Abstract
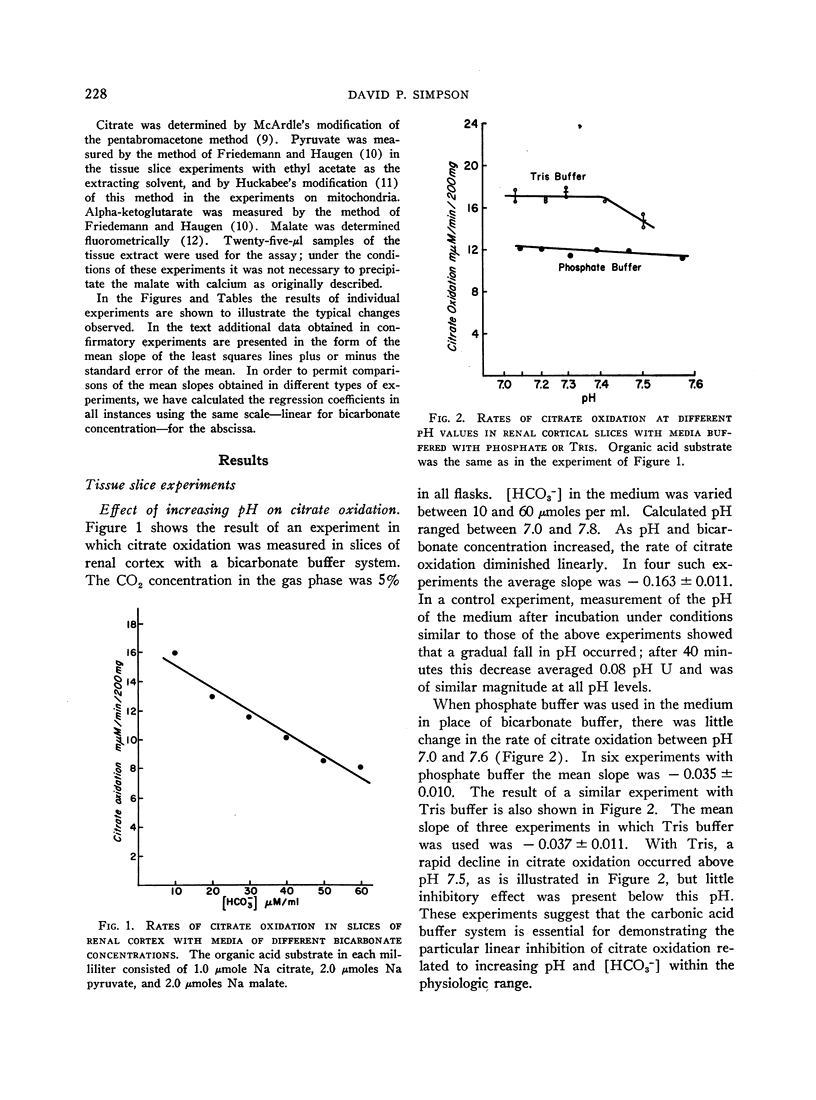
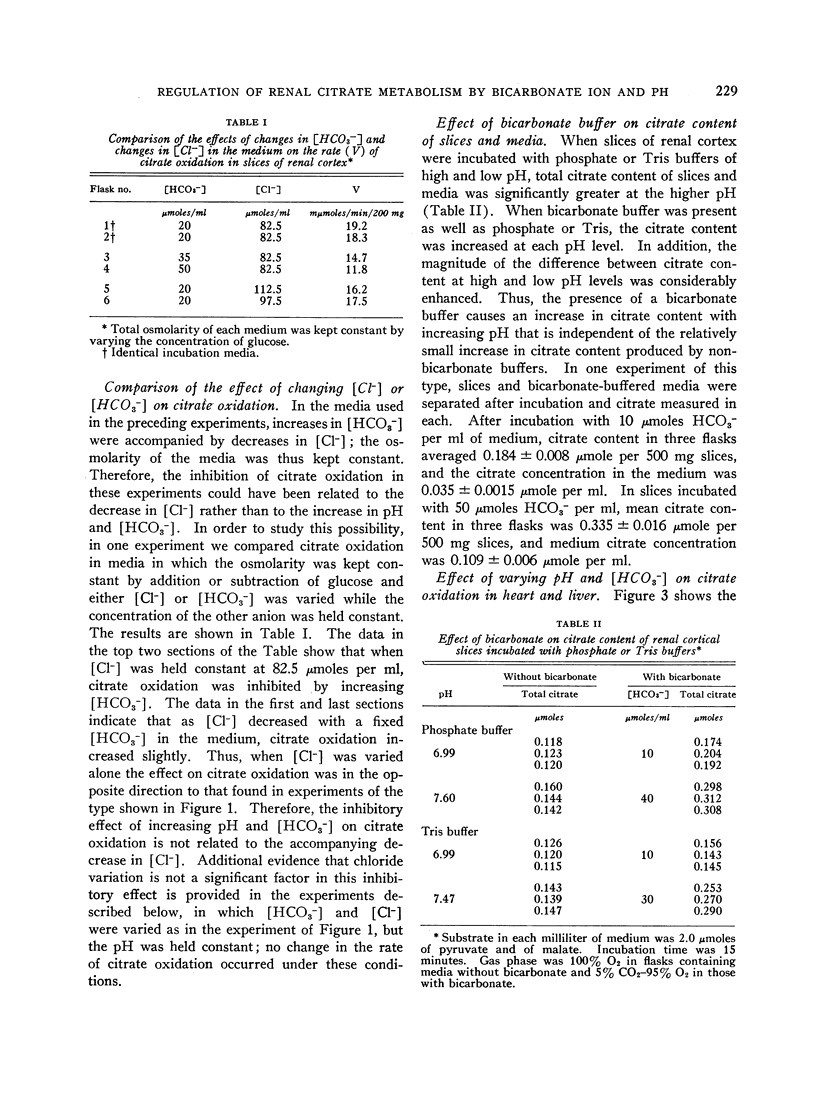
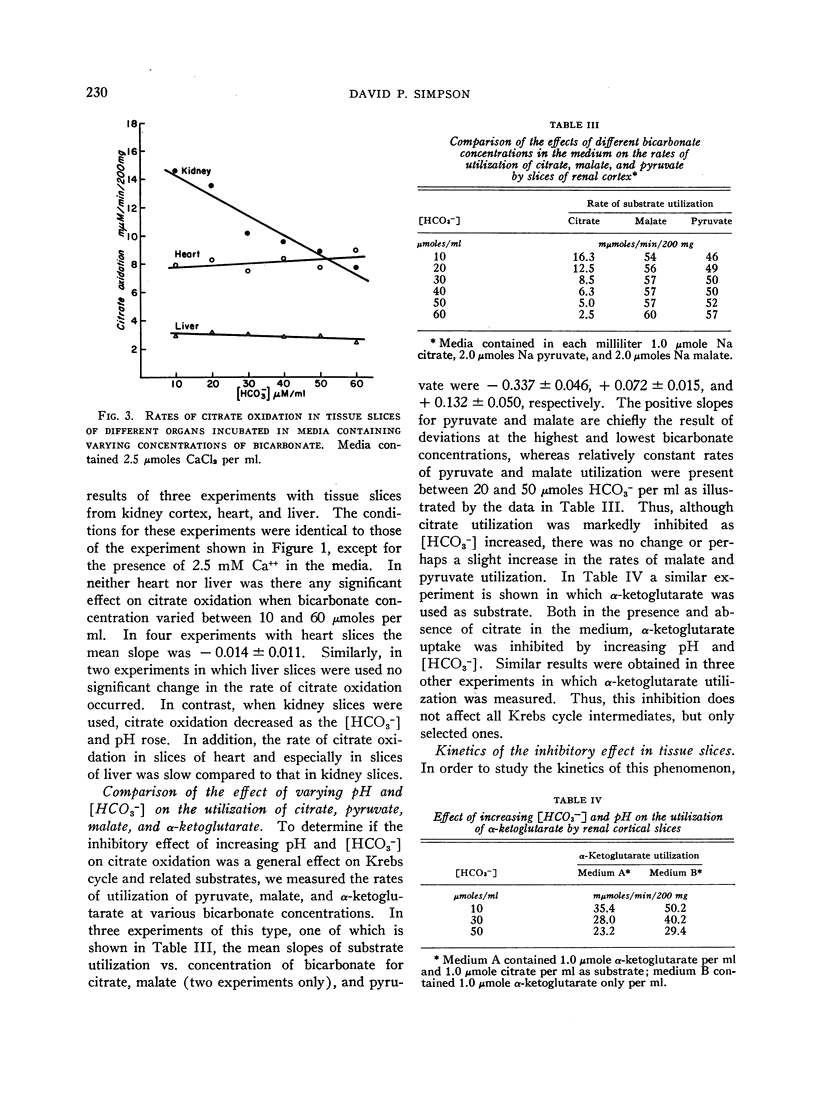
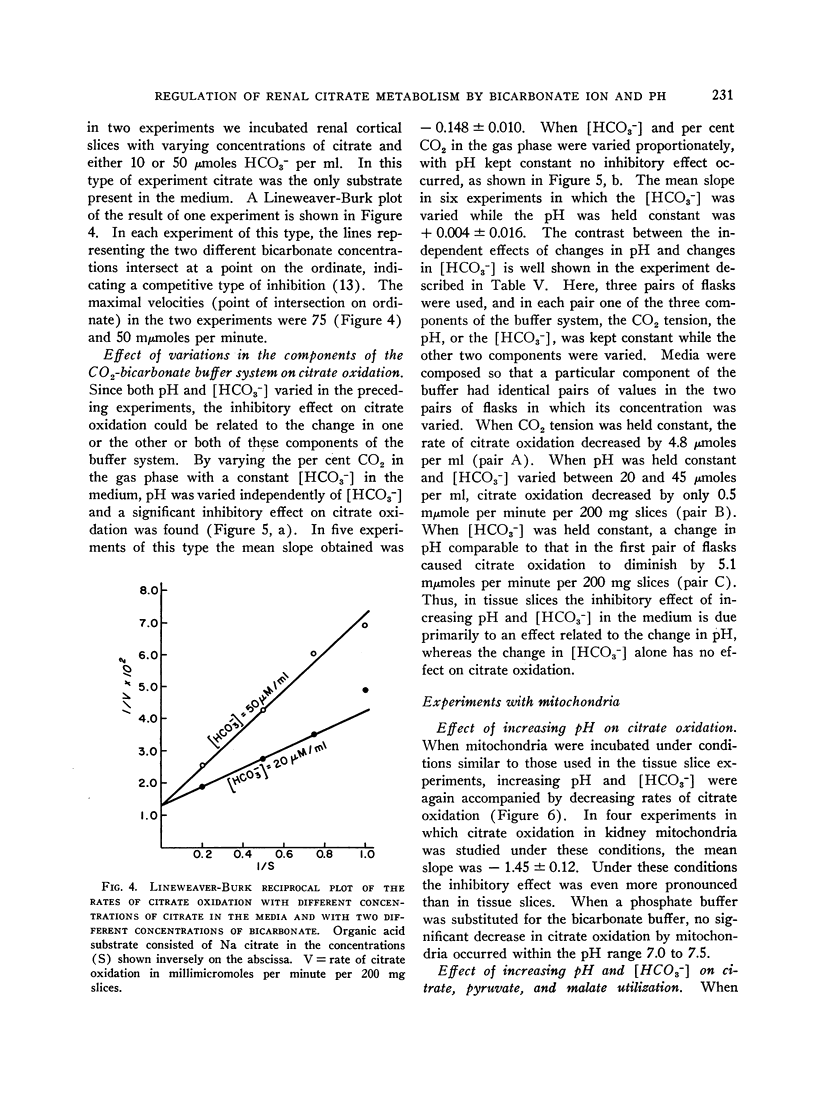
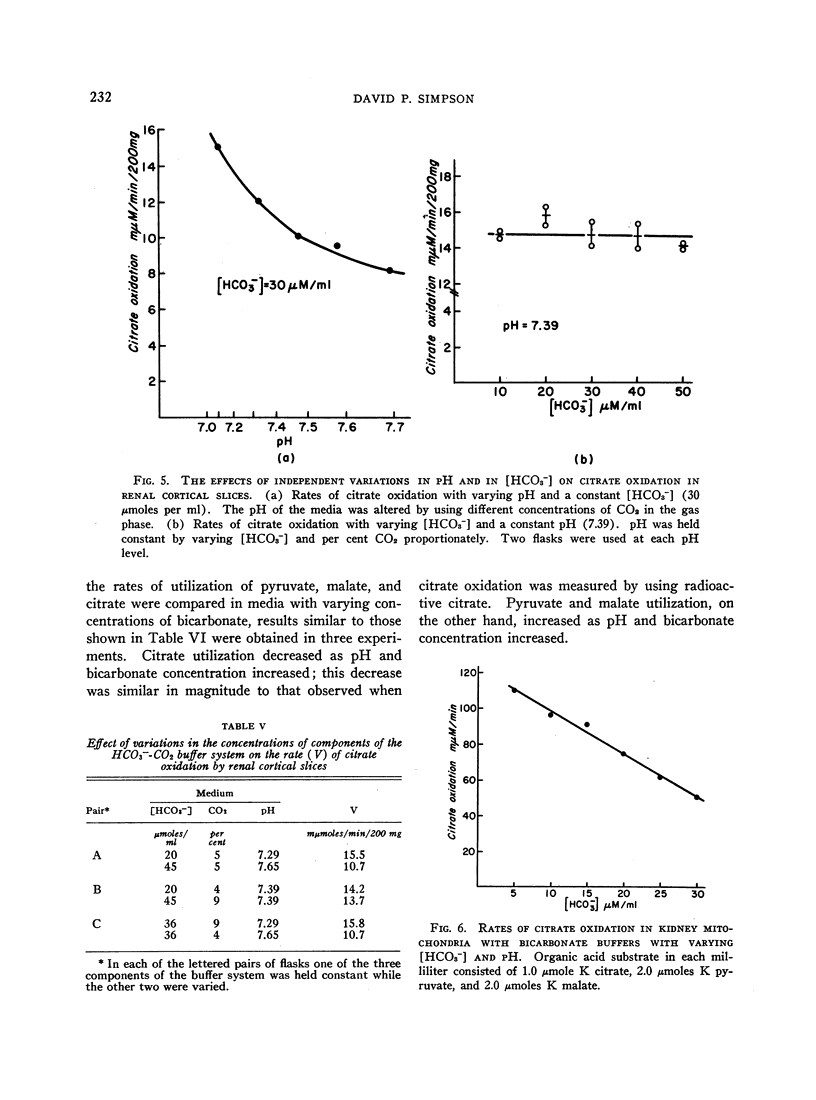
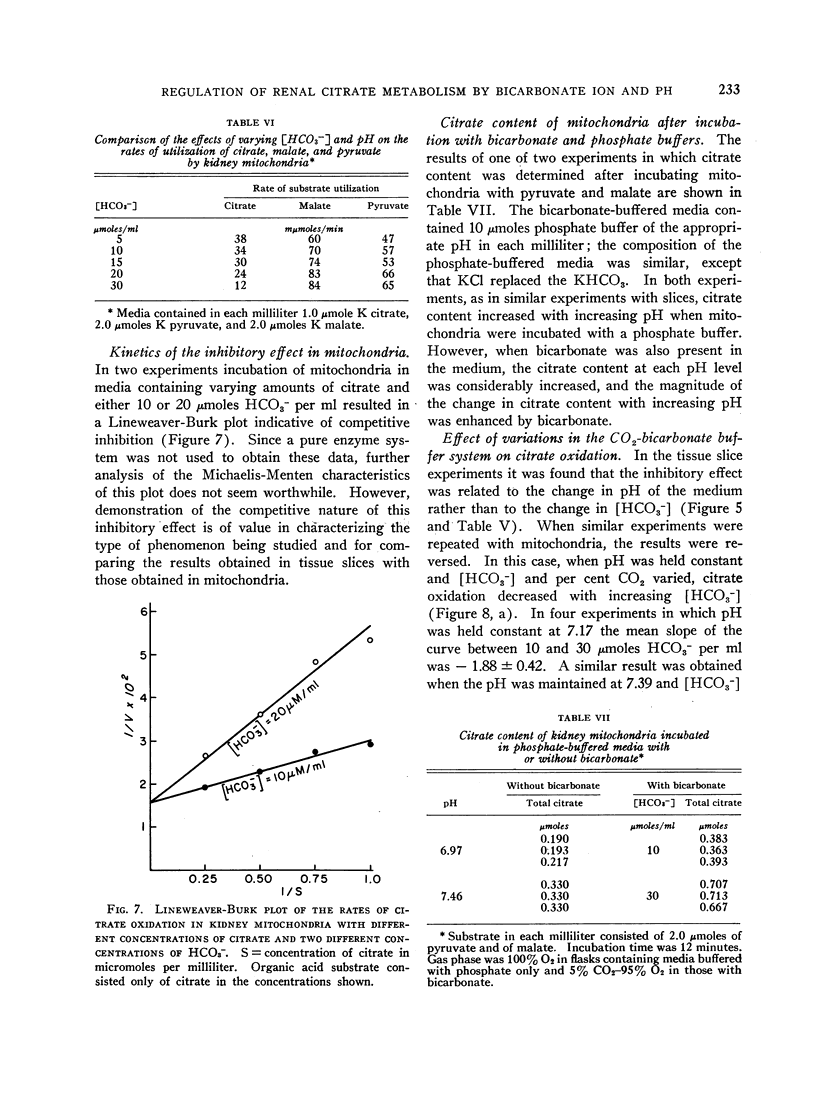
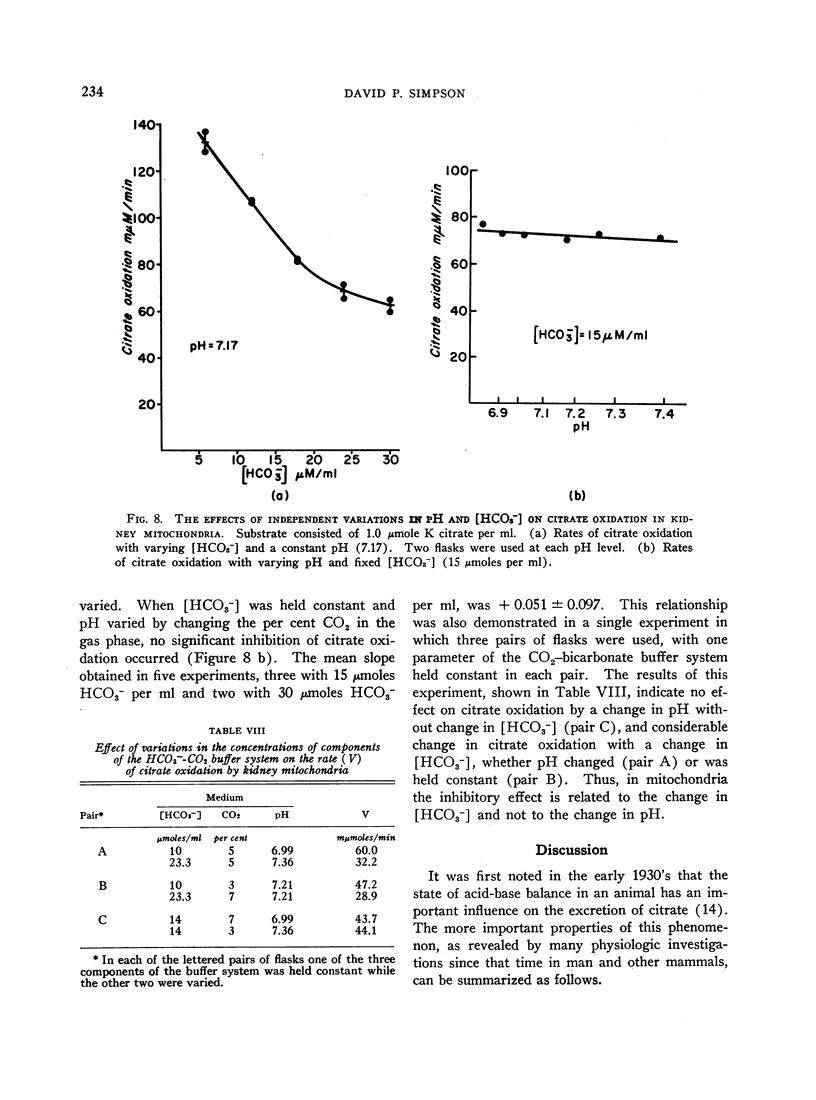
The effect of acid-base balance on the oxidation and utilization of citrate and other organic acids has been studied in tissue slices and isolated kidney mitochondria. The results show that: 1) With bicarbonate-buffered media, citrate oxidation and utilization are inhibited in slices of renal cortex and in kidney mitochondria when [HCO3-] and pH are increased within the physiologic range (pH 7.0 to 7.8; 10 to 60 μmoles HCO3- per ml). When phosphate or Tris buffers are used, no comparable effect on citrate oxidation occurs when pH is varied. 2) This effect is not demonstrable in heart or liver slices when a physiologic buffer is used. 3) α-Ketoglutarate utilization is inhibited in slices of renal cortex under similar conditions. Pyruvate and L-malate utilization are not inhibited in slices or mitochondria. 4) Citrate content in slices of renal cortex incubated with a high [HCO3-] is considerably greater than the concentration found with a low [HCO3-] in the medium. This effect is not duplicated by pH change in a nonbicarbonate buffer system. In mitochondria citrate content is also increased markedly at high bicarbonate concentrations. 5) The kinetic characteristics of the inhibition of citrate oxidation are those of a competitive type of inhibition. 6) When pH was varied with a constant [HCO3-] in the media, citrate oxidation was inhibited by increasing pH in slices of renal cortex but not in mitochondria. On the other hand, when [HCO3-] was increased without change in pH, no decrease in citrate oxidation occurred in slices, but a marked inhibitory effect was found when mitochondria were used.
From a comparison of these results with those previously obtained in intact animal experiments, we conclude that the inhibition of citrate oxidation caused by increasing pH and [HCO3-] in slices of renal cortex and kidney mitochondria is an in vitro representation of the inhibition of citrate reabsorption in the nephron that occurs in metabolic alkalosis. Thus, citrate clearance increases in metabolic alkalosis because of inhibition of oxidation of reabsorbed citrate within cells of the renal tubules. This inhibition is the result of an inhibitory effect of bicarbonate ion on citrate oxidation in mitochondria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON H. M., MUDGE G. H. The effect of potassium on intracellular bicarbonate in slices of kidney cortex. J Clin Invest. 1955 Nov;34(11):1691–1697. doi: 10.1172/JCI103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN J. J., WITTMANN E. Renal utilization and excretion of alpha-ketoglutarate in dog: effect of alkalosis. Am J Physiol. 1963 May;204:795–811. doi: 10.1152/ajplegacy.1963.204.5.795. [DOI] [PubMed] [Google Scholar]
- COOKE R. E., SEGAR W. E., REED C., ETZWILER D. D., VITA M., BRUSILOW S., DARROW D. C. The role of potassium in the prevention of alkalosis. Am J Med. 1954 Aug;17(2):180–195. [PubMed] [Google Scholar]
- CRAWFORD M. A., MILNE M. D., SCRIBNER B. H. The effects of changes in acid-base balance on urinary citrate in the rat. J Physiol. 1959 Dec;149:413–423. doi: 10.1113/jphysiol.1959.sp006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD M. A. The effects of fluoroacetate, malonate and acid-base balance on the renal disposal of citrate. Biochem J. 1963 Jul;88:115–120. doi: 10.1042/bj0880115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS B. M., MACINTYRE I., MACPHERSON C. R., MILNE M. D. Alkalosis in sodium and potassium depletion; with especial reference to organic acid excretion. Clin Sci. 1957 Feb;16(1):53–65. [PubMed] [Google Scholar]
- FOURMAN P., ROBINSON J. R. Diminished urinary excretion of citrate during deficiencies of potassium in man. Lancet. 1953 Sep 26;265(6787):656–657. doi: 10.1016/s0140-6736(53)90375-4. [DOI] [PubMed] [Google Scholar]
- GORDON E. E. Effect of acute metabolic acidosis and alkalosis on acetate and citrate metabolism in the rat. J Clin Invest. 1963 Feb;42:137–142. doi: 10.1172/JCI104700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON E. E., SHEPS S. G. Effect of acetazolamide on citrate excretion and formation of renal calculi. N Engl J Med. 1957 Jun 27;256(26):1215–1219. doi: 10.1056/NEJM195706272562602. [DOI] [PubMed] [Google Scholar]
- GROLLMAN A. P., HARRISON H. C., HARRISON H. E. The renal excretion of citrate. J Clin Invest. 1961 Jul;40:1290–1296. doi: 10.1172/JCI104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Inhibition of urine citrate excretion and the production of renal calcinosis in the rat by acetazoleamide (diamox) administration. J Clin Invest. 1955 Nov;34(11):1662–1670. doi: 10.1172/JCI103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNDON R. F., FREEMAN S. Renal citric acid utilization in the dog. Am J Physiol. 1958 Feb;192(2):369–372. doi: 10.1152/ajplegacy.1958.192.2.369. [DOI] [PubMed] [Google Scholar]
- HERRIN R. C., LARDINOIS C. C. Renal clearance of citric acid in the dog. Proc Soc Exp Biol Med. 1958 Feb;97(2):294–297. doi: 10.3181/00379727-97-23720. [DOI] [PubMed] [Google Scholar]
- HUCKABEE W. E. Control of concentration gradients of pyruvate and lactate across cell membranes in blood. J Appl Physiol. 1956 Sep;9(2):163–170. doi: 10.1152/jappl.1956.9.2.163. [DOI] [PubMed] [Google Scholar]
- MCARDLE B. A modified method for the microdetermination of citric acid. Biochem J. 1955 Aug;60(4):647–649. doi: 10.1042/bj0600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieth H., Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966 Mar 19;209(5029):1244–1245. doi: 10.1038/2091244a0. [DOI] [PubMed] [Google Scholar]
- Runeberg L., Lotspeich W. D. Krebs cycle acid excretion with isotopic split renal function techniques. Am J Physiol. 1966 Aug;211(2):467–475. doi: 10.1152/ajplegacy.1966.211.2.467. [DOI] [PubMed] [Google Scholar]
- SIMPSON D. P. EFFECT OF ACETAZOLAMIDE ON CITRATE EXCRETION IN THE DOG. Am J Physiol. 1964 Apr;206:883–886. doi: 10.1152/ajplegacy.1964.206.4.883. [DOI] [PubMed] [Google Scholar]
- SIMPSON D. P. INFLUENCE OF PLASMA BICARBONATE CONCENTRATION AND PH ON CITRATE EXCRETION. Am J Physiol. 1964 Apr;206:875–882. doi: 10.1152/ajplegacy.1964.206.4.875. [DOI] [PubMed] [Google Scholar]
- SIMPSON D. P. TISSUE CITRATE LEVELS AND CITRATE UTILIZATION AFTER SODIUM BICARBONATE ADMINISTRATION. Proc Soc Exp Biol Med. 1963 Nov;114:263–265. doi: 10.3181/00379727-114-28647. [DOI] [PubMed] [Google Scholar]
- STERN J. R., OCHOA S., LYNEN F. Enzymatic synthesis of citric acid. V. Reaction of acetyl coenzyme A. J Biol Chem. 1952 Sep;198(1):313–321. [PubMed] [Google Scholar]
- THUNBERG T. Occurrence and significance of citric acid in the animal organism. Physiol Rev. 1953 Jan;33(1):1–12. doi: 10.1152/physrev.1953.33.1.1. [DOI] [PubMed] [Google Scholar]



