Abstract
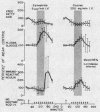
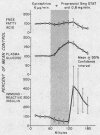
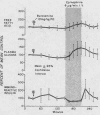
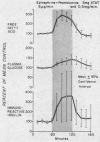
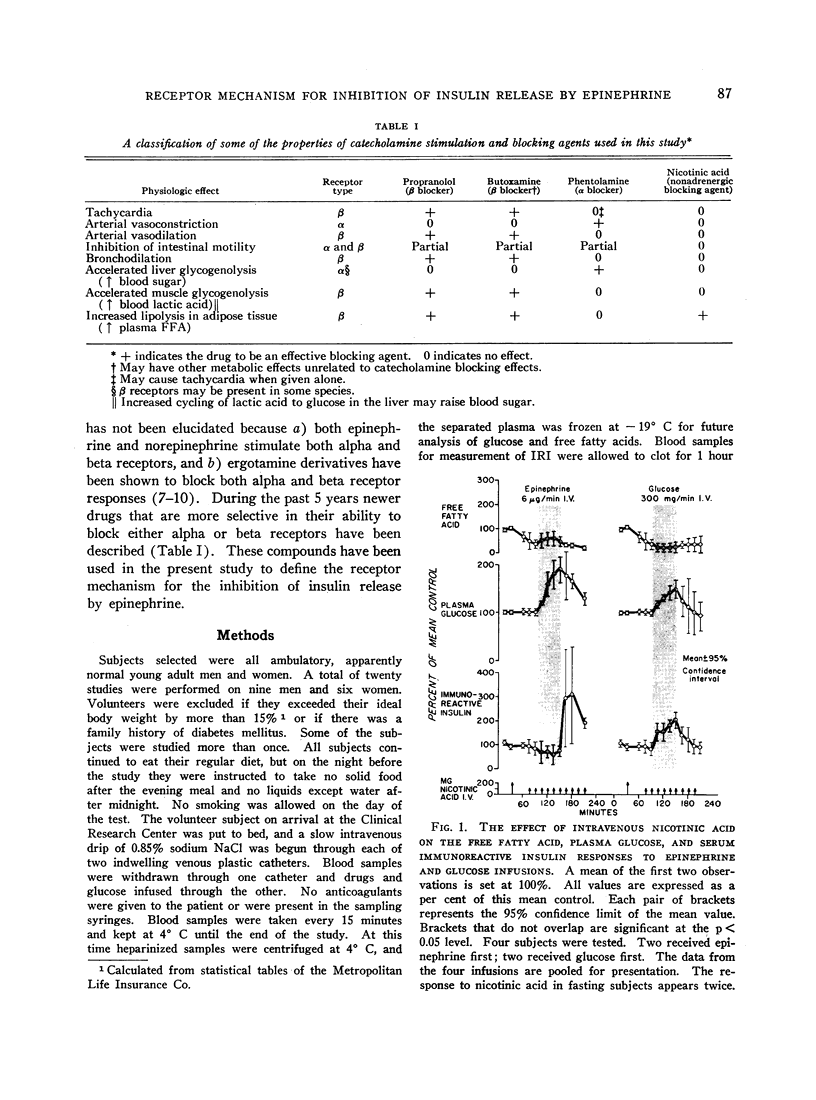
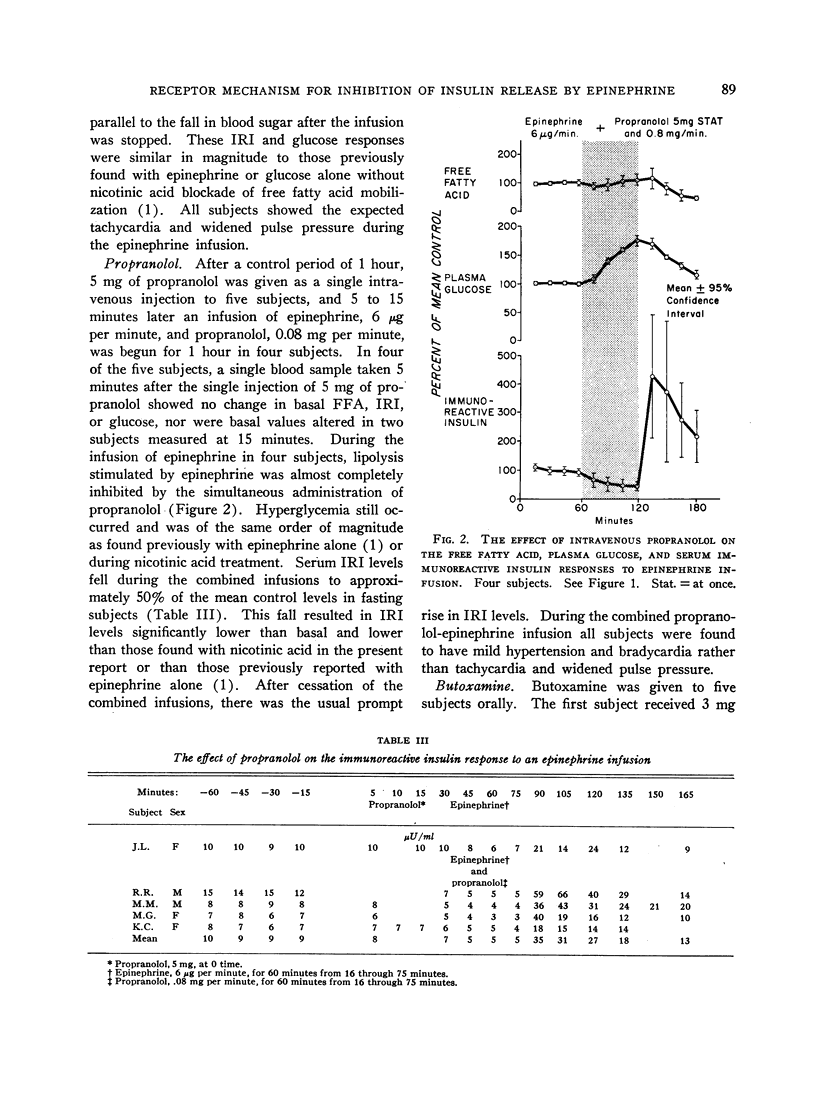
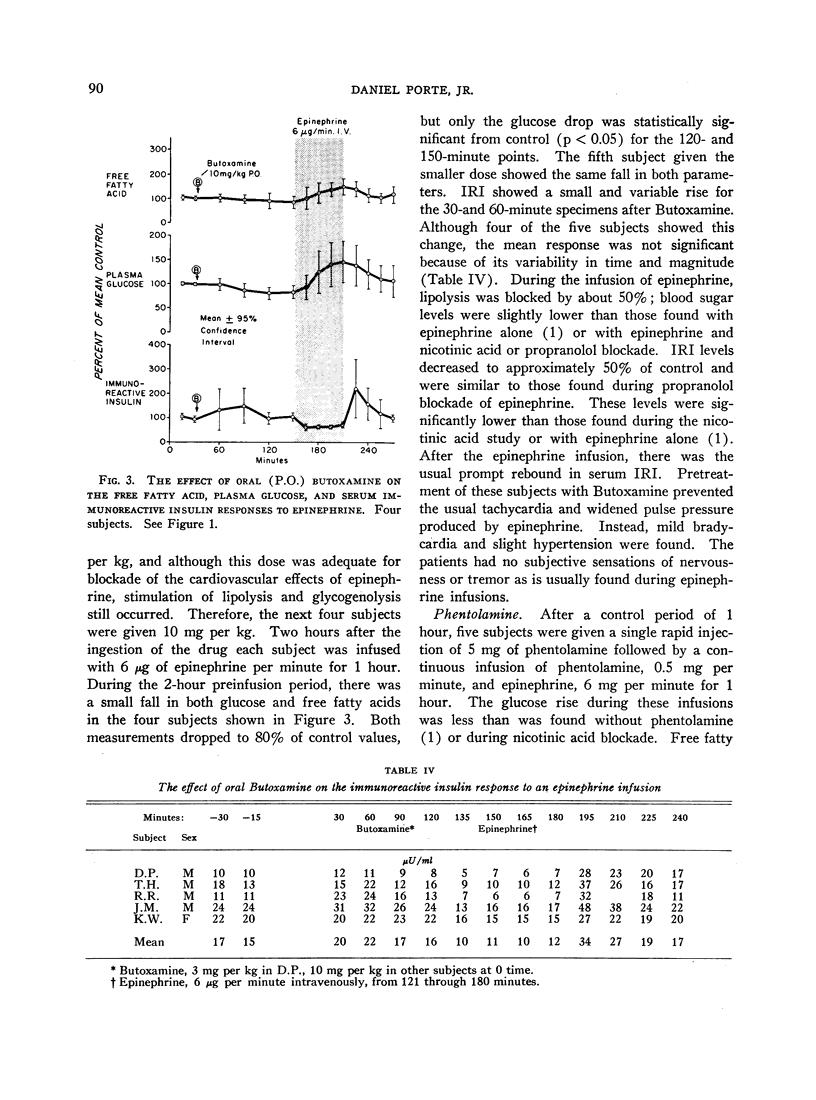
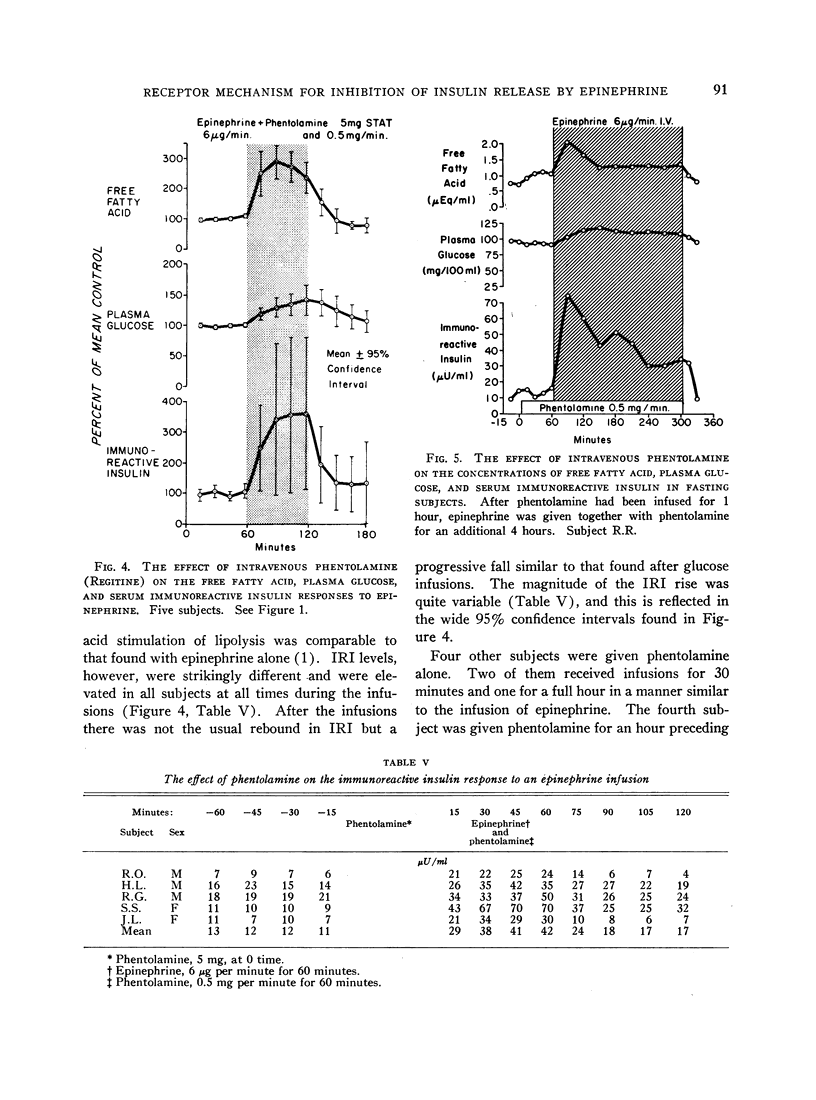
Normal adult men and women have been infused with epinephrine, 6 μg per minute, during lipolytic blockade with nicotinic acid, beta-adrenergic blockade with propranolol and Butoxamine, and alpha-adrenergic blockade with phentolamine. Epinephrine infusion was associated with low serum levels of immunoreactive insulin (IRI) except when phentolamine was given simultaneously. These findings are compatible with an alpha receptor mechanism for the epinephrine inhibition of insulin release. Phentolamine had no blocking effects on the tachycardia and widened pulse pressure or lipolytic stimulation by epinephrine, whereas both propranolol and Butoxamine blocked lipolysis, tachycardia, and widened pulse pressure. These findings are consistent with an alpha receptor blocking action for phentolamine and beta receptor blocking action for propranolol and Butoxamine. Inhibition of lipolysis by nicotinic acid did not alter IRI or glucose responses to epinephrine. It is concluded that the lipolytic effect of epinephrine is unrelated to its effects on IRI release. Lipolytic blockade by nicotinic acid also did not change IRI or glucose in fasting subjects or their responses to a glucose infusion, 300 mg per minute. These observations appear to conflict with the Randle hypothesis (the glucose-fatty acid cycle) and raise some doubt as to whether plasma FFA concentrations are direct determinants of glucose or IRI concentrations in normal man.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING W. W., KENNY A. D. THE EFFECT OF FASTING ON THE HYPERGLYCAEMIC RESPONSES TO CATECHOL AMINES IN RATS. Br J Pharmacol Chemother. 1964 Apr;22:267–274. doi: 10.1111/j.1476-5381.1964.tb02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN H. M., KNOBIL E. Effect of adrenergic blocking agents on fatty acid mobilization during fasting. Proc Soc Exp Biol Med. 1959 Nov;102:493–495. doi: 10.3181/00379727-102-25293. [DOI] [PubMed] [Google Scholar]
- HARVEY S. C., WANG C. Y., NICKERSON M. Blockade of epinephrine-induced hyperglycemia. J Pharmacol Exp Ther. 1952 Mar;104(3):363–376. [PubMed] [Google Scholar]
- LEVY B., AHLQUIST R. P. An analysis of adrenergic blocking activity. J Pharmacol Exp Ther. 1961 Aug;133:202–210. [PubMed] [Google Scholar]
- MCELROY W. T., Jr, SPITZER J. J. Effects of adrenergic blocking agents on plasma free fatty acid concentrations. Am J Physiol. 1961 Feb;200:318–322. doi: 10.1152/ajplegacy.1961.200.2.318. [DOI] [PubMed] [Google Scholar]
- MORAN N. C., PERKINS M. E. An evaluation of adrenergic blockade of the mammalian heart. J Pharmacol Exp Ther. 1961 Aug;133:192–201. [PubMed] [Google Scholar]
- NAKANO J., KUSAKARI T. COMPETITIVE ANTAGONISM BETWEEN ISOPROTERENOL AND A NEW BETA-RECEPTOR ADRENERGIC BLOCKING AGENT, PROPRANOLOL. Proc Soc Exp Biol Med. 1965 Jun;119:350–352. doi: 10.3181/00379727-119-30177. [DOI] [PubMed] [Google Scholar]
- Pilkington T. R., Lowe R. D., Foster R., Robinson B. F., Antonis A. Effect of sympathomimetic compounds with beta-adrenergic effects on plasma free fatty acids in man. J Lipid Res. 1966 Jan;7(1):73–76. [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr, Williams R. H. Inhibition of insulin release by norepinephrine in man. Science. 1966 May 27;152(3726):1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- VRIJ C., Jr, GHO B. K., DE GROOT C. A., WEBER J. F. The effect of isopropyl-nor-adrenaline and nor-adrenaline on the glycogen-content of skeletal muscle and liver of the rat. Acta Physiol Pharmacol Neerl. 1956 Mar;4(4):547–554. [PubMed] [Google Scholar]