Abstract
Objective
To compare the volumes of the caudate nucleus, using traditional volumetry and a three-dimensional brain mapping technique, in a group of individuals with late-life depression and a group of age- and education-equated nondepressed comparison subjects.
Design
Cross-sectional.
Setting
University Medical Center
Participants
Twenty-three nondemented subjects with late-life depression and 15 age- and education-equated elderly comparison subjects (depressed mean years of age: 70.5 ± 5.7 SD, comparison subjects = 69.9 years ± 6.4) with no history of psychiatric or neurologic disease.
Measurements
Structural magnetic resonance imaging. Three-dimensional (3-D) surface models were created from manually traced outlines of the caudate nucleus from spoiled gradient echo images. Models were geometrically averaged across subjects and statistical maps created to localize any regional volume differences between groups.
Results
Relative to comparison subjects, depressed subjects had significantly lower mean volumes for both the left (p = 0.029) and right (p = 0.052) caudate nucleus as well as total caudate volume (p = 0.032). Total volumes were 13.1% less in the depressed group (13.5% on the left and 12.6% on the right). 3-D maps further localized these reductions to the caudate head. Volume reductions were correlated with depression severity, as measured by the 17-item Hamilton Depression Rating Scale.
Conclusion
Late-life depression is associated with left and right caudate nucleus reduction especially in anterior portions. Among depressed subjects, greater caudate reduction was associated with more severe depression. These results are consistent with growing evidence that the anterior caudate nucleus, especially the head, may be structurally and functionally abnormal in affective disorders.
Keywords: Geriatric depression, late-life depression, caudate nucleus
There is a relationship between late-life depression (LLD) and indices of structural integrity of the central nervous system. Computed tomography studies have shown that older depressed subjects have decreased parenchymal tissue density as well as central and cortical atrophy (reviewed by Morris and Rapoport1). Magnetic resonance imaging (MRI) studies have generally replicated these findings and have revealed diffuse high signal hyperintensities in the subcortical white matter in elderly depressed subjects, especially in the frontal lobe, and among those with late-onset depression (i.e., first lifetime episode occurring > age 60) (see e.g.,2–8).
LLD-associated atrophy is regionally specific and includes the frontal lobe in general,9 as well as the orbitofrontal cortex,10,11 the gyrus rectus, and the anterior cingulate10 as well as the striatum, including the putamen12 and caudate nucleus.4,12 Also there are frequent basal ganglia lesions,11,13–16 especially in the striatal putamen and caudate nucleus.8,17
Some structural abnormalities may be associated with specific clinical characteristics. For example, late-onset depression may be associated with structural neuroimaging abnormalities such as white matter hyperintensities in the deep frontal lobe3,5,6,18 and hyperintensities in the basal ganglia generally8 and the caudate nucleus specifically.4 Right frontostriatal atrophy is associated with chronic, treatment-resistant depression.19 Studies such as these, along with findings showing an association between LLD and executive dysfunction (reviewed by Alexopoulos20), lend support to the current prominent model suggesting that structural brain changes are associated with frontostriatal dysfunction and LLD, especially late-onset depression (see e.g.,21,22).
The caudate nucleus, situated within the striatum in the basal ganglia, serves as a major relay station between the frontal cortex and the thalamus, and may play an important role in LLD. Caudate nucleus abnormalities are frequently found in LLD,4,12 and are often associated with clinical characteristics, including psychomotor speed,23 late-age of depression onset,4 and treatment resistance.19 A recent study found an association between caudate nucleus volume and the short allele of the 5HTT serotonin transporter gene.24 Moreover, depression is very common in individuals with disorders of the basal ganglia in general (see e.g.,25) and the caudate nucleus in particular (e.g., Huntington's disease26). The caudate nucleus is a key structure involved in regulating mood, some aspects of cognition, motor function, and motivation.27 Growing awareness of the associations between structural brain changes such as reduced caudate nucleus volume and affective disturbances in late-life, emphasizes the need to better understand the underlying neurobiologic substrates to better inform development of preventive interventions. The development of new imaging methods permits increasingly accurate measures of subcortical structures like the caudate nucleus and localization of volume differences.
Traditional manual tracing of regions-of-interest and voxel-based morphometry28 methods offer advantages for studying the neuropathologic substrates of LLD, but other techniques such as 3-D anatomical surface modeling may provide additional, unique information. In particular, 3-D anatomical models can visualize the spatial profile of any systematic atrophic changes in clinical syndromes, and can relate structural volume and relevant clinical phenomena. Here we aimed to produce 3-D surface mesh reconstructions of the caudate nucleus in a group of LLD subjects and a group of age- and education-equated comparison subjects without history of neurologic or psychiatric disorder. As suggested by a recent report encouraging the use of cutting-edge magnetic resonance (MR) technology in studies of LLD to measure with greater specificity the location, type, and extent of morphometric brain abnormalities,29 we examined the presence, extent and locus of caudate reduction in the two groups by creating average surface models for each group. We also assessed the association between caudate nucleus volume and selected clinical features—including age at and duration since, onset of depression as well as depression severity.
METHODS
Subjects
The 23 depressed subjects were all evaluated in the University of Pittsburgh's Advanced Center for Intervention and Services Research for Late-Life Mood Disorders (ACISR/LLMD). The MR scans for the 15 comparison subjects were obtained through the ACISR/LLMD and the Imaging Methods and Analysis in Geriatrics (IMAGe) Research group data repository (Note 1). The inclusion criteria that apply to all ACISR/LLMD and IMAGe subjects participating in this study were: 1) Age ≥60; 2) MMSE <17; 3) Meet DSM–IV criteria for major depression; and 4) Hamilton Depression Rating Scale (17-item) ≥15. The study exclusion criteria were: 1) Lifetime history of any psychiatric disorder (comparison subjects only); 2) Lifetime history of psychosis; 3) Acute or unstable medical illness; 4) History of neurologic disorder (including dementia) or traumatic brain injury; 5) History of substance abuse or dependence in the last 6 months. The depressed and comparison subjects did not differ in terms of age, education, or general cognitive ability as measured by the Mini-Mental Status Exam and the Dementia Rating Scale. Relevant demographic and clinical characteristics are included in Table 1.
TABLE 1.
Subjects: Descriptive Information
| Comparison Subjects (N = 15) | LLD Subjects (N = 23) | Test Statistic (df), p value | |
|---|---|---|---|
| Age, years, mean (SD) | 69.9 (6.4) | 70.5 (5.7) | t = −0.27 (36), 0.78 |
| % Male | 60 (n = 9) | 39 (n = 9) | χ2 = 0.096 (36), 0.92 |
| Education, years, mean (SD) | 15.0 (2.3) | 14.9 (3.0) | t = 2.36 (1), 0.12 |
| % White | 80 (n = 12) | 95.7 (22) | χ2 = 2.36 (1), 0.12 |
| Mini-Mental Status Examination, mean (SD) | 29.0 (1.4) | 28.8 (1.6) | t = 0.434 (36), 0.67 |
| Dementia Rating Scale, mean (SD) | 140.2 (2.7) | 19.0 (4.5) | t = 1.78 (36), 0.08 |
| HDRS (17-item), mean (SD) | 1.8 (1.8) | 19.0 (4.5) | t = −13.9(36), <0.001 |
| Age of first depression episode, years, mean (SD) | — | 46.4 (19.9) | — |
| % Late onset (age ≥60) | — | 39.1 (n = 7) | — |
| % Single episode | — | 39.1 (n = 9) | — |
The data for the present study were collected during depressed subjects' baseline, pretreatment evaluation. Details on subject recruitment and evaluation are described elsewhere.30–32 The broad-based pretreatment assessment included clinical, psychosocial, and neurobiologic measures. Diagnosis of major depression was established by a Structured Clinical Interview for Axis-I DSM–IV Disorders (SCID-IV33) interview administered by formally trained Master's and Doctoral level clinicians and a consensus diagnostic conference attended by the raters and at least three research geriatric psychiatrists. No subject met DSM–IV criteria for dementia. MRI scanning was performed within 2–3 weeks of the baseline evaluation and on a few subjects, during the first couple of weeks of controlled selective serotonin reuptake inhibitor treatment. The literature linking caudate changes and psychotropic medications involves dopaminergic antipsychotic medications (we excluded subjects with a history of psychosis), and we are aware of no published reports of an association between selective serotonin reuptake inhibitor use and caudate volume. The 15 comparison subjects were selected from the ACISR/LLMD cohort based on age and education, and their MRI scans were retrieved from the IMAGe data repository.
MRI Acquisition
The anatomical MRI data were acquired using a 1.5T GE scanner in three dimensions to obtain 124 thin, contiguous images throughout the entire brain. The contrast maximized the gray-white matter and CSF differences (TR = 25, TE = 5, slice thickness = 1.5 mm, 0 mm gap, 40 degrees flip angle, FOV = 24 × 18).
MRI Data Analysis
All MR images were processed with a series of manual and automated procedures detailed in prior reports and summarized below. Individual brain volumes were first reoriented into the axial plane, and then spatially realigned with the International Consortium for Brain Mapping nonlinear average brain template, ICBM152, using FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl/) Linear Image Registration Tool (FLIRT). The left and right caudate nuclei from each brain were traced using the Tracer program from the UCLA Laboratory of Neuroimaging (http://www.loni.ucla.edu/Software/Software_Detail.jsp?software_ id=1034) by a research associate (J.S.), who was blind to all demographic information, including group membership. J.S. was trained to criterion using an independent set of MR images. Intrarater reliability was subsequently assessed by independently tracing the same caudate nuclei from 10 different brains twice, and yielded an intraclass correlation in excess of 0.90.
Anatomical segmentation was performed using a standard neuroanatomical atlas of the caudate nucleus35 according to previously described criteria.36 Caudate models were delineated in contiguous coronal brain sections using standard guidelines.36,37 Anatomic landmarks were followed in all three orthogonal viewing planes using the interactive segmentation software, LONI Tracer.
Anatomical mesh modeling methods were then used to match equivalent caudate nucleus surface points, obtained from the manual tracings, across subjects and groups.38 The manually derived contours were made uniform by modeling them as a 3-D parametric surface mesh39 allowing measurements to be made at corresponding surface locations in each subject. This procedure also allows the averaging of caudate surface morphology across all individuals belonging to a group and determines the amount of variation between corresponding surface points relative to the group averages.
To assess global caudate reduction, the total volume of each 3-D model was measured. To measure local reduction, a medial 3-D curve was derived from each individual caudate model down its central axis. The distance of each surface point from this center-line measures the radial size of the caudate. Statistical maps were generated indicating local group differences in radial caudate nucleus volume. Regression analyses were run at each surface point to map linkages between radial size and group membership (Fig. 1 A and B). The local p value was plotted onto the surface at each point showing between-group differences; overall corrected p values were also assigned to the maps using permutation testing to give an overall significance value for the effect, adjusting for multiple comparisons.38–44
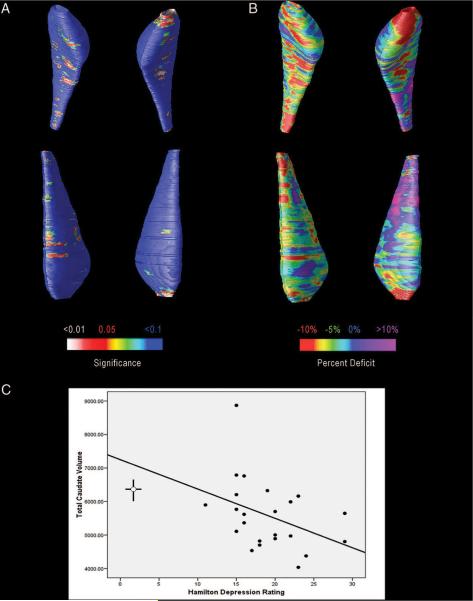
FIGURE 1. Maps of Caudate Reduction in Depressed Subjects, Adjusted for Age.
Notes: The caudate is oriented so that it faces the reader and right and left are reversed. To better localize atrophic changes that might account for the overall 13% mean reduction in caudate volume, average surface maps were compared between the depressed and control groups. [A] Shows regions in red colors, that show evidence of reduction in radial size in the depressed group versus controls. The significance maps are assessing, at each point on the caudate surface, the t statistic for the group difference between patients and controls, for the radial distance between that point and a medial axis threading down the center of the caudate in each subject. As such, the t statistic has 37 df, i.e., one less than the total number of subjects in the study. The same distribution, with the same number of degrees of freedom, is used for the assessments of volume reduction. [B] Shows that the level of reduction reaches 10% in the most anterior caudate regions. [C] Shows a scatterplot, revealing the significant correlation between depression severity and caudate volumes, with the best fit line. The crossed lines on the left-hand side indicate the mean and 95% CI for both the Hamilton Depression Rating Scale-17 and caudate volumes of the comparison subjects (HDRS-17 mean: 1.80; CI: 0.90–1.8, Caudate mean: 6419, CI: 6746–6096).
We performed permutation testing by computing the proportion (surface area) of the statistical map with statistics lower than p = 0.05. An empirical null distribution for this proportion was computed from random assignments of LLD and comparison subjects to groups, and the global p value was then defined as the fraction of the random assignments in which the proportion exceeded that found in the true assignment of LLD and comparison subjects to groups. The number of permutations N was chosen to be 100,000, to control the standard error SEp of omnibus probability p, which follows a binomial distribution B (N, p) with known standard error.40 When N = 8,000, the approximate margin of error (95% confidence interval) for p is around 5% of p; to further improve upon this, we ran 100,000 permutations, with 0.05 chosen as the significance level. This could be regarded as an approximate rather than an exact permutation test.
RESULTS
Volumetric Results
Relative to comparison subjects, depressed subjects had significantly lower mean volumes for both the left (t (36) = 2.28, p = 0.029, r = 0.36) and right (t (36) =2.01, p = 0.052, r = 0.32) caudate nucleus as well as total caudate volume (t (36) =2.23, p = 0.032, r = 0.35). Total volumes were 13.1% less in the depressed group (13.5% on the left and 12.6% on the right). Although the p value of 0.052 for the right caudate nucleus does not quite meet the traditional cutoff for significance, the effect sizes are similar, ranging from 0.32 to 0.36. Therefore, LLD was associated with significant reduction within both the left and right caudate nucleus.
Figure 1A shows regions of the caudate nucleus, especially in the head, where the mean radial size of the caudate was reduced in the depressed versus the comparison group. The upper representation shows an inferior view of the caudate nucleus, and the lower representation shows a view from above. Surprisingly, permutation testing did not confirm the significance of these local reductions (corrected p = 0.106, for the top left caudate, and corrected p = 0.107 for the top right caudate nucleus). Permutation testing provides stringent correction for multiple comparisons and further analysis (Fig. 1B) showed that the mean reduction in radial size was around 10% (red colors) for most of the caudate bilaterally, and greatest deficits were seen in the most anterior regions. Posterior regions of the left caudate nucleus did not show net reductions in the depressed group (purple colors). The significant reduction observed for overall volumes is therefore likely attributable to reduction in regions with radial contraction in Fig. 1B (red and green colors).
Permutation testing provides a global significance value for pattern of reductions in radial thickness of the caudate. As in several prior studies (see e.g.,38), we computed a corrected p value for the maps, by calculating the proportion of voxels on the surface of the structure where the significance of the intergroup differences falls below 0.05. Under the null hypothesis, this proportion would be 5% on average, and we computed an empirical reference (null) distribution for this proportion from 100,000 random maps created by randomly assigning LLD and comparison subjects to two groups. Surprisingly, the pattern of radial reduction was not significant after multiple comparisons correction, although the volume difference was robust. Even though a volume deficit was present, the radial measure of thickness may not be optimal for detecting it. This implies that the volume reduction was not consistently occurring in the same region of the caudate, which would tend to cause a consistent effect on the volumes but not on the maps. Most likely, the radial thickness of the caudate was subtly reduced, but the biological variance in thickness, and the error associated with manual segmentations may be high enough to preclude detecting it with sufficient effect size to pass the strong multiple comparisons correction implicit in permutation testing. Because the thickness measures in the maps are distinct from volume measures, it is possible but unusual, for the volumes but not the maps, to confirm significant reductions.
To evaluate the association between a range of clinical variables of interest and caudate nucleus volume, using data from all subjects, Pearson Product Moment correlations were calculated between the individual caudate volumes and age, years of education, Hamilton Depression Rating Scale-17 score, and for depressed subjects only, age at first lifetime depressive episode as well as duration since first lifetime episode. The only variable that was significantly correlated with caudate nucleus volume was depression severity as measured by the Hamilton Depression Rating Scale-17 (r = −0.396, df = 38, p = 0.014; see Fig. 1C for scatterplot).
DISCUSSION
The present study used 3-D anatomical surface modeling techniques, as well as traditional volumetry, to investigate caudate nucleus structural integrity in LLD. There were three principal findings. First, reduction in the caudate nucleus can be examined in detail using 3-D anatomical surface modeling techniques, revealing the profile of systematic differences in depressed subjects. Second, elderly depressed subjects had significant caudate volume reductions (around 13%) bilaterally compared with elderly comparison subjects with no psychiatric history. These differences were localized primarily to the head of the caudate nucleus, left greater than right. Third, caudate volume was correlated with depression severity.
An important story is emerging from studies in depressed individuals, examining the relationships between caudate nucleus volume and depression severity, cognitive functioning and serotonin transporter genotype. The present study shows that lower caudate volume is associated with more severe depression in elderly subjects, similar to those in midlife.45 In LLD, lower caudate volume may also be associated with psychomotor slowing,23 which appears to be the core cognitive deficit, accounting for a large proportion of impairment in most other cognitive domains.31 These findings taken together, suggest that the caudate nucleus plays an important role in both the mood and cognitive characteristics of some depressed elders, although we know little about the temporal or pathophysiologic nature of caudate nucleus reduction in LLD; it is unknown whether severe depression leads to cell loss or vice versa. Moreover, it is also unknown whether lower caudate nucleus volume is causally related to severe depression or whether it is related to developmental differences (implying a predispositional risk factor) or neurodegenerative changes (implying an ongoing pathological process). Caudate nucleus involvement has not been well-studied in neurodegenerative disorders, but three recent imaging studies46–48 have reported localized atrophy of the caudate head in Alzheimer disease. Also there is evidence that the caudate nucleus may be damaged by vascular disease in at least some elders with depression,16,49 and there may be a genetic link in some individuals with LLD due to an association between the serotonin transporter gene s-allele and lower caudate volume.24
This study further suggests that the critical volume loss may not be in the caudate nucleus in general but may be localized within the anterior portion, and more specifically, the head, particularly on the left. Notably, the head of the caudate is particularly rich in serotonergic fibers.50,51 This fits well with recent models emphasizing the relation between this sub-region of the caudate, which is innervated by the amygdala (see e.g.,52), and mood regulation (see Refs. 27 and 53 for reviews).
The prominent reduction in the most anterior part of the caudate also fits well with the vascular depression hypothesis of LLD (see Ref. 54 for a recent review). The vascular depression hypothesis posits that a subgroup of individuals with LLD (especially those with late-onset depression) experience disruption of prefrontal systems that mediate both mood and executive functions, by either single vascular lesions or an accumulation of lesions.21,22 We cannot identify the etiology of the reduced volume in the anterior caudate in our LLD subjects. Nevertheless, in the context of the particularly strong connections between the anterior caudate and frontal cortical areas, our finding lends support to the notion that striatal lesions may disconnect subcortical regions particularly involved in mood from frontal cortical regions that mediate executive functions.
Our analysis may have been aided by the fact that the depressed subjects were all recruited from a university-based depression research center. That is, in all cases, the depressed subjects, the primary caregiver, or a family member determined that the individual's change in mood was sufficiently severe to seek medical attention. If we had selected cases for analysis from a population study, for example, we would have been able to evaluate central nervous system structural changes before their clinical expression, and then the locus and extent of caudate reduction might differ from that observed here.
The primary limitations of this study include the relatively small group sizes and the sole focus on caudate nucleus measures. Nevertheless, the depressed subjects in this study were all carefully evaluated within the ACISR/LLMD which has a long record of using well-established research diagnostic criteria. An unexpected finding was that the maps localizing reduction (Fig. 1A) were not significant after stringent correction for multiple comparisons (permutation testing), although maps of the percent deficit reached 10% anteriorly (Fig. 1B) and overall volume reductions were significant. In past studies maps have proven to be as powerful, or more powerful, than traditional volumetry for detecting volume reductions, but, as was the case here, maps can miss an effect detected volumetrically if the effect is weak at each specific location on the structure (leading to nonsignificant point-wise p values), but nevertheless is strong enough to produce a significant overall volume reduction. In other studies, these 3-D mesh techniques have generally been more powerful for identifying cross-sectional differences in anatomy between clinical groups (e.g., the caudate nucleus in Fragile × Syndrome and the hippocampus in epilepsy, schizophrenia, autism, and methamphetamine users),37,43,44,55,56 and have provided important longitudinal information about change in status in Alzheimer disease, mild cognitive impairment, and normal brain development.36,38,57 Their use here helped to identify potential loci of systematic differences between LLD and nondepressed comparison subjects, which may be useful for future analysis of effects of treatment with various therapeutic or prevention strategies.
Acknowledgments
This research was supported by United States Public Health Service grants MH01684, MH072947, AG05133, MH52247, MH071944, MH71955. Algorithm development was supported by grants from the National Institute for Biomedical Imaging and Bioengineering, the National Center for Research Resources, the National Institute on Aging, and the National Institute for Child Health and Human Development (EB01651, RR019771, AG016570, HD050735 to P.M.T.) and a P41 NCRR grant to A.W.T.
The Imaging Methods and Analysis in Geriatrics (IMAGe) Research Group is a multidisciplinary research group including scientists from the University of Pittsburgh and the Carnegie-Mellon University. As of 2007, the group included: Howard Aizenstein, James Becker, Rishi Bhalla, Meryl Butters, Weiying Dai, Denise Davis, Chethan Devireddy, Mary Ganguli, Peter Gianaros, Ariel Gildengers, Christin Glorioso, Marco Inzitari, J. Richard Jennings, Seonggi Kim, Julie Kmiec, Charles Lee, Yanxi Liu, Oscar Lopez, Brian Lopresti, Chester Mathis, Carolyn Meltzer, James Mountz, Eric Nofzinger, Konsale Prasad, Julie Price, Cyrus Raji, Caterino Rosano, Bedda Rosario-Rivera, Eric Schwartz, Elizabeth Skidmore, Robert Tamburo, Costin Tanase, Leonid Teverovskiy, Nickolas Turco, Steven Uttecht, Wei Wang, Nora Watson, Lisa Weissfeld, David Wolk, Minjie Wu, Scott Ziolko.
References
- 1.Morris P, Rapoport SI. Neuroimaging and affective disorder in late life: a review. Can J Psychiatry. 1990;35:347–354. doi: 10.1177/070674379003500415. [DOI] [PubMed] [Google Scholar]
- 2.Coffey CE, Figiel GS, Djang WT, et al. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan KR, McDonald WM, Doraiswamy PM, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243:41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 5.Lesser IM, Boone KB, Mehringer CM, et al. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 6.Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 7.Taylor WD, MacFall JR, Provenzale JM, et al. Serial MR imaging of volumes of hyperintense white matter lesions in elderly patients: correlation with vascular risk factors. AJR Am J Roentgenol. 2003;181:571–576. doi: 10.2214/ajr.181.2.1810571. [DOI] [PubMed] [Google Scholar]
- 8.Tupler LA, Krishnan KR, McDonald WM, et al. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53:665–676. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 10.Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Payne ME, Steffens DC, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54:529–533. doi: 10.1016/s0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan KR, McDonald WM, Escalona PR. Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald BS, Kramer-Ginsberg E, Krishnan RR, et al. MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1996;153:1212–1215. doi: 10.1176/ajp.153.9.1212. [DOI] [PubMed] [Google Scholar]
- 14.Rabins PV, Pearlson GD, Aylward E, et al. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry. 1991;148:617–620. doi: 10.1176/ajp.148.5.617. see comments. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien JT, Ames D. White matter lesions in depression and Alzheimer's disease. Br J Psychiatry. 1996;169:671. doi: 10.1192/bjp.169.5.671a. [DOI] [PubMed] [Google Scholar]
- 16.Steffens DC, Helms MJ, Krishnan KR, et al. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30:2159–2166. doi: 10.1161/01.str.30.10.2159. [DOI] [PubMed] [Google Scholar]
- 17.Steffens DC, Tupler LA, Ranga K, et al. Magnetic resonance imaging signal hypointensity and iron content of putamen nuclei in elderly depressed patients. Psychiatry Res. 1998;83:95–103. doi: 10.1016/s0925-4927(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 18.Nebes RD, Reynolds CF, Boada F, et al. Longitudinal increase in the volume of white matter hyperintensities in late-onset depression. Int J Geriatr Psychiatry. 2002;17:526–530. doi: 10.1002/gps.635. [DOI] [PubMed] [Google Scholar]
- 19.Shah PJ, Glabus MF, Goodwin GM, et al. Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br J Psychiatry. 2002;180:434–440. doi: 10.1192/bjp.180.5.434. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos GS. Role of executive function in late-life depression. J Clin Psychiatry. 2003;64(suppl 14):18–23. [PubMed] [Google Scholar]
- 21.Alexopoulos GS, Meyers BS, Young RC, et al. The “vascular depression” hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 23.Naismith S, Hickie I, Ward PB, et al. Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. Am J Psychiatry. 2002;159:2096–2098. doi: 10.1176/appi.ajp.159.12.2096. [DOI] [PubMed] [Google Scholar]
- 24.Hickie IB, Naismith SL, Ward PB, et al. Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. J Affect Disord. 2007;98:137–142. doi: 10.1016/j.jad.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Miller KM, Okun MS, Fernandez HF, et al. Depression symptoms in movement disorders: comparing Parkinson's disease, dystonia, and essential tremor. Mov Disord. 2007;22:666–672. doi: 10.1002/mds.21376. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen JS, Nehl C, Hoth KF, et al. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005;17:496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- 27.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 29.Hoptman MJ, Gunning-Dixon FM, Murphy CF, et al. Structural neuroimaging research methods in geriatric depression. Am J Geriatr Psychiatry. 2006;14:812–822. doi: 10.1097/01.JGP.0000238588.34205.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 31.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 32.Mulsant BH, Pollock BG, Nebes R, et al. A twelve-week, double-blind, randomized comparison of nortriptyline and paroxetine in older depressed inpatients and outpatients. Am J Geriatr Psychiatry. 2001;9:406–414. [PubMed] [Google Scholar]
- 33.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis I Disorders SCID I: Clinician Version, Administration Booklet. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 34.Woods RP. Multitracer: a java-based tool for anatomic delineation of grayscale volumetric images. Neuroimage. 2003;19:1829–1834. doi: 10.1016/s1053-8119(03)00243-x. [DOI] [PubMed] [Google Scholar]
- 35.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- 36.Becker JT, Hayashi KM, Meltzer CC, et al. Alteration in hippocampal and caudate nucleus structure in HIV/AIDS revealed by three-dimensional surface mesh analysis. Neurology. 2005;64:A409. Abstract. [Google Scholar]
- 37.Gothelf D, Furfaro JA, Eckert MA, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and FMRP. Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PM, Hayashi KM, de Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 39.Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- 40.Edgington ES. Randomization Tests. 3rd ed. Marcel Dekker; New York: 1995. [Google Scholar]
- 41.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker JT, Davis SW, Hayashi KM, et al. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- 43.Lin JJ, Salamon N, Dutton RA, et al. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1094–1097. doi: 10.1212/01.wnl.0000179003.95838.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pillay SS, Renshaw PF, Bonello CM, et al. A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Psychiatry Res. 1998;84:61–74. doi: 10.1016/s0925-4927(98)00048-1. [DOI] [PubMed] [Google Scholar]
- 46.Rombouts SA, Barkhof F, Witter MP, et al. Unbiased whole-brain analysis of gray matter loss in Alzheimer's disease. Neurosci Lett. 2000;285:231–233. doi: 10.1016/s0304-3940(00)01067-3. [DOI] [PubMed] [Google Scholar]
- 47.Karas GB, Burton EJ, Rombouts SA, et al. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003;18:895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 48.Frisoni GB, Testa C, Zorzan A, et al. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannestad J, Taylor WD, McQuoid DR, et al. White matter lesion volumes and caudate volumes in late-life depression. Int J Geriatr Psychiatry. 2006;21:1193–1198. doi: 10.1002/gps.1640. [DOI] [PubMed] [Google Scholar]
- 50.Azmitia EC, Whitaker-Azmitia PM. Awakening the sleeping giant: anatomy and plasticity of the brain serotonergic system. J Clin Psychiatry. 1991;52(suppl):4–16. [PubMed] [Google Scholar]
- 51.Jacobs BL. Serotonin and behavior: emphasis on motor control. J Clin Psychiatry. 1991;52(suppl):17–23. [PubMed] [Google Scholar]
- 52.Drevets WC, Gautier C, Price JC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 53.Voorn P, Vanderschuren LJ, Groenewegen HJ, et al. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Kales HC, Maixner DF, Mellow AM. Cerebrovascular disease and late-life depression. Am J Geriatr Psychiatry. 2005;13:88–98. doi: 10.1176/appi.ajgp.13.2.88. [DOI] [PubMed] [Google Scholar]
- 55.Nicolson R, DeVito TJ, Vidal CN, et al. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Res. 2006;148:11–21. doi: 10.1016/j.pscychresns.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Nugent TF, Herman DH, Ordonez A, et al. Dynamic mapping of hippocampal development in childhood onset schizophrenia. Schizophr Res. 2007;90:62–70. doi: 10.1016/j.schres.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Gogtay N, Nugent TF, III, Herman DH, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]