3 | −178.8 (4) | C3—C4—C7—N1 | 2.5 (6) |
| O1—C1—C2—C3 | −1.6 (6) | C7—N1—C8—C2 | −1.9 (8) |
| Zn1—O3—C3—C4 | −179.0 (2) | C9—N1—C8—C2 | 178.3 (5) |
| Zn1—O3—C3—C2 | −1.1 (5) | C3—C2—C8—N1 | 1.4 (7) |
| C8—C2—C3—O3 | −177.0 (4) | C1—C2—C8—N1 | −177.4 (4) |
| C1—C2—C3—O3 | 1.7 (6) | C8—N1—C9—C10 | 90.7 (7) |
| C8—C2—C3—C4 | 1.0 (6) | C7—N1—C9—C10 | −89.1 (7) |
| C1—C2—C3—C4 | 179.7 (4) | C6—N4—C11—C12 | −148.9 (4) |
| O3—C3—C4—C7 | 175.2 (4) | C14—N4—C11—C12 | 51.8 (5) |
| C2—C3—C4—C7 | −2.9 (6) | C13—N5—C12—C11 | 53.7 (5) |
| O3—C3—C4—C5 | −6.0 (6) | Zn1iv—N5—C12—C11 | −178.8 (3) |
| C2—C3—C4—C5 | 175.9 (4) | N4—C11—C12—N5 | −52.7 (5) |
| C6—N3—C5—C4 | −0.8 (7) | C12—N5—C13—C14 | −55.0 (4) |
| C7—C4—C5—N3 | −5.2 (7) | Zn1iv—N5—C13—C14 | 176.0 (2) |
| C3—C4—C5—N3 | 175.9 (4) | C6—N4—C14—C13 | 145.9 (4) |
| C7—N2—C6—N4 | 174.8 (4) | C11—N4—C14—C13 | −54.5 (5) |
| C7—N2—C6—N3 | −6.2 (7) | N5—C13—C14—N4 | 56.5 (5) |
| C14—N4—C6—N2 | −9.3 (6) |
Symmetry codes: (i) −x+1, −y, −z+1; (ii) −x, y−1/2, −z+1/2; (iii) x+1, −y+1/2, z+1/2; (iv) −x, y+1/2, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H5N···O2v | 0.91 (5) | 2.28 (5) | 3.168 (5) | 166 (4) |
Symmetry codes: (v) −x+1, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB5062).
References
- Huang, J., Hu, W.-P. & An, Z. (2008). Acta Cryst. E64, m547. [DOI] [PMC free article] [PubMed]
- Johnson, C. K. (1976). ORTEPII Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- Mizuki, Y., Fujiwara, I. & Yamaguchi, T. (1996). J. Antimicrob. Chemother.37 Suppl. A, 41–45. [DOI] [PubMed]
- Rigaku (1998). RAPID-AUTO Rigaku Corporation, Tokyo, Japan.
- Rigaku/MSC (2002). CrystalStructure Rigaku/MSC, The Woodlands, Texas, USA.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809036939/hb5062sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809036939/hb5062Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report