Abstract
By adapting microfabrication techniques originally developed in the microelectronics industry novel device for drug delivery, tissue engineering and biosensing have been engineered for in vivo use. Implant microfabrication uses a broad range of techniques including photolithography, and micromachining to create devices with features ranging from 0.1 to hundreds of microns with high aspect ratios and precise features. Microfabrication offers device feature scale that is relevant to the tissues and cells to which they are applied, as well as offering ease of en masse fabrication, small device size, and facile incorporation of integrated circuit technology. Utilizing these methods, drug delivery applications have been developed for in vivo use through many delivery routes including intravenous, oral, and transdermal. Additionally, novel microfabricated tissue engineering approaches propose therapies for the cardiovascular, orthopedic, and ocular systems, among others. Biosensing devices have been designed to detect a variety of analytes and conditions in vivo through both enzymatic-electrochemical reaction and sensor displacement through mechanical loading. Overall, the impact of microfabricated devices has had an impact over a broad range of therapies and tissues. This review addresses many of these devices and highlights their fabrication as well as discusses materials relevant to microfabrication techniques.
Keywords: Photolithography, Micromachining, Tissue Engineering, Biosensors, Drug Delivery
Introduction
Microfabrication is a collection of methods that creates micro-meter scaled features in a variety of materials. The most common microfabrication method, lithography, was discovered by Nicéphore Niépce in the 1820’s where 0.5 to 1 millimeter features were transferred by photolithography. Since then, photolithography and other microfabrication techniques have been used to produce devices with features sizes of 0.18 micron feature size or less.1 One biomedical application of these techniques is for the fabrication of in vivo implants. The development of microfabricated implants is advantageous in many respects, including the ability to fabricate highly engineered, miniaturized devices with biologically-relevant scaled features, integrate circuit technology and use high-throughput manufacturing practices.
Microfabrication can be used to create physiologically relevant materials that mimic the scale cells experience in vivo. It has been reported that nano- and micro-scale materials lead to increased cellular integration, adhesion, proliferation, differentiation, and signaling.2–4 Additionally, work has shown that receptor mediated phagocytosis is determined, in part, by micro-particle shape and size.5 Not only is the overall particle surface area important for ligand and cell presentation but also the degree to which the particles fits the contours of the cells membrane.5, 6 This body of research indicates that mechanical cues on the micro- and nano-scale can influence cellular phenotype and motivates the application of microfabricated surfaces for biomedical applications and implantation.
Perhaps one of the most advantageous elements of microfabrication is the ability to control the design of surface topography. For example, traditional light photolithography allows for development of surface features ranging in size from one micron up to the centimeter scale. Electron beam (E-beam) photolithography has resolution on the order of nanometers. The aspect ratio of the fabricated surfaces can be highly manipulated to allow for constrained geometry. Aspect ratios over 25 have been reported for microfabrication of polyester extrusion dies1 and ratios of 30 for SU-8 features.7 High aspect ratio microfabricated features can alter cell phenotype, proliferation, and differentiation.8, 9 Besides precise control over aspect ratio, microfabrication allows management of shape and spacing in order to optimize surface characteristics such as binding molecule functionalization,5, 6 drug release kinetics10, 11 and surface fouling characteristics12.
The novelty of microfabrication arises mainly in the miniaturization of features, a design element that was not available until the 1950’s with the advent of integrated circuit technology and not applied to biology until the 1990’s.13 Miniaturization can aid in fitting the implant into a small space, such as between tissue. Implant size is on the order of 50 microns for arterial stents, 4 microns and up for pressure and flow sensors, 45 microns for nerve stimulating microelectrodes, and 50 microns for liver reconstructs.14 The minute size of microfabricated devices offers limitless possibilities for application in in vivo environments.
Because much of the early microfabrication technology was extrapolated from integrated circuit development, integration of electronic elements into microfabricated implant design is straightforward and facile, especially for implantable biosensors. For example, a sensor signal can be amplified or processed easily with circuit integration.15 Furthermore, integrated circuits can be used to generate a stimulus to evoke a response in nerve or tissue cells in prosthetics.14 The combination of integrated circuits with implanted devices offers a high level of synergy not necessarily afforded on the macro-scale.
The mass fabrication techniques developed for integrated circuit technology carry over to many or all of the features of microfabricated implants. Whether on the bench-top or in the factory, the same methods used to generate computer chips can be used to produce microfabricated biotechnological elements en masse. One silicon wafer can be processed and contain drug-eluting as well as sensing elements to be implanted in the body.16, 17 High-throughput, reproducible mass fabrication of these implantable elements allow for low-cost fabrication and precise replication control.
Microfabricated implants can be applied to almost every cell type of the body. Implants have short term uses for applications on the order of hours and long term applications that can last for days to decades. This review will focus on the microfabricated elements of both short- and long-term implants as applied to the most common tissues of the body, including cardiovascular, oral, neural, and orthopedic. Microfabricated technologies as applied to therapeutics delivery, tissue engineering, and biosensors will be highlighted as well as a general overview of the techniques employed to fabricate these devices. In addition to the information presented in this review, supplementary information can be gleaned from a variety of other review articles focused on whole implant microsystems14, nanostructured tissue scaffolds18, medical application of micro-and nano-technologies15, 19–22 and tissue or application specific implants.22–33
Microfabrication Techniques
Microfabrication is a process in which micro-scale features are constructed on the surface of a bulk material or patterned into the bulk material itself. The bulk material can be any material but the most prolific materials are silicon, glass, and various polymers. The most commonly applied microfabrication technique is photolithography. The process of photolithography involves the transfer of a pattern from a mask to a thin film via the localization of light. By combining steps of masked exposure and thin film application, multi-layered resists can be formulated with high aspect ratios. Higher resolution photolithographic techniques, such as x-ray and e-beam lithography, can lead to smaller feature size as well as more intricate topography. Often photolithography is incorporated in a multi-technique micromachining process for the development of bulk materials. The most common micromachining techniques are chemical etching and surface micromachining. Chemical etching can be either wet, with acids and bases, or dry, commonly with plasmas, and removes material from the bulk surface. In contrast, surface micromachining adds to the bulk substrate surface generally by chemical reaction, such as chemical vapor deposition or oxide formation. The location of etching and micromachining is easily manipulated by photolithographic addition of polymer resists. Micromachining and photolithography can also be used in another microfabrication process, micromolding. Micromolding consists of a master with features to be transferred to the polymer. The initial master substrate can be microfabricated using a variety of methods, including electrochemical microfabrication, lithography, and micromachining. The pattern of the master can be replicated through techniques like injection molding, hot embossing, and plastics casting.1 Plastics casting is commonly used for microfabricated implants. By casting a bio-friendly polymer like PDMS, patterns can be transferred to a secondary polymer such as biodegradable PLGA or PCL. These techniques are used in isolation or combination to create implantable biomedical devices. Typically microfabricated implants are developed in silicon or similar materials, due to ease of manufacturing. After proof of concept has been shown, the implant can then be modified to be cast in additional materials through techniques such as micromolding.
Substrates
Silicon
Silicon is the most traditional material for microfabrication due to its use historically in integrated circuit development. Although methods such as wet etching can be performed on materials like silicon and quartz, silicon is commonly chosen because of its electrical properties, high yield stress, hardness, and Young’s modulus, especially in microelectromechanical systems (MEMS).1 The material is available in a variety of single-crystal, thin (0.25–10mm) wafers of various crystalline orientations.1, 15 In biomedical applications, many silicon-based devices, such as implantable sensors,34, 35 devices for drug delivery,36–38 and electrical stimulation, 39–41 are already under consideration for use within the human body. There are numerous additional examples of MEMS-based biomedical devices.14, 33, 42–49 Micro- and nano-structured silicon, such as porous and nanowired silicon, has previously been employed as a foundation for sensor technologies.50–58 It has been shown that topographically-modified silicon can elicit specific physiological responses within the body, from bioactivity 59–62 to biodegradation,63–66 displaying the ability to use the material for engineering of cellular response.
With implants, biocompatibility as well as immunogenicity needs to be studied for a surface due to the chronic inflammation foreign body response (cellular encapsulation) and the desire to maintain patient comfort. Biocompatibility is defined as the ability of a material to perform with an appropriate response in a specific application whereas immunogenicity is defined as the ability of the surface to invoke an immune response, such as inflammation. Micro- and nano-structured silicon has been shown to be biocompatible and non-immunogenic. A small number of studies have been performed with immune cells on silicon or silane modified surfaces.67, 68 A study from the Desai group has concluded that micro-peaked silicon is more immunogenic than other micro- and nano-structured silicon surfaces based on human monocyte cytokine production, cell viability, and shape factor evaluation. Additionally, the immunogenicity and biocompatibility of flat, nano-channeled and nanoporous silicon are approximately equivalent to tissue culture polystyrene. None of the silicon surfaces were as immunostimulatory as lipopolysaccharide (LPS). In addition, it was determined that the formation of reactive oxygen species is not a prerequisite for inflammation from silicon based surfaces.69 Silicon is not only a user-friendly material, but also can be formulated to be biocompatible and not invoke a significant immune response.
Glass
Like silicon, glass or silicon oxide is available in a variety of sizes and compositions, such as fused silica and borosilicate wafers. Of these two materials, silica offers ease in microscopic imaging.15 Additional microfabrication techniques are being developed continuously to enhance the role of micro- and nano-structured glass.70, 71 The use of glass in microfabricated implants is not as universal as silicon, but its use is increasing as micro- and nano-scale technology develops new fabrication methods.
With regards to glass biocompatibility, bioactive silicon oxide based implants have been used since the 1970’s, primarily in dentistry, but also in orthopedics.72 The immunogenicity of glass has been observed with immune cells on both flat and microstructured surfaces. Macrophage cell morphology has showed increased size and cell spreading, consistent with the high level of cytokine secretion induced by the bioactive glass surface;73 however, few studies have been performed on micro- or nano-structured glass. Studies have reported that a difference was not observed between the adherence of cells on textured versus flat glass.74 Also, glass has been shown to be non-cytotoxic in particulate form at low concentrations (< 1mg/mL).75 With the increasing development of micro-structured glass surfaces, as well as the proven biocompatibility of such surfaces, the application of these surfaces for implanted devices is inevitable.
Polymers
Often the most economical substrate material for microfabrication is polymer based. Many traditional polymer manufacturing techniques, such as injection molding and embossing, can be carried over into the microfabrication realm at a much smaller scale.15 Often polymers are introduced through photolithographic techniques. The most common photolithographic polymers are commercially available SU-8 and PMMA. Patterning of additional polymers can be accomplished using micro-molding of SU-8, PMMA, or silicon fabricated masters. The range of polymers available adds an additional design element to the microfabrication process by allowing the developer to tailor specific material properties such as hydrophobicity, biodegradability, and biocompatibility. Furthermore, features such as drug release kinetics, biodegradability, and cell incorporation can be altered with variables such as molecular weight, chemical structure, and/or cross-linking density. A variety of biocompatible polymers are currently used in medical applications, including polyglycolic acid (PGA), polylactic acid (PLA), polycaprolactone (PCL), poly(glycerol-sebacate) (PGS) and poly(DL-lactide-co-glycolide) (PLGA). Chen et al. reviews some of these polymers and presents average degradation rates and tensile modulus.76
One concern with polymer based microfabricated implants is biocompatibility in particular, the immunogenicity of the material. With respect to biocompatibility, adherence and viability for many cell types on several different polymer substrates have been reported in literature. Table 1 reports studies of cell adherence on polymers that have been patterned with microfabrication techniques. A decrease in cell number was observed with rat epitenon cells on polystyrene (PS) nanometer-sized pillars.77 Also, bladder smooth muscle cells (BSMCs) grown on nanostructured PLGA had increased cell adhesion and numbers.152 With regards to immunogenicity, neutrophils grown on roughened PS surfaces, both in vitro and vivo, have decreased cell viability. Decreased neutrophil viability could be due to lack of stimulation, rather than due to increased activation.78 Overall, many of the polymer surfaces, including those that are micro-structured, do not elicit a significant immune response.
Table 1.
Immune related and other cell studies with polymers applied in microfabrication techniques.
| Polymer | Acronym | Cell | Notes |
|---|---|---|---|
|
Expanded poly (tetrafluoroethylene) |
ePTFE | Macrophages150, 151 |
|
| Poly(dimethyl siloxane) | PDMS | ECs, Macrophages, Fibroblasts151 |
|
| Poly(caprolactone) | PCL | Bladder smooth muscle (BSMC) 152 Fibroblasts153–155 Smooth Muscle Cell (SMC) ECs154 |
|
|
Poly(ethylene terephthalate) |
PET | Macrophages74, 156 |
|
| Polyethylene | PE | ECs, Macrophages, Fibroblasts151 |
|
| Poly(lactic-co-glycolic acid) | PLGA | BSMC152 |
|
| Poly-methylmethacrylate | PMMA | ECs, Fibroblasts, Macrophages151 |
|
| Polystyrene | PS | Neutrophil 87 Epitenon cells94 Macrophages150, 151 Mononuclear cells 173 |
|
| Polyurethane | PU | BSMC152 |
|
Microfabricated Implant Technology
Therapeutics Delivery
Microfabricated therapeutic delivery devices can be implanted for short, up to a few hours, and longer term, several days to months, depending on the application. Short term application, such as transdermal microneedles which can be applied to the skin to deliver DNA30 and then removed in a matter of seconds. Whereas devices that are used to treat chronic diseases, such as osmotic pumps to deliver therapeutic proteins79, can be implanted into the body for longer term, on the order of months or more. Independent of implant timescale, the ability to control device design as well as drug eluting properties is advantageous in therapeutic delivery. Microfabrication allows for design control and drug elution. For example, an asymmetric particle can be microfabricated to deliver drug in a concentrated fashion at the device/intestinal interface (Fig. 1 and 2).33, 80, 81 Also, pore size can be micromachined from silicon to allow for single molecule release of drug, permitting zero order kinetics (Fig. 2).36, 82 A representative sample of implanted therapeutic devices developed through microfabrication are presented in Table 2. These devices are applied in a variety of tissue systems and disease states where several of the obstacles associated with therapeutic delivery can be overcome with microfabrication design. A review of micro- and nano-electromechanical devices for drug delivery is given by Staples et al.48
Figure 1.
Bi-polymeric particles. (A) Process flow diagram for bi-polymeric particles. (B) Optical micrograph of hydrogel loaded particles. (C) Fluorescent micrograph of FITC-bovine serum albumin loaded hydrogel particles. (D) Fluorescent micrograph of multilayered hydrogel prepared with DNP-BSA, FITC-BSA and Texas-red-BSA (from outmost layer to inmost). The grey dotted-line box highlights the reservoir area and the red dotted-line box the outer area of the microdevice. (E) A fluorescent micrograph of combined filters of hydrogel-filled microdevices. (F) Schematic depicting the release of drug from spherical and microdevice particles.95
Figure 2.
Microfabrication of immunoisolation silicon nano-channeled membrane. (A) Process flow diagram adapted from Desai et al.82 The diffusion channel is 20–100 nm in thickness. (B) Optical micrograph of nano-channeled membrane with pores 78 nm in diameter. (C) Scanning electron micrograph of silicon nano-channeled membrane. Scale Bar = 1 µm
Table 2.
Microfabricated implants used for drug delivery. An * indicated related references.
| Tissue System | Description | Microfabrication Method(s) |
Feature Size | Reference |
|---|---|---|---|---|
| Cardio-Vascular | Biodegradable and biocompatible mesoporous silicon particles that have well-controlled shapes, sizes and pores are loaded with Q-dots and single walled carbon nanotubes for combination imaging and therapeutic delivery. |
Micromachining and photolithography |
5 µm in diameter with 50 nm pores |
87 |
| Multiple | A biodegradable PLGA 3-layer device comprised of a diffusion control layer via micro-orifices, diffusion layer, and drug reservoir layers. |
Micromolding | Orifices on the order of 100 µm |
109 |
| Orthopedic | bFGF was delivered 40 ng/day on average over four weeks from a biodegradable osmotic pump PLGA MEMs device. |
Micromolding | 50×50 µm2 reservoir, 25 µm deep |
79 |
| Multiple | Drug eluting microparticles of PEG, PLA and poly-(pyrrole) molded from photocurable perfluoropolyether. |
Photolithography and Micromolding |
>200 nm | 85 |
| Oral Drug Delivery | Asymmetric, multilayer microdevices for oral delivery of made of SU-8, PMMA and PLGA with a protected reservoir for therapeutic delivery. |
Photolithography | 150×150×8 µm3 |
32, 33, 80, 81, 91– 93, 95 |
| Oral Drug Delivery | An asymmetric silicon microdevice for oral delivery of therapeutics with a protected reservoir. |
Micromachining | 50×50×2 µm3 thick with 25×25×1 µm3 deep wells |
94 |
| Hepatic | A silicon nanochanneled delivery system for zero order release of IFN-α. |
Micromachining | Nanochannel of 100 nm |
114 16, 110, 112, 113* |
| Dermal | PLA, PGA and PLGA microneedles increased dermal permeability almost 3 orders of magnitude. |
Micromolding | 570 µm high needle with beveled diameter of 10 µm |
99 30, 97–102* |
| Multiple | A solid-state silicon microchip that provides controlled release of single or multiple chemical substances through the electrochemical dissolution of thin anode membranes covering micro-reservoirs filled with chemicals in solid, liquid or gel form. |
Photolithography, CVD, and micromachining |
Reservoirs on the order of 70 microns |
38 48, 116, 117* |
| Cardio-Vascular | Silicon based needles that penetrate into the internal elastic lamina of normal arteries or atherosclerotic plaques to deliver DNA. |
Micromachining | 80 µm high, with a radius of curvature of <0.1 µm |
86 97, 98* |
| Neural | Silicon based multi-channel neural probe for therapeutic delivery on the order of 100 pL in 1 second. |
Micromachining | 10 µm features with channel length of 4 mm |
107 |
Intravenous Delivery
Inherently non-protected, intravenously-delivered drugs are diluted upon introduction into the blood stream. Also, the drug is delivered systemically, distributing toxicity and harmful side effects in the process. By incorporating the drug into a delivery vehicle, advantages such as targeting, protection, and prevention from dilution are more closely achieved. Currently nanoparticles and liposomes are used ubiquitously for intravenous delivery, but despite cancer phenomenon like the enhanced permeability and retention (EPR) effect, a majority of these particles adversely accumulate in the liver and spleen.83 Indeed when injected intravenously, microfabricated poly(ethylene glycol) (PEG) hydrogels on the micro- and nano-meter scale, developed in the DeSimone laboratory, accumulated preferentially in the liver (Fig. 3).84 To make the particles, the DeSimone laboratory creates a silicon master from traditional micromachining techniques (photolithographic application of resist layer with wet etching of the surface) that is then used to create a perfluoropolyether (PFPE) mold to micromold isolated particles on a non-wetting substrate. These particles can be made of a variety of materials including PEG, triacrylate resin, poly(lactic acid), and poly(pyrrole).85 Most microfabricated implants, due to size, can not be inserted into the intravascular regime because of risk of capillary blockage. However, silicon based microneedles have been implanted into atherosclerotic plaques to deliver DNA.86 Also, mesoporous silicon particles have been microfabricated to both image and deliver therapeutics. Q-dots and FITC conjugated single wall nanotubes were added to the silicon particles through surface chemistry. The imaging specificity of these particles was measured on endothelial cells, indicating their potential for intravenous therapy.87 Most likely, further development for intravenous delivery through microfabricated implants will be enhanced as more microfabricated elements reach into nanometer scales.
Figure 3.
Microfabrication of micro- and nano-scale particles through PRINT method. (A) Process flow diagram for fabrication of PFPE mold. Adapted from Rolland et al.85 (B) Schematic of particle formation with PFPE stamp through modified micromolding. (C)-(E) Scanning electron micrographs of microfabricated PEG particles of various sizes and shapes.
Oral Delivery
Oral delivery of therapeutics requires drug stability in harsh pH and flow conditions as well as permeation through a thick mucous layer and intestinal epithelial cell tight junctions. These conditions typically prevent the adsorption of most macromolecules, and the ones that are capable of reaching the blood stream are on the order of 3%.88 Many orally delivered pharmaceuticals overcome these obstacles by administering copious amounts of drug throughout the lumen of the intestine. However this approach is not practical for expensive or toxic drugs, such as ones used to treat cancer. Non-microfabricated approaches with nanoparticles and liposomes have had limited success and can be restricted by the hydrophobicity of the therapeutic. Additionally, the therapeutic is often non-specifically delivered to healthy tissue throughout the intestine.89, 90 An ideally designed oral delivery vehicle would incorporate a protected drug reservoir, asymmetric drug delivery, cyto-adhesion, small device design to resist shear, and cell permeability enhancers. The Desai laboratory has designed an oral delivery vehicle that incorporates many of these features (Fig. 1 and Supplementary Figure 1).32, 33, 80, 81, 91–94 These devices were initially developed in silicon through micromachining techniques.94 Later modifications included multilayer SU-8 devices developed photolithographically, and PMMA and PLGA devices.81 (Supplementary Figure 1) Building on the multi-layered device, a bi-polymeric device can be formulated with photolithography. Poly (ethylene glycol) dimethacrylate (PEGMA) has been incorporated into SU-8 microdevices for oral delivery of chemotherapeutics.95 (Fig. 1) Acrylated PEG polymers were covalently bound to free radicals present on a SU-8 surface.96 One advantage of an oral delivered vehicles is if the vehicle is larger than several microns, and therefore not endocytosed by the cell, it will be eliminated in a matter of hours by the gastrointestinal tract, avoiding organ accumulation observed with intravenous injected particles. Another potential advantage is that drug release can occur on a relatively short time scale, the order of hours rather than weeks, months or years. Certainly, oral delivery of microfabricated devices is a field that holds a lot of promise.
Transdermal delivery
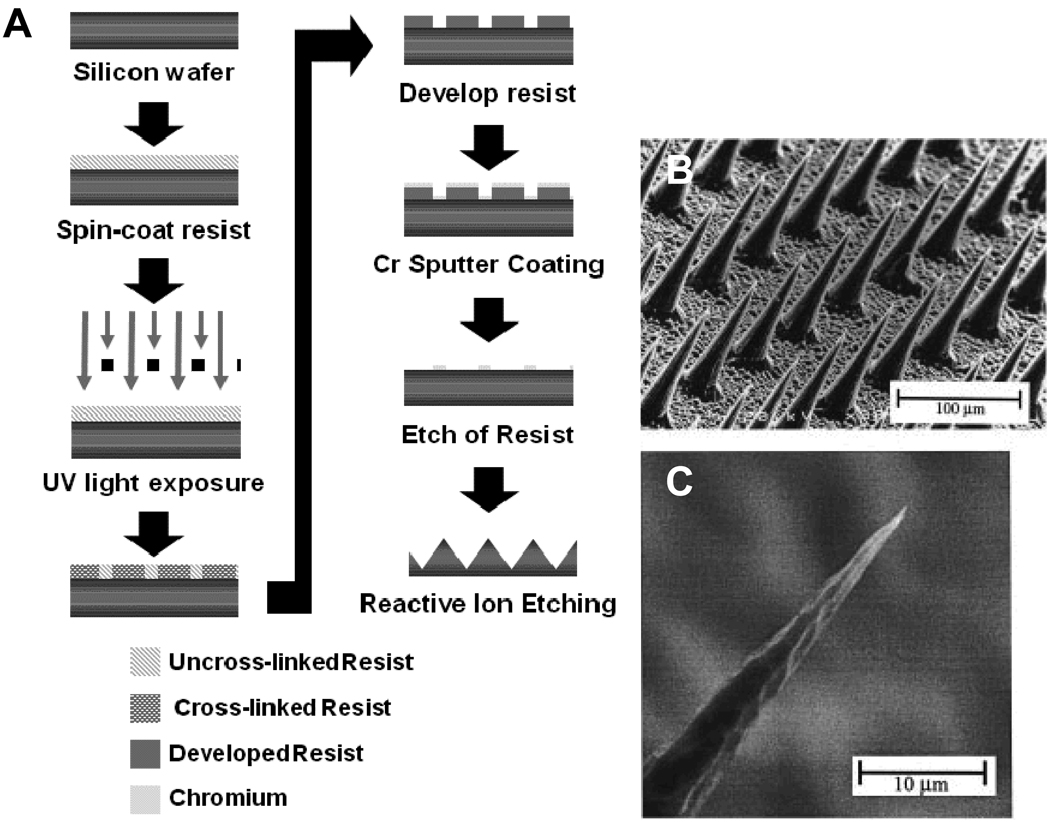
Due to the high density of vascular beds in the dermis, delivering drugs through the epidermis to this region can aid in therapeutic applications. Currently, non-microfabricated techniques include transdermal patches for birth control and nicotine addiction treatments. Microfabricated silicon and polymer based needles have been used to enhance dermal permeability as well as deliver therapeutics.30, 97–102 (Fig. 4) The microneedles work like thousands of tiny syringes, on the scale of 10–100 microns in diameter, that permeate the epidermal layer and deliver compounds to the dermis below. Besides the aforementioned application of these microneedles for vascular delivery,86 they have also been used for ocular,100 vaccines and DNA86, 97–99 delivery. In addition to silicon construction, the microneedles can be microfabricated through micromolding to form platforms of biodegradable carboxymethylcellulose102, amylopectin102, polylactic acid,99 polyglycolic acid,99 and PLGA.99 Microfabricated microneedles can aid in the en masse application of vaccines and treatments at relatively low costs.
Figure 4.
Micromachining of microneedles made of silicon. (A) process flow diagram for microfabrication of silicon microneedles. Adapted from Henry et al. (B) and (C) scanning electron micrographs of microneedles.97
Cell Based Drug Delivery Therapy
For the treatment of diseases like diabetes, the therapeutic approach needs to be a dynamic one that incorporates both sensing, for example blood glucose, and drug delivery, e.g. insulin. Replacing the cells damaged in such a disease is one way to replicate this dynamic environment. Unfortunately, foreign cells implanted in the body, are commonly attacked and destroyed by the immune system, so a protective system need to be fabricated to facilitate such an implant. Ideally, the system would have a limited diffusion barrier for the analyte and cell nutrients to diffuse in and the therapeutic to diffuse out. Additionally, this diffusion barrier would need to be impermeable to immune cells and antibodies. Desai et al. has developed a microfabricated membrane that can be incorporated into a device that both limits immune cell signaling and allows for adequate diffusion (Fig. 2).11, 82, 103–105 Nano-channels on the surface of the membrane allow for quick diffusion of glucose and other nutrients, yet inhibit transport of antibodies. Micromachining was used to fabricate the immunoisolating membrane for cellular encapsulation applications. During the microfabrication process, the thickness of the silicon oxidation layer controls the thickness of the diffusion pore and can vary from 10–100 nm with a uniform reproducibility of around 5% across a 4 inch wafer.82, 106 This membrane can be incorporated into a silicon device with a cell reservoir for islet cell transplant.11, 103
Other delivery routes
Long term implantation of microfabricated drug delivery devices has been used in a variety of tissue systems and disease states. Through traditional micromachining techniques, a silicon based multi-channel probe connected to a micropump can be used to deliver therapeutics directly into the neural tissue at a rate of 100 pL/sec to stimulate or monitor neural tissue.107 To deliver basic fibroblast growth factor (bFGF), researchers have micromolded a biodegradable pump out of PLGA for treatment of bone tissue.79 Although a considerable amount of microfabricated implants have focused on active delivery through micropumps108, passive diffusion is also common for therapeutic delivery. A layered system has been developed through micromolding where diffusion is regulated through micro-orifices on the order of 100 microns.109 The pore size of the device can be controlled through micromolding, which can be advantageous for drug release kinetics. By controlling the pore size of drug delivery devices, traditional concentration dependent diffusion kinetics can be turned into zero-order kinetics, such as can be seen with silicon membranes16, 110–114 and is predicted to be available with drug release from titanium nanotubes115. A system wherein triggered release of therapeutics, has been developed through silicon micromachining, photolithographic and chemical vapor deposition processes. Single or multiple reservoirs are incorporated into a silicon device. The reservoirs are gold coated and serve as the anode with a microfabricated cathode also present on the chip. A small electric potential is placed across the chip, dissolving the thin gold membrane and releasing the therapeutic payload.38 This technology has been fabricated from PLGA116, and been modified to include a sensor aspect for triggered release117 as well as releasing of MRI imaging reagents.117
Other delivery routes, such as nasal, buccal, and pulmonary, have not yet found suitable microfabricated implant devices, perhaps due to the potential harm incurred with vehicles on the order of one micron or larger in some of these systems. Additionally, therapeutic delivery systems incorporating vaccines have not been explored beyond the application of microneedles.118 Future applications which reduce the resolution scale of techniques such as photolithography and micromolding, can perhaps lead to technologies for alternative delivery routes not yet explored.
Tissue Engineering
The matrix surrounding cells is on the micro- and nano-scale. Development of micro-scaled tissue engineering scaffolds lends itself well to microfabrication techniques due to the wide variety of materials, many biodegradable, which can be formulated with such methods. Microfabricated implants have been engineered for tissue engineering applications in a variety of systems including vascular, bone, neural, respiratory and urinary (see Table 3). The chief advantage of microfabricated implants for tissue engineering is illustrated in the phenomena of contact guidance.119 For example, fibroblasts grown on microfabricated polystyrene channels (1–20 µm wide and 0.5–5.4 µm deep) will align along the length of the channels only when contact between cells and the bottom of the grooves is present.120 This highlights the need for precise engineering of surface micro-structure, as facilitated through microfabrication.
Table 3.
Microfabricated tissue engineering implants. An * indicates related references.
| Tissue System | Description | Microfabrication Method(s) |
Feature Size | Reference |
|---|---|---|---|---|
| Cardio-Vascular | ECs grown on nano- and micro-structured titanium comparable, as well as controls consisting of random nanostructured features. EC alignment on nanometer-patterned titanium surfaces. |
Micromachining | 750 nm to 100 µm | 124 |
| Cardio-Vascular | Microrods fabricated from SU-8 contained in a 3D collagen matrix with fibroblasts, reduced cell proliferation. |
Photolithography | 100 × 15 × 15 µm3 (L × H × W) |
129 |
| Ocular | Retinal progenitor cells differentiated and migrated on PMMA scaffolds with either smooth or porous surface topography. |
Lithography and Micromachining |
6 µm thickness 11 µm diameter pours |
134 135* |
| Orthopedic | Human connective tissue progenitor cells were grown on PDMS substrates with post and channel micro-textures. Cells grown with posts exhibited more contoured morphology with closely packed cytoskeletal actin microfilaments and increased proliferation. Cells in channels were more densely packed then with posts. |
Micromachining and Micromolding |
Post: 7–10 µm high, 5–40 µm in diameter Channels: 11 mm wide by 45 mm high |
131 |
| Cardio-Vascular | VSMC aspect ratio, alignment, and oriented remodeling of the underlying extracellular matrix enhanced with PDMS grooved scaffolds. |
Lithography and Micromolding |
Width of 19, 48, and 79 µm by 12 µm spacing by 5 µm groove depth |
121 157* |
| Cardio-Vascular | Enhanced patterning of HUVECs with a PEG coated inter- channel space. |
Micromolding | 460 µm channels | 125 |
| Cardio-Vascular | VSMC alignment similar on PLGA-leached and non-porous micro-patterned scaffolds. |
Micromolding | 48 µm grooves; 5 µm deep; 12 mm spacing |
121, 122 |
| Orthopedic | More hydrophilic, porous and nanoscaled roughened NaOH treated PLGA scaffold displayed enhanced chondrocyte functions. |
Micromolding | Average pore diameter 215 µm |
130 158–162* |
| Cardio-Vascular | Human retinal endothelial cell relocate inside the microfabricated silicon grooves twice as fast in response to vascular endothelial growth factor (VEGF) stimulation. |
Micromachining | 50 µm | 126 |
| Ocular | Human corneal epithelial cells on microfabricated nanochannels of silicon elongated and aligned along patterns of grooves and ridges, whereas on smooth substrates, cells were mostly round. |
E-beam lithography and CVD |
20 nm and 200 nm |
132 133* |
| Urinary | Both PLGA and PU nano-scale roughness increased bladder SMC adhesion. |
Micromolding | Roughness of PLGA (206 nm) and PU (368 nm) |
136 |
| Cardio-Vascular | Fibroblasts on micropegged surface had reduced proliferation while myocytes had unaltered proliferation. |
Photolithography and Micromolding |
10 µm high, 30×100 µm Spacing |
128 127, 163–166* |
| Cardio-Vascular | Microfludic gels of collagen, collagen–chitosan, matrigel and fibrin created a biomimetic arterial structure. |
Micromolding | Channels 500 µm wide and 300 µm high |
123 167–170* |
| Cardio-Vascular | Fibroblasts cultured on microgroves polystyrene showed that the number of cells was not dependent on the highest amount of surface area, although the strongest alignment was with these surfaces. Also that deep channels resulted in bridging and shallow channels resulted in contact guidance. |
Micromolding | 1–20 µm wide and 0.5–5.4 µm deep |
120 |
Vascular and Cardiac Microfabricated Tissue Engineering Constructs
Researchers have utilized vascular and cardiac tissue constructs to mimic cell orientation as well as develop therapeutic tissue engineering applications for disease states such as myocardial infarction. In a vessel wall, smooth muscle cells are aligned in distinct patterns that result in specific vasocontraction patterns. Alignment of vascular cells has been shown by limiting the cell’s growth area. Vascular smooth muscle cells (VSMCs) are aligned by PDMS121 and PLGA121, 122 constructed microchannels. To create the microchannels photolithography was applied to fabricate topographical features of 48 micron groves spaced 12 microns apart and 5 microns deep, generating a master for micromolding. A PDMS mold was fabricated, molten PCL was added and pressure on the order of 10 psi was administered to produce an even PCL thickness of approximately 50 microns. The PCL scaffold was then carefully pealed off the PDMS mold.121, 122 By incorporating extra cellular matrix proteins in gels, with microfabricated channels, the three main cellular components of vessel walls (fibroblasts, VSMCs and endothelial cells) were patterned in co-culture to create a vessel wall model (Fig. 5).123 Each microfabricated layer was tailored to specify the orientation of that particular cell type and then the semi-porous surfaces were layered to recreate the layered structure of the tissue. In addition to cell alignment in microfabricated polymer constructs, endothelial cells (ECs) have shown alignment on nanostructured titanium surfaces.124 Similarly, human umbilical vein endothelial cells (HUVECs) also align when constrained by PEG coated channels.125 The relocation of endothelial cells into the microfabricated channels was shown to be twice as fast when vascular endothelial growth factor (VEGF) was introduced into the channel.126
Figure 5.
Micromolding fabrication of PCL tissue engineering scaffolds. (A) Process flow diagram for development of micro-fabricated PCL films. Adapted from Sarkar et al.121, 122 (B) Scanning electron micrograph of non-porous PCL scaffold. (C) Scanning electron micrograph of cross section of PCL scaffold.
Cardiac tissue engineering therapies have been developed with microfabricated pegs on a surface and detached pegs (microrod). Formation of in vivo like cardiac myocyte sarcomeric striations were initially reported on micro-pegged and grooved surfaces with posts approximately 5 microns high.127 On similarly pegged surfaces, scar-tissue forming fibroblasts displayed reduced proliferation while cardiomyocytes had similar proliferation to flat controls.128 The same trend in reduced fibroblast proliferation was observed with microrods of similar dimension.129 Both the pegged and microrod system have clear application in the cardiac tissue therapy; however, the microrod system allows for easier administration of a micropatterned surface, perhaps through syringe injection.
Other Tissue Engineering Constructs
Adhesion of bone and bone precursor cells is of importance with orthopedic implants. More than 30,000 orthopedic implant surgeries19 are revised each year, which could be reduced with increased implant fixation and cellular adherence. Chondrocytes grown on nano-porous PLGA scaffolds also displayed enhanced cellular function.130 Similarly, connective tissue progenitor cells displayed increased proliferation and more in vivo like morphology when grown on surfaces with PDMS posts. The same cells grown in PDMS channels displayed more dense cell packing.131 Based on these studies, the addition of microstructure to implant surfaces could aid in orthopedic implant fixation to the surrounding tissue.
Microfabricated tissue engineering constructs have also been applied in a variety of other tissue systems including ocular and urinary. Corneal epithelial cells on microfabricated silicon nanochannels elongated along the length of the channel and were mostly round on smooth substrates.132, 133 Retinal progenitor cells grown on microfabricated PMMA (Figure 3) displayed signs of cell differentiation and migrated off the surface of the scaffold to insert into the tissue layer. Tao et al. used both photolithography and reactive ion etching to create poly(methyl methacrylate) (PMMA) thin films for retinal progenitor cell ocular implantation.134 In the creation of the scaffold, photolithography is used to create a resist mask for the etching process. The resist and PMMA is etched to create circular holes in PMMA. (Supplementary Figure 2) The original PMMA scaffold has been microfabricated with biodegradable poly(glycerol-sebacate) to further develop this novel retinal tissue engineering scaffold.135 The high incidence of bladder cancer and urinary incontinence results in the need for tissue engineered bladders. On PLGA and PU nano-roughened surfaces, BSMC adhesion was shown to be increased.136
Microfabricated tissue engineering implants have had some success in a variety of tissue systems. Concerns still exist with regards to scaffold degradability. Many of the techniques used to form initial constructs of non-degradable polymer tissue engineering scaffolds can be easily altered to develop similar platforms using degradable polymers, such as PLGA and PCL. In addition to biodegradability concerns, the incorporation of therapeutic release could aid in the success of the implant. By adding the release of therapeutics or chemical signals from microfabricated tissue engineering implants, stem cell fate and survival can further be defined, as well as the mechanical cues of the scaffold itself. Microfabrication of a scaffold that can incorporate biodegradability, therapeutic release and mechanical cell signaling can significantly further the development of clinically applied therapies.
Biosensors
Microfabrication of biosensors is beneficial because of the small sample volume required, as well as the device being small. By definition, a biosensor is a device that transforms or detects a biological signal and converts it into a more easily detectable one. Detection of one or more analytes in the complex moiety of biological fluids such as blood, and interstitial fluid can lead to non-specific binding, fouling and sensor drift, in addition to adverse effects such as embolism formation in some tissue systems. The foreign body response is one such event that can lead to sensor failure. Over the course of a month, an implanted sensor can become fouled with proteins and cells on the order of 10 to 100 microns in thickness.137 This cellular encapsulation forms a mass transfer barrier between the sensor and the analyte. Methods to prevent or limit capsule formation include anti-fouling polymers like PEG,138 biomimics such as phospholipids,139, 140 flow based systems,141 membranes,141 and nanostructured surface topography, like nanowires.12 The precise engineering afforded with microfabrication can aid in the incorporation of these non-fouling elements. For example, patterned areas can be modified with photolithography or micromachining to form preferential areas for surface chemistry, or micro- and nano-scaled features can be directly added to the sensor surface. A variety of microfabricated biosensors are presented in Table 4. There are two main types of microfabricated biosensors: electrochemical and mechanical loading. Electrochemical sensors can have a microfabricated body with micro-patterned electrodes and enzymatic regions. Biosensors that work through mechanical loading turn the biological signal into a detectable mechanical movement that can be detected.
Table 4.
Microfabricated biosensor based implants.
| Analyte | Description | Microfabrication Method(s) |
Feature Size | Reference |
|---|---|---|---|---|
|
Atherosclerotic pressure sensor |
Formation of atherosclerotic plaque was measured in vivo with a BioMEMs pressure sensor. |
Micromachining | 1 to 20 microns | 35 |
| Ethanol | Ethanol with methyl phenyl mercapto propyl silicone functionalized silicon microcantilevers in rats. |
Micromachining | 120 µm long | 148 |
| Glucose | Micro- and nano-pore platinum and silicon substrates were used as electrochemical substrates in conjunction with glucose oxidase. |
Electrochemical Microfabrication and Micromachining |
10 µm pore, 80 µm deep | 143 |
| Glucose | Glucose oxidase is patterned on a flexible polymer substrate (Kapton film) that is then rolled to fit inside a blood vessel. The enzyme is upstream and the sensing elements down stream. |
Photolithography | 20 µm | 144 |
|
Neural Action Potentials |
Neural probes fabricated that perform in vivo recording acute neural action potentials. |
Micromachining | 50 µm by 128 µm in cross- section |
145 |
|
Neural Action Potentials |
The array consisted of 64 gold electrodes on an 8×8 grid for EEG measurements. |
Photolithography and Micromachining |
150 µm diameter spaced 750 µm |
146 |
| Bacterial Growth | Biofilm formation through bacterial receptor-ligand biosensor binding and integrated reservoirs release antibiotics and destroy bacteria with an electric field. For orthopedic applications. |
Micromachining | 300 µm | 149 |
| Spinal Pressure |
In vivo analysis of silicon MEMs spinal pressure sensor showed no necrosis of foreign body response surrounding the implant. |
Electrochemical Microfabrication and Micromachining |
150 × 150 µm2 | 34 |
| L-glutamate | Glutamate oxidase was used to electrochemically monitor glutamate in the brains of rats. |
Micromachining | 50 by 150 µm 2 | 142 |
Electrochemical Sensors
The ease of incorporating integrated circuitry into microfabricated devices facilitates biosensors development, particularly electrochemical sensors. A majority of the biochemical electrochemical sensors developed focus on the incorporation of an oxidase enzyme that reduces in the presence of oxygen to form hydrogen peroxide. Some of the drawbacks of enzymatic based biosensors are the degradation of the enzyme in vivo and oxygen diffusion to the enzyme/electrode surface. The small feature size of microfabricated elements can limit one of these obstacles by reducing the oxygen path length to the electrode surface by producing high aspect ratio elements. Commonly, such sensors are made through traditional micromachining techniques wherein electrodes can be microfabricated at discrete locations and scales. Glutamate oxidase bound to the end of a syringe to detect a L-glutamate, a common neurotransmitter, is one example of a biosensor that could be directly implanted into the brain tissue.142 To increase the surface area available to detect electrochemical signaling, micro- and nano-porous platinum was used as a base substrate for glucose oxidase functionalization to detect blood glucose levels for diabetes treatment.143 A novel modification of the traditional flat geometry used to create microfabricated electrochemical biosensors is where Kapton film is rolled up in the vascularature to detect blood glucose levels in vivo. When rolled, the areas for enzymatic reaction are upstream of the sensing electrodes.144 In addition to the electrical signal detected from an enzymatic reaction, electrochemical sensors can detect biological electrical signals such as neuronal action potentials.145 Acute neural action potentials can be amplified and detected using microfabrication techniques to create a multi-plexed electrode system. Micromachining facilitates the multi-electrode formulation needed for detection of action potential signals, such as the 8 by 8 electrode array created for electroencephalography (EEG) measurements (Fig. 7). The multi-electrode array is created with surface micromachining techniques and photolithography. By masking with a photoresist and sputter coating with various metal alloys, multiple electrically isolated features can be created.146 The ease of incorporating integrated signaling and the engineering of microscale electrodes leads to beneficial development of electrochemical biosensors.
Mechanical Loading Biosensors
Mechanical loading of a thin layer, usually silicon based, can be measured via the piezoresistive effect or laser diffraction. Piezoresistive effect is characterized by a change in electrical resistance as a result of mechanical stress. Quartz and silicon are examples of piezoelectric materials. A silicon-based piezoresistive pressure sensor was developed through micromachining and implanted in a caprine model. After six-months of implantation, the sensor showed no signs of necrosis in the surrounding tissue or an adverse foreign body response.34 A similar type sensor could be implanted in the atherosclerotic plaque to measure stenosis.35 With piezoresistive sensors, non-specific binding can result in indiscriminate signaling. The addition of non-fouling layers or blocking with proteins beforehand can aid in reducing this phenomena. Mechanical movement of a biosensor can also be measured with laser diffraction by using a micromechanical cantilever (microcantilever). Microcantilevers are fabricated through micromachining, wherein silicon is etched away leaving a diving-board like platform on the order of hundreds of microns in length and tens of microns in width (Supplementary Figure 3). As the cantilever is loaded mechanically, surface stresses cause it to deflect, and this deflection can be monitored by reflecting a laser off the tip of the cantilever, much like atomic force microscopy. These microcantilevers have been used ex vivo to detect any number of analytes, including prostate-specific antigen.147 In vivo, microcantilevers have been inserted into the peritoneal cavity of rats to detect ethanol concentrations.148 One combination biosensor and drug delivery system is the antibacterial system developed by Ehrlich et al.149 A microcantilever’s surface is used to detect changes in viscosity as the result of glucose displacing dextran’s binding to the lectin concanavalin A. Glucose is specifically released near the sensor due to the binding of a bacterial quorum-sensing molecule (ribonucleic acid III-activating protein, RAP) to an engineered receptor protein (target of RNAIII-activating protein, TRAP), resulting in the activation of a galactosidase function that cleaves polysaccharides in the substrate into glucose monomers. Activation of cantilever deflection due to viscosity changes from RAP-TRAP binding opens two reservoirs that release inhibitory compounds and antibiotics to lyse bacterial cells. Such a sensor would be implanted during an orthopedic implant surgery to reduce bacterial growth and therefore prevent implant rejection.149 One advantage of deflection based sensors is that they do not require labeling or reaction for detection. Simple mechanical force on the sensor results in a discernable signal, as long as sensing specificity can be confirmed.
Numerous other microfabricated biosensors exist for non-implanted, lab on a chip use.15 Many of these sensing elements can be formulated into a bio-microelectromechanical system (BioMEM) for such-point-of-care systems. These systems are typically designed to be inexpensive and disposable, whereas the microfabricated biosensors here are meant to be implanted for long-term care and monitoring.
Conclusions
Microfabricated implants have had influence in a variety of fields including drug delivery, tissue engineering and biosensing. Development of these implants, particularly for drug delivery and tissue engineering, has been tested mostly with in vitro or ex vivo models. In vivo considerations such as mechanical forces, biodistribution and removal from the body have had limited study with regards to microfabricated implants. Application in an in vivo environment would also require further consideration of material construction and biodegradability. Many of the implants are initially developed in materials like silicon, tested in vitro and then developed further with biodegradable materials. Considering that the field of microfabricated implants is less than two decades old, limited development of these technologies is certainly reasonable and in vivo models are certain to be more reported in the near future.
In addition to further study in animal models, incorporation of implants that accomplish all or multiples of these areas, drug delivery, tissue engineering and sensing, would be desirable. Ehrlich et al. have accomplished incorporation of bacterial quorum sensing as well as antibiotic delivery, as one example of multiple functions on a single implant.149 Also, tissue engineering scaffolds that incorporate the delivery of growth factors to stimulate cell and tissue growth would be a step towards an improved therapy.126 Besides incorporating drug delivery, tissue engineering and sensing, the addition of imaging would also be advantageous. One example is the work from the Ferrari laboratory that incorporates imaging and drug delivery with mesoporous silicon spheres.87 Further development of multiple function devices will certainly allow for microfabricated implants to have a broader biological impact.
Microfabricated elements allow for design at a biologically relevant scale that can easily include integrated circuit technology on a low-cost and high-throughput platform. Although microfabrication might not be relevant for all tissue systems and biotechnological applications, the features of the broad techniques available can produce viable devices, as described here. As time passes and additional microfabrication techniques are developed to produce features at a smaller scale with additional materials, the field of microfabricated implants will no doubt result in more sophisticated and complicated structures.
Supplementary Material
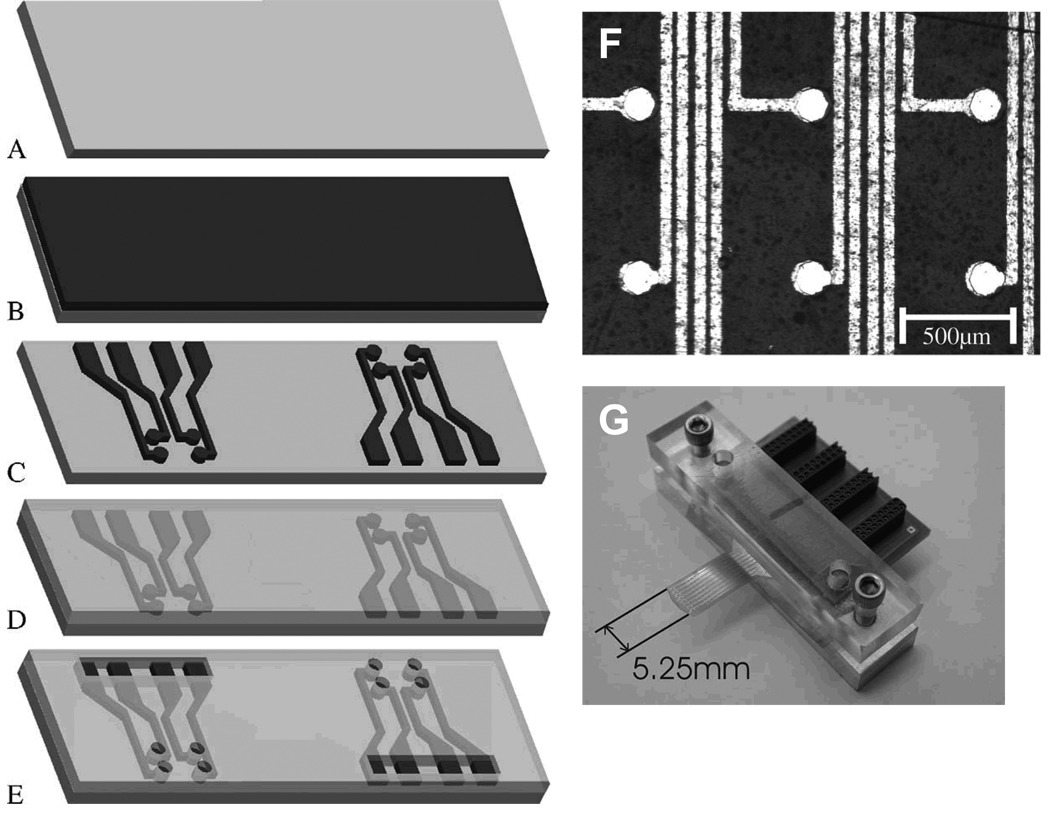
Figure 6.
Microfabrication of multi-electrode array for electroencephalography measurements. (A) A 50 micron thick Kapton film. (B) Sputter coating of 5 nanometers of titanium-tugsten alloy followed by 300 nm of gold. (C) Patterning of alloy through photolithography, resulting in a gold circuit consisting of an 8×8 grid of 64 electrodes and connection pads. (D) Spin coating of SU-8 over entire surface. (E) Photolithographic patterning of SU-8 to expose each electrode and connection pads. (F) Scanning electron micrograph of electrode surface. The light areas are the gold substrate and the black areas the Kapton film. (G) Electrodes with circuit board. Adapted from Hollenberg et al.146
Acknowledgments
The authors would like to Daniel Bernards, Eric Bachelder, Rahul Thakar, Mark Steedman, Adam Mendelsohn, and Kayte Fischer.
Biographies

Dr. Ainslie is a Post-doctoral scholar at the University of California, San Francisco. Her research is on the microfabrication of oral drug delivery vehicles and the immune response of nanobiomaterials. Previously, she studied biosensors at the Navel Research Laboratory in Washington, DC and completed a PhD at The Pennsylvania State University in the application of nanobiomaterials for anti-fouling implant surfaces.

Dr. Desai is a Professor at the University of California, San Francisco. Her research combines method and materials originally used for MEMs to create implantable biohydrid devices for cell encapsulation, templates for cell and tissue regeneration, and novel protocols for the surface modifications of biomaterials. She is an associate editor of Langmuir, Biomedical Microdevices, and Sensor Letters.
References
- 1.Madou M. Fundamentals of Microfabrication: The Science of Miniaturization. ed. 2nd. New York, NY: CRC Press; 2002. p. 723. [Google Scholar]
- 2.Lim JY, Donahue HJ. Tissue Engineering. 2007;13:1879. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 3.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung DR, Kapur R, Adams T, Giuliano KA, Mrksich M, Craighead HG, Taylor DL. Critical Reviews in Biotechnology. 2001;21:111. doi: 10.1080/20013891081700. [DOI] [PubMed] [Google Scholar]
- 5.Champion JA, Katare YK, Mitragotri S. J Control Release. 2007;121:3. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champion JA, Mitragotri S. Proc Natl Acad Sci U S A. 2006;103:4930. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vora KD, Peele AG, Shew BY, Harvey EC, Hayes JP. Microsystem Technologies-Micro-and Nanosystems-Information Storage and Processing Systems. 2007;13:487. [Google Scholar]
- 8.Lopez CA, Fleischman AJ, Roy S, Desai TA. Biomaterials. 2006;27:3075. doi: 10.1016/j.biomaterials.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Saha K, Pollock JF, Schaffer DV, Healy KE. Curr Opin Chem Biol. 2007;11:381. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin F, Walczak R, Boiarski A, Cohen M, West T, Cosentino C, Shapiro J, Ferrari M. J Control Release. 2005;102:123. doi: 10.1016/j.jconrel.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Desai TA, West T, Cohen M, Boiarski T, Rampersaud A. Advanced Drug Delivery Reviews. 2004;56:1661. doi: 10.1016/j.addr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Ainslie KM, Sharma G, Dyer MA, Grimes CA, Pishko MV. Nano Letters. 2005;5:1852. doi: 10.1021/nl051117u. [DOI] [PubMed] [Google Scholar]
- 13.Ogura E, Abatti PJ, Moriizumi T. IEEE Trans Biomed Eng. 1991;38:721. doi: 10.1109/10.83583. [DOI] [PubMed] [Google Scholar]
- 14.Receveur RAM, Lindemans FW, de Rooij NF. Journal of Micromechanics and Microengineering. 2007;17:R50. [Google Scholar]
- 15.Voldman J, Gray ML, Schmidt MA. Annual Review of Biomedical Engineering. 1999;1:401. doi: 10.1146/annurev.bioeng.1.1.401. [DOI] [PubMed] [Google Scholar]
- 16.Leoni L, Desai TA. Advanced Drug Delivery Reviews. 2004;56:211. doi: 10.1016/j.addr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Gardner P. Expert Opin Drug Deliv. 2006;3:479. doi: 10.1517/17425247.3.4.479. [DOI] [PubMed] [Google Scholar]
- 18.Norman JJ, Desai TA. Ann Biomed Eng. 2006;34:89. doi: 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- 19.Liu HA, Webster TJ. Biomaterials. 2007;28:354. doi: 10.1016/j.biomaterials.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Nie SM, Xing Y, Kim GJ, Simons JW. Annual Review of Biomedical Engineering. 2007;9:257. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 21.Pope-Harman A, Cheng MMC, Robertson F, Sakamoto J, Ferrari M. Medical Clinics of North America. 2007;91:899. doi: 10.1016/j.mcna.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Polla DL, Erdman AG, Robbins WP, Markus DT, Diaz-Diaz J, Rizq R, Nam Y, Brickner HT, Wang A, Krulevitch P. Annual Review of Biomedical Engineering. 2000;2:551. doi: 10.1146/annurev.bioeng.2.1.551. [DOI] [PubMed] [Google Scholar]
- 23.Peckham PH, Knutson JS. Annual Review of Biomedical Engineering. 2005;7:327. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 24.Weiland JD, Liu WT, Humayun MS. Annual Review of Biomedical Engineering. 2005;7:361. doi: 10.1146/annurev.bioeng.7.060804.100435. [DOI] [PubMed] [Google Scholar]
- 25.Wilson BS, Lawson DT, Muller JM, Tyler RS, Kiefer J. Annual Review of Biomedical Engineering. 2003;5:207. doi: 10.1146/annurev.bioeng.5.040202.121645. [DOI] [PubMed] [Google Scholar]
- 26.West JL, Halas NJ. Annual Review of Biomedical Engineering. 2003;5:285. doi: 10.1146/annurev.bioeng.5.011303.120723. [DOI] [PubMed] [Google Scholar]
- 27.Rutten WLC. Annual Review of Biomedical Engineering. 2002;4:407. doi: 10.1146/annurev.bioeng.4.020702.153427. [DOI] [PubMed] [Google Scholar]
- 28.Maynard EM. Annual Review of Biomedical Engineering. 2001;3:145. doi: 10.1146/annurev.bioeng.3.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Annual Review of Biomedical Engineering. 2000;2:9. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 30.McAllister DV, Allen MG, Prausnitz MR. Annual Review of Biomedical Engineering. 2000;2:289. doi: 10.1146/annurev.bioeng.2.1.289. [DOI] [PubMed] [Google Scholar]
- 31.Loeb GE. Annual Review of Neuroscience. 1990;13:357. doi: 10.1146/annurev.ne.13.030190.002041. [DOI] [PubMed] [Google Scholar]
- 32.Tao SL, Desai TA. Drug Discovery Today. 2005;10:909. doi: 10.1016/S1359-6446(05)03489-6. [DOI] [PubMed] [Google Scholar]
- 33.Tao SL, Desai TA. Advanced Drug Delivery Reviews. 2003;55:315. doi: 10.1016/s0169-409x(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara LA, Fleischman AJ, Togawa D, Bauer TW, Benzel EC, Roy S. Biomedical Microdevices. 2003;5:297. [Google Scholar]
- 35.Steeves CA, Young YL, Liu Z, Bapat A, Bhalerao K, Soboyejo ABO, Soboyejo WO. Journal of Materials Science-Materials in Medicine. 2007;18:25. doi: 10.1007/s10856-006-0659-8. [DOI] [PubMed] [Google Scholar]
- 36.Desai TA, Hansford D, Ferrari M. Journal of Membrane Science. 1999;159:221. [Google Scholar]
- 37.Ma B, Liu S, Gan ZY, Liu GJ, Cai XX, Zhang HH, Yang ZG. Microfluidics and Nanofluidics. 2006;2:417. [Google Scholar]
- 38.Santini JT, Jr, Cima MJ, Langer R. Nature. 1999;397:335. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 39.Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. Ieee Transactions on Biomedical Engineering. 1991;38:758. doi: 10.1109/10.83588. [DOI] [PubMed] [Google Scholar]
- 40.Chow AY, Pardue MT, Chow VY, Peyman GA, Liang CP, Perlman JI, Peachey NS. Ieee Transactions on Neural Systems and Rehabilitation Engineering. 2001;9:86. doi: 10.1109/7333.918281. [DOI] [PubMed] [Google Scholar]
- 41.Santiesteban FMM, Swanson SD, Noll DC, Anderson DJ. Ieee Transactions on Biomedical Engineering. 2006;53:547. doi: 10.1109/TBME.2005.864497. [DOI] [PubMed] [Google Scholar]
- 42.Dario P, Carrozza MC, Benvenuto A, Menciassi A. Journal of Micromechanics and Microengineering. 2000;10:235. [Google Scholar]
- 43.LaVan DA, McGuire T, Langer R. Nature Biotechnology. 2003;21:1184. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 44.Menz W, Guber A. Minimally Invasive Neurosurgery. 1994;37:21. doi: 10.1055/s-2008-1053444. [DOI] [PubMed] [Google Scholar]
- 45.Mokwa W. Measurement Science & Technology. 2007;18:R47. [Google Scholar]
- 46.Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Advanced Drug Delivery Reviews. 2004;56:185. doi: 10.1016/j.addr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Schurr MO. Minimally Invasive Therapy & Allied Technologies. 2007;16:75. doi: 10.1080/13645700701266909. [DOI] [PubMed] [Google Scholar]
- 48.Staples M, Daniel K, Cima MJ, Langer R. Pharm Res. 2006 doi: 10.1007/s11095-006-9906-4. [DOI] [PubMed] [Google Scholar]
- 49.Wallrabe U, Ruther P, Schaller T, Schomburg WK. International Journal of Artificial Organs. 1998;21:137. [PubMed] [Google Scholar]
- 50.Lillis B, Jungk C, Iacopino D, Whelton A, Hurley E, Sheehan MM, Splinter A, Quinn A, Redmond G, Lane WA, Mathewson A, Berney H. Biosensors & Bioelectronics. 2005;21:282. doi: 10.1016/j.bios.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Park IY, Li ZY, Li XM, Pisano AP, Williams RS. Biosensors & Bioelectronics. 2007;22:2065. doi: 10.1016/j.bios.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 52.De Stefano L, Rotiroti L, Rendina I, Moretti L, Scognamiglio V, Rossi M, D'Auria S. Biosensors & Bioelectronics. 2006;21:1664. doi: 10.1016/j.bios.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Balarin A, Gamulin O, Ivanda A, Djerek V, Celan O, Music S, Ristic A, Furic K. Journal of Molecular Structure. 2007;834:465. [Google Scholar]
- 54.Chambon E, Florentin E, Torchynska T, Gonzalez-Hernandez J, Vorobiev Y. Microelectronics Journal. 2005;36:514. [Google Scholar]
- 55.Jelinek I, Chvojka T, Vrkoslav V, Jindrich J, Lorenc M, Niznansky D, Nemec I, Kral V, Dian J. Chimia. 2005;59:222. [Google Scholar]
- 56.Natarajan B, Ramakrishnan V, Vasu V, Ramamurthy S. Surface Review and Letters. 2006;13:351. [Google Scholar]
- 57.Peng KQ, Hu JJ, Yan YJ, Wu Y, Fang H, Xu Y, Lee ST, Zhu J. Advanced Functional Materials. 2006;16:387. [Google Scholar]
- 58.Reddy RRK, Chadha A, Bhattacharya E. Biosensors & Bioelectronics. 2001;16:313. doi: 10.1016/s0956-5663(01)00129-4. [DOI] [PubMed] [Google Scholar]
- 59.Wilk SJ, Petrossian L, Goryll A, Thorriton TJ, Goodnick SM, Tang JM, Eisenberg RS. Biosensors & Bioelectronics. 2007;23:183. doi: 10.1016/j.bios.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 60.Chen SQ, Zhu ZQ, Zhu JZ, Zhang JA, Shi YL, Ke Y, Wang WM, Wang XH, Feng XA, Luo LQ, Li S. Applied Surface Science. 2004;230:418. [Google Scholar]
- 61.Hing KA, Revell PA, Smith N, Buckland T. Biomaterials. 2006;27:5014. doi: 10.1016/j.biomaterials.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 62.Weis RP, Montchamp JL, Coffer JL, Attiah DG, Desai TA. Disease Markers. 2002;18:159. doi: 10.1155/2002/727014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edquist BT, White TB, Fulmer GR, Thornberry DR, Porter LA. Abstracts of Papers of the American Chemical Society. 2006;231:292. [Google Scholar]
- 64.Fulmer GR, Thornberry DR, Rhodes SD, Porter LA. Abstracts of Papers of the American Chemical Society. 2005;229:U514. [Google Scholar]
- 65.Thornberry DR, Fulmer GR, Rhodes SD, Porter LA. Abstracts of Papers of the American Chemical Society. 2005;229:U679. [Google Scholar]
- 66.White TB, Edquist BT, Fulmer GR, Thornberry DR, Porter LA. Abstracts of Papers of the American Chemical Society. 2006;231 [Google Scholar]
- 67.Anderson JM, Defife K, McNally A, Collier T, Jenney C. J Mater Sci Mater Med. 1999;10:579. doi: 10.1023/a:1008976531592. [DOI] [PubMed] [Google Scholar]
- 68.Jenney CR, DeFife KM, Colton E, Anderson JM. J Biomed Mater Res. 1998;41:171. doi: 10.1002/(sici)1097-4636(199808)41:2<171::aid-jbm1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 69.Ainslie KM, Tao SL, Popat KC, Desai TA. ACS Nano. 2008;2:1076–1084. doi: 10.1021/nn800071k. [DOI] [PubMed] [Google Scholar]
- 70.Coltro WK, Piccin E, Fracassi da Silva JA, Lucio do Lago C, Carrilho E. Lab Chip. 2007;7:931. doi: 10.1039/b702931d. [DOI] [PubMed] [Google Scholar]
- 71.Nagarajan S, Bosworth JK, Ober CK, Russell TP, Watkins JJ. Chem. Mater. 2007 Epub. [Google Scholar]
- 72.Thomas MV, Puleo DA, Al-Sabbagh M. J Long Term Eff Med Implants. 2005;15:585. doi: 10.1615/jlongtermeffmedimplants.v15.i6.20. [DOI] [PubMed] [Google Scholar]
- 73.Bosetti M, Hench L, Cannas M. J Biomed Mater Res. 2002;60:79. doi: 10.1002/jbm.1282. [DOI] [PubMed] [Google Scholar]
- 74.Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM. Cytokine. 2002;18:311. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 75.Yamawaki H, Iwai N. Circulation Journal. 2006;70:129. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- 76.Lu Y, Chen SC. Adv Drug Deliv Rev. 2004;56:1621. doi: 10.1016/j.addr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Craighead HG, James CD, Turner AMP. Current Opinion in Solid State & Materials Science. 2001;5:177. [Google Scholar]
- 78.Panush RS. J Clin Lab Immunol. 1981;5:113. [PubMed] [Google Scholar]
- 79.Ryu W, Huang Z, Prinz FB, Goodman SB, Fasching R. J Control Release. 2007;124:98. doi: 10.1016/j.jconrel.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 80.Tao SL, Desai TA. Journal of Controlled Release. 2005;109:127. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 81.Tao SL, Desai TA. Advanced Materials. 2005;17:1625. [Google Scholar]
- 82.Desai TA, Chu WH, Tu JK, Beattie GM, Hayek A, Ferrari M. Biotechnology and Bioengineering. 1998;57:118. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 83.Mornet S, Vasseur S, Grasset F, Duguet E. Journal of Materials Chemistry. 2004;14:2161. [Google Scholar]
- 84.Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. paper presented at the Controlled Release Socitey; Long Beach, CA. July 7–11, 2007.2007. [Google Scholar]
- 85.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. J Am Chem Soc. 2005;127:10096. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 86.Reed ML, Wu C, Kneller J, Watkins S, Vorp DA, Nadeem A, Weiss LE, Rebello K, Mescher M, Smith AJ, Rosenblum W, Feldman MD. J Pharm Sci. 1998;87:1387. doi: 10.1021/js980085q. [DOI] [PubMed] [Google Scholar]
- 87.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MMC, Decuzzi P, Tour JM, Robertson F, Ferrari M. Nature Nanotechnology. 2008;3:151. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 88.Eaimtrakarn S, Itoh Y, Kishimoto JI, Yoshikawa Y, Shibata N, Takada K. Int J Pharm. 2001;224:61. doi: 10.1016/s0378-5173(01)00738-4. [DOI] [PubMed] [Google Scholar]
- 89.Fahr A, Liu X. Expert Opinion on Drug Delivery. 2007;4:403. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 90.Mustata G, Dinh SM. Critical Reviews in Therapeutic Drug Carrier Systems. 2006;23:111. doi: 10.1615/critrevtherdrugcarriersyst.v23.i2.20. [DOI] [PubMed] [Google Scholar]
- 91.Tao SL, Lubeley MW, Desai TA. Journal of Biomedical Materials Research Part A. 2003;67A:369. doi: 10.1002/jbm.a.10047. [DOI] [PubMed] [Google Scholar]
- 92.Tao SL, Lubeley MW, Desai TA. Journal of Controlled Release. 2003;88:215. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 93.Tao SL, Popat K, Desai TA. Nat Protoc. 2006;1:3153. doi: 10.1038/nprot.2006.451. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed A, Bonner C, Desai TA. Journal of Controlled Release. 2002;81:291. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 95.Ainslie K, Kraning C, Desai T. Lab Chip. doi: 10.1039/b800604k. In Press, DOI: 10.1039/b800604k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Bachman M, Sims CE, Li GP, Allbritton NL. Langmuir. 2006;22:2719. doi: 10.1021/la053188e. [DOI] [PubMed] [Google Scholar]
- 97.Henry S, McAllister DV, Allen MG, Prausnitz MR. J Pharm Sci. 1999;88:948. doi: 10.1021/js990783q. [DOI] [PubMed] [Google Scholar]
- 98.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Proc Natl Acad Sci U S A. 2003;100:13755. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park JH, Allen MG, Prausnitz MR. J Control Release. 2005;104:51. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, Prausnitz MR. Invest Ophthalmol Vis Sci. 2007;48:4038. doi: 10.1167/iovs.07-0066. [DOI] [PubMed] [Google Scholar]
- 101.Hallow DM, Mahajan AD, Prausnitz MR. J Control Release. 2007;118:285. doi: 10.1016/j.jconrel.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JW, Park JH, Prausnitz MR. Biomaterials. 2008;29:2113. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Desai TA. Expert Opinion on Biological Therapy. 2002;2:633. doi: 10.1517/14712598.2.6.633. [DOI] [PubMed] [Google Scholar]
- 104.Desai TA, Hansford DJ, Ferrari M. Biomolecular Engineering. 2000;17:23. doi: 10.1016/s1389-0344(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 105.Mendelsohn A, Desai T. In: Inorganic Nanoporous Membranes for Immunoisolated Cell-Based Drug Delivery. Pedraz J, Orive G, editors. Austin, TX: Landes Bioscience; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Desai TA, Hansford DJ, Leoni L, Essenpreis M, Ferrari M. Biosensors & Bioelectronics. 2000;15:453. doi: 10.1016/s0956-5663(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 107.Chen J, Wise KD, Hetke JF, Bledsoe SC., Jr IEEE Trans Biomed Eng. 1997;44:760. doi: 10.1109/10.605435. [DOI] [PubMed] [Google Scholar]
- 108.Tsai NC, Sue CY. Sensors and Actuators a-Physical. 2007;134:555. doi: 10.1016/j.sna.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryu WH, Vyakarnam M, Greco RS, Prinz FB, Fasching RJ. Biomed Microdevices. 2007;9:845. doi: 10.1007/s10544-007-9097-8. [DOI] [PubMed] [Google Scholar]
- 110.Lesinski GB, Sharma S, Varker KA, Sinha P, Ferrari M, Carson WE., 3rd Biomed Microdevices. 2005;7:71. doi: 10.1007/s10544-005-6174-8. [DOI] [PubMed] [Google Scholar]
- 111.La Flamme KE, Mor G, Gong D, La Tempa T, Fusaro VA, Grimes CA, Desai TA. Diabetes Technol Ther. 2005;7:684. doi: 10.1089/dia.2005.7.684. [DOI] [PubMed] [Google Scholar]
- 112.Leoni L, Attiah D, Desai TA. Sensors. 2002;2:111. [Google Scholar]
- 113.Leoni L, Boiarski A, Desai TA. Biomedical Microdevices. 2002;4:131. [Google Scholar]
- 114.Leoni L, Desai TA. Ieee Transactions on Biomedical Engineering. 2001;48:1335. doi: 10.1109/10.959329. [DOI] [PubMed] [Google Scholar]
- 115.Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA. Biomaterials. 2007;28:4880. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 116.Grayson AC, Cima MJ, Langer R. Biomaterials. 2005;26:2137. doi: 10.1016/j.biomaterials.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 117.Daniel KD, Kim GY, Vassiliou CC, Jalali-Yazdi F, Langer R, Cima MJ. Lab Chip. 2007;7:1288. doi: 10.1039/b705143c. [DOI] [PubMed] [Google Scholar]
- 118.Giudice EL, Campbell JD. Advanced Drug Delivery Reviews. 2006;58:68. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Walboomers XF, Croes HJE, Ginsel LA, Jansen JA. Journal of Biomedical Materials Research. 1999;47:204. doi: 10.1002/(sici)1097-4636(199911)47:2<204::aid-jbm10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 120.Walboomers XF, Monaghan W, Curtis ASG, Jansen JA. Journal of Biomedical Materials Research. 1999;46:212. doi: 10.1002/(sici)1097-4636(199908)46:2<212::aid-jbm10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 121.Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. Acta Biomaterialia. 2005;1:93. doi: 10.1016/j.actbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Sarkar S, Lee GY, Wong JY, Desai TA. Biomaterials. 2006;27:4775. doi: 10.1016/j.biomaterials.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 123.Tan W, Desai TA. Biomaterials. 2004;25:1355. doi: 10.1016/j.biomaterials.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 124.Lu J, Rao MP, MacDonald NC, Khang D, Webster TJ. Acta Biomaterialia. 2008;4:192. doi: 10.1016/j.actbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 125.Sharma S, Desai TA. Journal of Nanoscience and Nanotechnology. 2005;5:235. doi: 10.1166/jnn.2005.030. [DOI] [PubMed] [Google Scholar]
- 126.Kulkarni SS, Orth R, Ferrari M, Moldovan NI. Biosensors & Bioelectronics. 2004;19:1401. doi: 10.1016/j.bios.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 127.Motlagh D, Senyo S, Desai TA, Russell B. Journal of Molecular and Cellular Cardiology. 2002;34:A32. [Google Scholar]
- 128.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. American Journal of Physiology-Cell Physiology. 2003;285:C171. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 129.Norman JJ, Collins JM, Sharma S, Russell B, Desai TA. Tissue Eng. 2007 doi: 10.1089/tea.2007.0077. [DOI] [PubMed] [Google Scholar]
- 130.Park GE, Pattison MA, Park K, Webster TJ. Biomaterials. 2005;26:3075. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Mata A, Boehm C, Fleischman AJ, Muschler GF, Roy S. Int J Nanomedicine. 2007;2:389. [PMC free article] [PubMed] [Google Scholar]
- 132.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. J Cell Sci. 2003;116:1881. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, J C. Murphy, Biomaterials. 2006;27:3945. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tao S, Young C, Redenti S, Zhang Y, Klassen H, Desai T, Young MJ. Lab Chip. 2007;7:695. doi: 10.1039/b618583e. [DOI] [PubMed] [Google Scholar]
- 135.Neeley WL, Redenti S, Klassen H, Tao S, Desai T, Young MJ, Langer R. Biomaterials. 2008;29:418. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thapa A, Miller DC, Webster TJ, Haberstroh KM. Biomaterials. 2003;24:2915. doi: 10.1016/s0142-9612(03)00123-6. [DOI] [PubMed] [Google Scholar]
- 137.Padera RF, Colton CK. Biomaterials. 1996;17:277. doi: 10.1016/0142-9612(96)85565-7. [DOI] [PubMed] [Google Scholar]
- 138.Amirpour ML, Ghosh P, Lackowski WM, Crooks RM, Pishko MV. Analytical Chemistry. 2001;73:1560. doi: 10.1021/ac000907f. [DOI] [PubMed] [Google Scholar]
- 139.Bird RlR, Hall B, Chapman D, Hobbs KEF. Thrombosis Research. 1988;51:471. doi: 10.1016/0049-3848(88)90113-2. [DOI] [PubMed] [Google Scholar]
- 140.Hayward JA, Chapman D. Biomaterials. 1984;5:135. doi: 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- 141.Wisniewski N, Reichert M. Colloids and Surfaces B: Biointerfaces. 2000;18:197. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 142.Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Journal of Neuroscience Methods. 2002;119:163. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 143.Seo HK, Park DJ, Park JY. Ieee Sensors Journal. 2007;7:945. [Google Scholar]
- 144.Li CY, Han JY, Ahn CH. Biosensors & Bioelectronics. 2007;22:1988. doi: 10.1016/j.bios.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 145.Oh SJ, Song JK, Kim JW, Kim SJ. Ieee Transactions on Biomedical Engineering. 2006;53:351. doi: 10.1109/TBME.2005.862568. [DOI] [PubMed] [Google Scholar]
- 146.Hollenberg BA, Richards CD, Richards R, Bahr DF, Rector DM. J Neurosci Methods. 2006;153:147. doi: 10.1016/j.jneumeth.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 147.Wu G, Datar RH, Hansen KM, Thundat T, Cote RJ, Majumdar A. Nat Biotechnol. 2001;19:856. doi: 10.1038/nbt0901-856. [DOI] [PubMed] [Google Scholar]
- 148.Cheney CP, Wig A, Farahi RH, Gehl A, Hedden DL, Ferrell TL. Applied Physics Letters. 2007;90 [Google Scholar]
- 149.Ehrlich GD, Stoodley P, Kathju S, Zhao YJ, McLeod BR, Balaban N, Hu FZ, Sotereanos NG, Costerton JW, Stewart PS, Post JC, Lin Q. Clinical Orthopaedics and Related Research. 2005:59. doi: 10.1097/00003086-200508000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thomsen P, Gretzer C. Current Opinion in Solid State and Materials Science. 2001;5:163. [Google Scholar]
- 151.Chang S, Popowich Y, Greco RS, Haimovich B. Journal of Vascular Surgery. 2003;37:1082. doi: 10.1067/mva.2003.160. [DOI] [PubMed] [Google Scholar]
- 152.Thapa A, Webster TJ, Haberstroh KM. J Biomed Mater Res A. 2003;67:1374. doi: 10.1002/jbm.a.20037. [DOI] [PubMed] [Google Scholar]
- 153.Tang ZG, Black RA, Curran JM, Hunt JA, Rhodes NP, F D. Williams, Biomaterials. 2004;25:4741. doi: 10.1016/j.biomaterials.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 154.Serrano MC, Pagani R, Manzano M, Comas JV, Portoles MT. Biomaterials. 2006;27:4706. doi: 10.1016/j.biomaterials.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 155.Serrano MC, Pagani R, Vallet-Regi M, Pena J, Ramila A, Izquierdo I, Portoles MT. Biomaterials. 2004;25:5603. doi: 10.1016/j.biomaterials.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 156.MacEwan MR, Brodbeck WG, Matsuda T, Anderson JM. J Biomed Mater Res A. 2005;74:285. doi: 10.1002/jbm.a.30316. [DOI] [PubMed] [Google Scholar]
- 157.Norman JJ, Desai TA. Tissue Engineering. 2005;11:378. doi: 10.1089/ten.2005.11.378. [DOI] [PubMed] [Google Scholar]
- 158.Smith LL, Niziolek PJ, Haberstroh KM, Nauman EA, Webster TJ. International Journal of Nanomedicine. 2007;2:383. [PMC free article] [PubMed] [Google Scholar]
- 159.Smith LJ, Swaim JS, Yao C, Haberstroh KM, Nauman EA, Webster TJ. International Journal of Nanomedicine. 2007;2:493. [PMC free article] [PubMed] [Google Scholar]
- 160.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Biomaterials. 2004;25:53. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 161.Vance RJ, Miller DC, Thapa A, Haberstroh KM, Webster TJ. Biomaterials. 2004;25:2095. doi: 10.1016/j.biomaterials.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 162.Pattison M, Webster TJ, Leslie J, Kaefer M, Haberstroh KM. Macromolecular Bioscience. 2007;7:690. doi: 10.1002/mabi.200600297. [DOI] [PubMed] [Google Scholar]
- 163.Deutsch J, Motiagh D, Russell B, Desai TA. Journal of Biomedical Materials Research. 2000;53:267. doi: 10.1002/(sici)1097-4636(2000)53:3<267::aid-jbm12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 164.Snyder JD, Desai TA. Journal of Biomaterials Science-Polymer Edition. 2001;12:921. doi: 10.1163/156856201753113105. [DOI] [PubMed] [Google Scholar]
- 165.Motlagh D, Hartman TJ, Desai TA, Russell B. Journal of Biomedical Materials Research Part A. 2003;67A:148. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 166.Motlagh D, Senyo SE, Desai TA, Desai TA. Biomaterials. 2003;24:2463. doi: 10.1016/s0142-9612(02)00644-0. [DOI] [PubMed] [Google Scholar]
- 167.Tan W, Desai TA. Biomedical Microdevices. 2003;5:235. [Google Scholar]
- 168.Tan W, Desai TA. Tissue Engineering. 2003;9:255. doi: 10.1089/107632703764664729. [DOI] [PubMed] [Google Scholar]
- 169.Tan W, Desai TA. Journal of Biomedical Materials Research Part A. 2005;72A:146. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- 170.Tan W, Krishnaraj R, Desai TA. Tissue Engineering. 2001;7:203. doi: 10.1089/107632701300062831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.