Abstract
The fovea is the retinal location responsible for our most acute vision. There are several methods used to localize the fovea, but the fovea is not always easily identifiable. Landmarks used to determine the foveal location are variable in normal subjects and localization becomes even more difficult in instances of retinal disease. In normal subjects, the photoreceptor axons that make up the Henle fiber layer are cylindrical and the radial orientation of these fibers is centered on the fovea. The Henle fiber layer exhibits form birefringence, which predictably changes polarized light in scanning laser polarimetry imaging. In this study 3 graders were able to repeatably identify the fovea in 35 normal subjects using near infrared image types with differing polarization content. There was little intra-grader, inter-grader, and inter-image variability in the graded foveal position for 5 of the 6 image types examined, with accuracy sufficient for clinical purposes. This study demonstrates that scanning laser polarimetry imaging can localize the fovea by using structural properties inherent in the central macula.
Keywords: scanning laser polarimetry, birefringence, Henle fiber layer
Introduction
The fovea occupies a relatively small, but crucial, retinal area, as it is the location responsible for our most acute spatial vision and best color discrimination. The fovea is not always easily identifiable, even in normal subjects. Structurally, the fovea is a shallow depression formed by the displacement of inner retinal cells, leaving only cone photoreceptors and the Henle fiber layer, the laterally displaced axons of the cone photoreceptors. Retinal vessels and capillaries are also absent in most normal individuals within 450–600 μm of the center of the macula, called the foveal avascular zone (FAZ; Bradley, Zhang, Applegate, Thibos, & Elsner, 1998; Early Treatment Diabetic Retinopathy Study Research Group, 1991). Displacement of retinal cells and absence of retinal vasculature in the center of the macula reduces light scatter, allowing higher visual sensitivity and acuity.
There are several methods used to determine the location of the fovea (Table 1), including ophthalmoscopy (Newcomb & Potter, 1981); fundus photography (Williams & Wilkinson, 1992); scanning laser ophthalmoscopy (Chen et al., 1992); imaging methods including fundus reflectometry and optical coherence tomography (OCT; Legarreta et al., 2008; Massin et al., 2001; Zagers, van de Kraats, Berendschot, & van Norren, 2002); fluorescein angiography (Wolf et al., 1990); entoptic methods (Bradley et al., 1998); and fixation stability measured with fundus imaging devices (Timberlake et al., 1986). Landmarks used to locate the relative position of the fovea with these techniques include the position of the optic disc, the foveal light reflex, the distribution of macular pigment, the configuration of the retinal vasculature, retinal thickness measurements, and the density of cone photopigment. Each of these methods has advantages but can be limited by normal variability of retinal structures, as well as changes associated with aging and retinal disease.
Table 1.
Methods used for foveal localization illustrating criteria for foveal localization and advantages and disadvantages of each method.
| Method for foveal localization | Criteria used to localize fovea | Advantages | Disadvantages |
|---|---|---|---|
| Ophthalmoscopy/fundus photography | Retinal vasculature | High availability | Flood illumination (low contrast) |
| Macular pigment and melanin | High intensity visible light(requiring pupil dilation) | ||
| Relative position to optic disc | Affected by structural variability in normal and retinal disease | ||
| Foveal light reflex | Often requires localization relative to other features | ||
| Low accuracy | |||
|
| |||
| Scanning laser ophthalmoscopy | Retinal vasculature | High contrast of retinal features(vasculature and optic disc) | Affected by structural variability in normal and retinal disease |
| Macular pigment and melanin | Ability to acquire high-resolution images | Pigment variability in normals and diseased retinas | |
| Relative position to optic disc | Ability to use specific light sources | Often requires localization relative to other features | |
| Foveal light reflex easily visible | Stronger foveal light reflex | ||
| Topography measurements (HRT) | |||
| Cone diameter or unresolvable cones (AOSLO) | |||
|
| |||
| Fundus reflectometry | Macular pigment reflectance | Uses inherent foveal properties(macular pigment, cone photopigment) | Indicates general region, not fovea per se |
| Cone foveal photopigment(unbleached) | Quantifiable criteria | Pigment variability in normals and diseased retinas | |
| Stiles Crawford effect | |||
| Subject fixation | |||
| Foveal light reflex | |||
| Focal decrease in autofluorescence | |||
|
| |||
| Optical coherence tomography | Foveal depression(retinal thickness) | Uses inherent foveal properties(foveal depression) | Foveal depression absent in many retinal diseases/hypoplasia |
| Fixation | High accuracy in normal populations | Affected by eccentric or inconsistent fixation for long sampling periods | |
| High availability | |||
| Quantitative | |||
|
| |||
| Fluorescein angiography | Retinal vasculature | High availability | Invasive, potential systemic side effects to dye |
| Foveal avascular zone | High contrast of retinal vasculature | Indicates general region based on foveal avascular zone, not fovea per se | |
| Choroidal fluorescence | Large variability in vascular structures, particularly in diabetes | ||
| Fovea not always centered on foveal avascular zone | |||
| Dark fundi and media opacities reduce contrast | |||
|
| |||
| Entoptic methods | Retinal vasculature | Inexpensive | Subjective |
| Foveal avascular zone | Non-invasive | Indicates general region based on foveal avascular zone, not fovea per se | |
| Large variability in foveal avascular zone (larger/altered in retinal disease) | |||
| Fovea not always located at the center of the foveal avascular zone | |||
|
| |||
| Fixation stability with fundus imaging | Scanning laser ophthalmoscope and optic disc as landmark | Match structure to subjective function | Subjective |
| Estimates variability of fixation | Expensive equipment | ||
| Non-invasive | Limited availability | ||
| Requires patient cooperation | |||
|
| |||
| Scanning laser polarimetry | Birefringence | Uses inherent foveal properties(birefringence) | Birefringence disrupted with cone axon atrophy |
| Retinal vasculature | Non-invasive | Not widespread availability for analysis of macular data | |
| Foveal light reflex | Robust | ||
In addition to the methods previously mentioned, scanning laser polarimetry (SLP) is a potential technique for determining the foveal position. In normal subjects, the photoreceptor axons in the Henle fiber layer are cylindrical and the radial orientation of these fibers is centered on the fovea. Like other highly organized biological structures, such as the nerve fiber layer and the cornea, the Henle fiber layer exhibits form birefringence (Dreher, Reiter, & Weinreb, 1992; Hemenger, 1989; Huang & Knighton, 2002; Zhou & Knighton, 1997). Form birefringence is a meridional variation in refractive index, which differentially retards transmitted light polarized along the “fast” and “slow” axes of a material. Form birefringence can be described by both the orientation of the axes and the magnitude of birefringence. When the central macula is imaged using polarized light, the interaction of the corneal birefringence with the Henle layer birefringence produces a characteristic macular cross pattern centered on the fovea. This technique has previously been used to monitor fixation and eye tracking in infants and young subjects (Gramatikov, Zalloum, Wu, Hunter, & Guyton, 2006, 2007; Hunter et al., 2004; Hunter, Patel, & Guyton, 1999; Hunter, Shah, Sau, Nassif, & Guyton, 2003; Nassif, Gramatikov, Guyton, & Hunter, 2003) and has been used to characterize damage due to age-related macular degeneration (Weber, Elsner, Miura, Kompa, & Cheney, 2007). The macular cross pattern remains intact, or partially intact, in many cases of eye disease, including exudative macular degeneration (Weber et al., 2007). Because mild to moderate disruption caused by retinal pathology does not necessarily destroy the birefringent properties of the Henle fiber layer, scanning laser polarimetry may be a robust measurement of foveal location.
In this study, three graders measured the foveal location in 35 subjects with normal retinas. We compared the graded foveal locations among graders using images with differing polarization content. We hypothesized that the birefringent properties of the Henle fiber layer could be used to accurately assess the foveal location, and in more difficult image grading, the magnitude and orientation of the birefringence would be more useful than the magnitude alone. We also hypothesized that the fovea could be repeatably and accurately located in near infrared images that contained landmarks typically used to locate the fovea, but image types showing fewer landmarks would produce more variable foveal localization.
Methods
Equipment
A confocal scanning laser polarimeter (GDx, Laser Diagnostic Technologies/Carl Zeiss Meditec, Dublin, CA) was used to acquire images for the foveal location grading, using the acquisition method described previously (Burns, Elsner, Mellem-Kairala, & Simmons, 2003; Elsner, Weber, Cheney, VanNasdale, & Miura, 2007; Mellem-Kairala, Elsner, Weber, Simmons, & Burns, 2005; Weber, Cheney, Smithwick, & Elsner, 2004; Weber et al., 2007). The GDx uses a 780-nm linearly polarized near infrared light source to scan a 15 × 15 deg raster on the retina. The instrument used in this study has a fixed birefringent element with a magnitude of 60 nm (single-pass retardance) and a fast axis oriented at 15° nasally downward to compensate for corneal birefringence. Corneal compensation using this technique is incomplete in most individuals but is not necessary for this foveal localization technique. Complete compensation of corneal birefringence would result in an annular pattern instead of a macular cross pattern in images demonstrating the birefringence magnitude of the Henle fiber layer and would result in a radially symmetric pinwheel pattern in images demonstrating the axis of phase retardation. The GDx has 2 detectors, a parallel detector collecting light with the same polarization as the input light; and a crossed detector, collecting light with polarization that is 90 degrees from the input polarization. Each detector produces images at 20 different input polarizations, for a total of 40 images per image series. Each image has 256 × 256 resolution with 8-bit grayscale. The image series has an acquisition time of approximately 0.9 seconds and is performed non-invasively and without mydriasis.
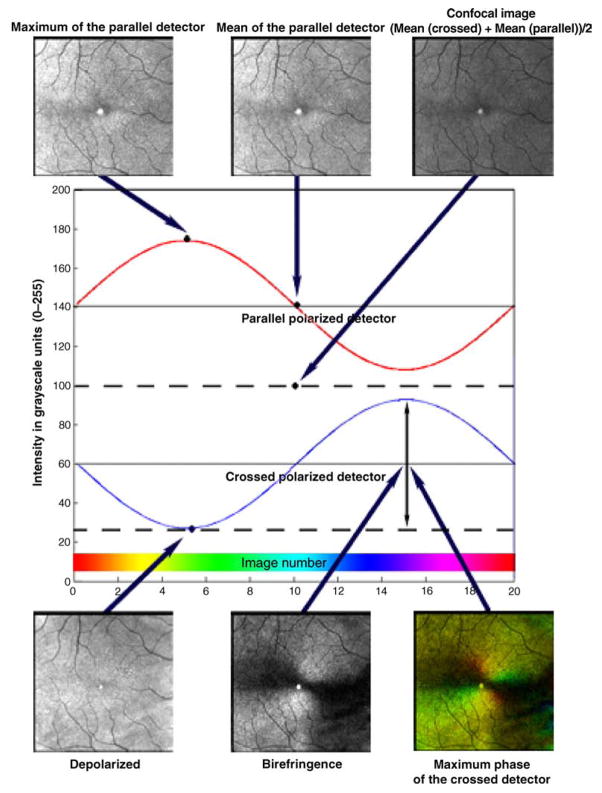
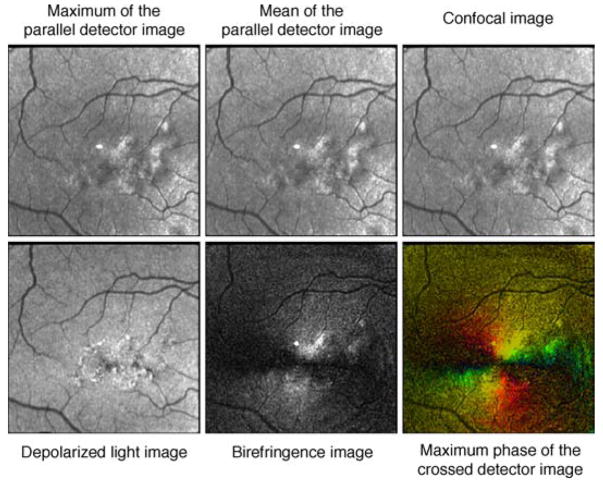
Raw image data are processed using custom Matlab routines (Matlab, Mathworks, Natick, MA), with six image types used for grading. These images differ based on polarization content for each pixel over the 20 input polarizations and emphasize different retinal structures resulting from light/tissue interactions (Burns et al., 2003; Elsner et al., 2007; Mellem-Kairala et al., 2005; Miura, Elsner, Cheney, Usui, & Iwasaki, 2007; Miura et al., 2005; Weber et al., 2004, 2007). Figure 1 demonstrates the six image types from a single data set in one individual and a representation of the computations used to create each image type. The Maximum of the Parallel Detector Image, top left, shows the highest amplitude of modulation, regardless of input polarization angle, thereby emphasizing contrast in the image relative to the most specularly reflected or polarization retaining structures. For comparison, we included the Mean of the Parallel Detector Image, top center, which is the average amount of light returning to the parallel detector. This average includes no input from the light that is rotated or scattered and subsequently acquired by the crossed detector. The Confocal Image, top right, is the average of the amount of light returning to both the parallel and crossed detectors. The Confocal Image is a polarization insensitive image that is analogous to images acquired using a confocal scanning laser ophthalmoscope. The Depolarized Light Image, bottom left, is defined as the minimum value of the modulation for light returning to the crossed detector, which removes any variation due to polarization across the 20 images. This minimizes features that are visualized via their polarization changes and similarly minimizes specular reflections, while maximizing deeper structures and those that have enhanced contrast from scattered light. The Birefringence Image, bottom center, is the amplitude of the modulation across polarization input angles of the light returning to the crossed detector. The Maximum Phase of the Crossed Detector Image, bottom right, indicates both the magnitude of birefringence and the input polarization orientation that produces the highest grayscale value in the crossed detector. This orientation is coded with the Cardinal Directions Color map. The modulation for the crossed detector is coded by intensity and saturation, and the polarization input angle producing the maximum amplitude coded by hue (Elsner, Miura, Stewart, Kairala, & Burns, 2003; Elsner et al., 2007). The Henle fiber layer has characteristic birefringent properties, which can be measured in both the Birefringence Image and the Maximum Phase of the Crossed Detector Image. The interaction of the Henle layer birefringence and the corneal birefringence produce the “bow-tie” macular cross pattern seen in both the Birefringence Image and the Maximum Phase of the Crossed Detector Image. In the normal eye, with a healthy nerve fiber layer, there is little contribution to the central portion of the macular cross from the retinal nerve fiber layer and therefore little influence of glaucoma is anticipated (Elsner, Weber, Cheney, & VanNasdale, 2008). Figure 2 shows that in a standard color fundus photograph, there is no macular cross, but the macular pigment and distribution of the retinal vessel arcades indicate the approximate location of the foveal center.
Figure 1.
Calculations used to derive the 6 image types used for foveal location grading based on a single data set. The GDx has 2 detectors and information is combined from each of the detectors or a combination of the 2 detectors. The intensity at each pixel location is fit to a sine wave (red line and blue line) using a Fast Fourier Transform, which acts as a low-pass filter for the data, prior to image calculations. The Maximum of the Parallel Detector Image (top left) shows the highest amplitude of modulation, regardless of input polarization angle, thereby emphasizing contrast in the image relative to the most specularly reflected or polarization retaining structures. The Mean of the Parallel Detector Image (top center) is the average amount of light returning to the parallel detector. The Confocal Image (top right) is the average of the amount of light returning to both detectors. The Confocal Image is a polarization insensitive image. The Depolarized Light Image (bottom left) is the minimum value of the modulation for light returning to the crossed detector, which removes any variation due to polarization across the 20 images. The Birefringence Image (bottom center) is the amplitude of the modulation across polarization input angles of the light returning to the crossed detector. The Maximum Phase of the Crossed Detector Image (bottom right) indicates both the amount of birefringence and is coded with the Cardinal Directions Color map as in Elsner et al. (2007). There is a bright reflection artifact inherent in this GDx that is at the center of each image.
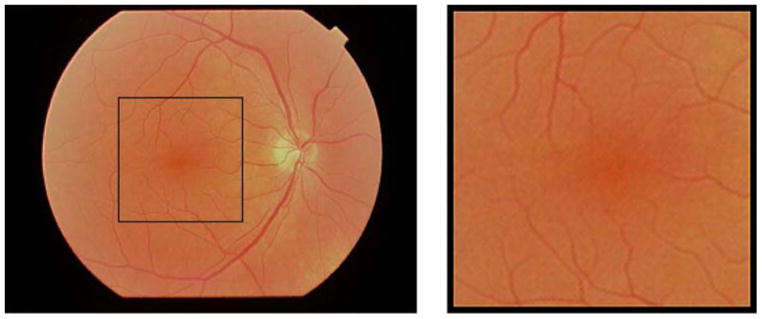
Figure 2.
Fundus photograph of a 56-year-old Caucasian male. The black rectangle in the left photograph indicates the size of the 15 × 15 degree retinal area sampled by the GDx. The area outlined by the black rectangle is shown on the right.
All images derived from the parallel detector, the Maximum of the Parallel Detector Image, the Mean of the Parallel Detector Image, and the Confocal Image, contain light that is specularly reflected and more easily demonstrates reflective landmarks like the foveal light reflex. The Maximum of the Parallel Detector Image contains the most specularly reflected light and because it most easily demonstrates the foveal light reflex, one of the easiest and most reliable landmarks for foveal detection, it was chosen as a standard image for comparison in inter-image variability analysis. The crossed detector receives relatively small amounts of light; thus, images derived from the crossed detector, the Depolarized Image, the Birefringence Image, and the Maximum Phase of the Crossed Detector Image, are dim. After processing, these images were enhanced using the Auto Levels function in Adobe Photoshop (Adobe Photoshop, San Jose, CA), which improved contrast and visualization during grading in an objective manner that did not favor one image type over another.
Subjects
Images were graded from 35 eyes of 35 subjects (13 males, 22 females, 19 to 90 years old: mean 33.5, standard deviation 14.7). Subjects were recruited from the Indiana University School of Optometry (Bloomington, IN, USA), the Department of Ophthalmology, University Hospital (Aachen, Germany), the Department of Ophthalmology, Tokyo Medical University (Tokyo, Japan), and the Schepens Eye Institute (Boston, MA, USA) following the human subjects protocol procedures at the respective institutions. Study participants were considered eligible for enrollment if a complete ophthalmologic examination had been completed within the past year, and no signs of ocular pathology were present. Subjects were required to have visual acuity of 20/20 or better, unless reduced acuity could be attributed to normal aging lens opacification. Patients with systemic diseases that carry a high likelihood of ocular manifestations were excluded.
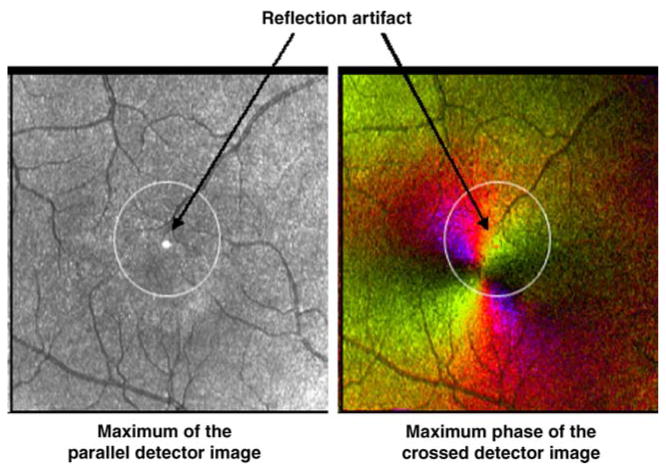
Two to three data sets were taken for each subject to ensure gradable images that were well focused, adequately and evenly illuminated, and without movement artifacts. When images were acquired, the macula was displaced from the center of the image to eliminate a reflection artifact inherent in the GDx system that could mask the foveal light reflex (Figure 3). This also ensured that the foveal location would be sufficiently displaced to an unpredictable location to eliminate grading bias. The best image for each subject was chosen for grading.
Figure 3.
A 5 deg, movable circle was available for the grader to define a central region based on vessel and contrast features, and the grader then selected a single foveal location within that circle. The Maximum of the Parallel Detector Image and Maximum Phase of the Crossed Detector Image are displayed here with the circle in the initial position prior to grader manipulation. There is a reflection artifact located in the center of each image that is the result of a reflection in the GDx.
Image grading
Three graders (DV, AE, and AW) determined the pixel location of the fovea in six image types for all 35 subjects. With a conversion factor of 1 deg of visual angle per 300 microns at the retina, each pixel is the equivalent of 17.6 microns. Pixel coordinates match for all images derived from the same data set. A 5 deg, movable circle was available for the grader to define a central region based on vessel and contrast features and then select a single foveal location (Figure 3).
Variability within graders
Images were graded for foveal location at two separate times, and data were divided into Time 1 and Time 2. The distance in graded foveal location between Time 1 and Time 2 was compared for each image to determine intra-grader variability. Statistical comparisons were made using STATVIEW (StatView, Abacus Concepts, Berkeley, CA, USA). Analysis of Variance (ANOVA) was used to compare difference in pixel location between Time 1 and Time 2.
Variability between graders
Each grader selected two foveal positions for each image (Time 1 and Time 2). The average location was used to compute the distance in foveal locations between graders, and the inter-grader comparisons (DV vs. AE, DV vs. AW, and AE vs. AW) were made for each image. ANOVA was used to compare the difference in foveal location between different graders for the same image.
Variability across image types
The distance between a grader’s average locations between Time 1 and Time 2 for different image types was used to make inter-image comparisons. Each image type was compared to all other image types, and ANOVA was used to compare the differences in foveal location between different image types.
Birefringence image comparisons
Analysis was also performed to determine whether the additional information provided by the Maximum Phase of the Crossed Detector Image, which contains both birefringence magnitude and orientation information, provides any benefit when compared to the Birefringence Image, which contains only birefringence magnitude information. We performed a separate analysis on the data sets that contained the 10 birefringence images with the greatest inter-grader variability. The Maximum of the Parallel Detector Image was chosen as the standard to compare measurement accuracy between the Maximum Phase of the Crossed Detector Image and the Birefringence image. The Maximum of the Parallel Detector Image was chosen because it includes information about the highest reflectivity of retinal structures. This image contains landmarks and features more commonly used to determine the foveal position, including the foveal light reflex in cases where it is present. The distance between the foveal location in the Birefringence image and the foveal location in the Maximum of the Parallel Detector Image, which provides color-coded information about the macular cross (Figure 1), was calculated for images from the same raw data set. Similarly, the distance between the foveal location in the Maximum Phase of the Crossed Detector Image and in the Maximum of the Parallel Detector Image was calculated. ANOVA was used to determine whether the Birefringence Image or the Maximum Phase of the Crossed Detector Image was more consistent with the Maximum of the Parallel Detector Images when determining foveal position.
Results
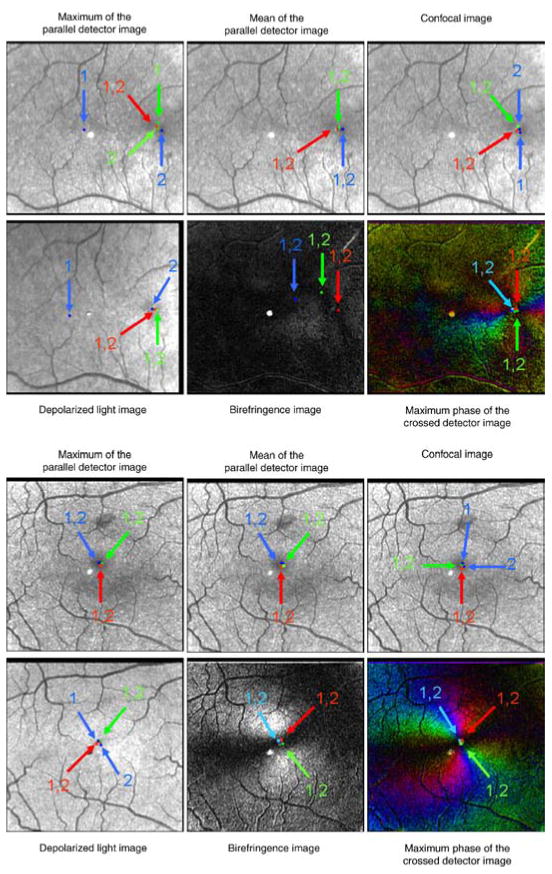
The fovea was typically well localized by the graders in terms of repeatability and agreement across individuals, with some differences among image types. Figure 4 shows the foveal locations for the subjects with the most overall variability (Figure 4, top) and least overall variability (Figure 4, bottom).
Figure 4.
Foveal locations for the subject with the most overall variability (top) and the subject with the least overall variability (bottom). Time 1 locations for the three graders are marked with an arrow and a “1”, and Time 2 locations are marked with an arrow and a “2” Foveal locations that are too close to be distinguished between Time 1 and Time 2 have arrows labeled with “1,2”. The light or dark spot in the center of the images is a reflection artifact inherent in the GDx.
Variability within graders
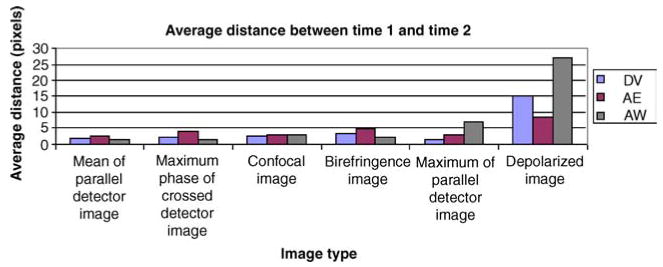
Figure 5 shows the average distance between Time 1 and Time 2 for each grader separated by image type. The mean of the parallel detector had the best repeatability, with an average distance of 1.89 pixels (33.3 microns) between Time 1 and Time 2. The depolarized light image had the worst repeatability with an average distance of 16.7 pixels (294 microns). When all six images are considered, the average distance between foveal locations for Time 1 and Time 2 was 5.19 pixels (91.3 microns). When the depolarized data are not considered, the average distance between Time 1 and Time 2 for the remaining five image types is 2.86 pixels (50.3 microns). There was a statistically significant difference between locations for Time 1 and Time 2 (ANOVA, p < 0.001). Planned comparisons, with Bonferroni correction, showed a statistically significant difference between the Time 1 and Time 2 distances for the Depolarized Image when compared to each of the other image types (p < 0.001 for each comparison), demonstrating a higher intra-grader variability for the Depolarized Image. There was no significant difference in the comparisons made among any of the other combinations of image types.
Figure 5.
The average pixel distance in foveal location between Time 1 and Time 2 for each image type separated by the 3 graders (DV, AE, and AW).
Variability between graders
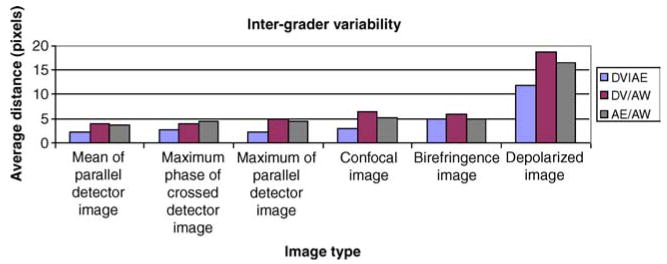
Figure 6 shows the average distance between the graders’ foveal location, separated by image type. The Depolarized Image had the largest amount of variability. The average difference was 11.8 pixels (208 microns) when comparing DV and AE, 18.7 pixels (329 microns) for DV and AW, and 16.5 pixels (290 microns) when comparing AE and AW. The smallest average distance between DV and AE was in the Maximum of the Parallel Detector Image, 2.16 pixels (38.0 microns). The smallest average distance between DV and AW was in the Mean of the Parallel Detector Image, 3.89 pixels (68.5 microns). The smallest average distance between AE and AW was in the Mean of the Parallel Detector Image, 3.67 pixels (64.6 microns). ANOVA on the comparison groups (DV vs. AE, DV vs. AW, and AE vs. AW) using all image types showed no statistically significant difference between graders (p = 0.605).
Figure 6.
Inter-grader variability demonstrating the distances for the average foveal location for the three graders DV, AE, and AW were compared for each of the image types.
Variability across image types
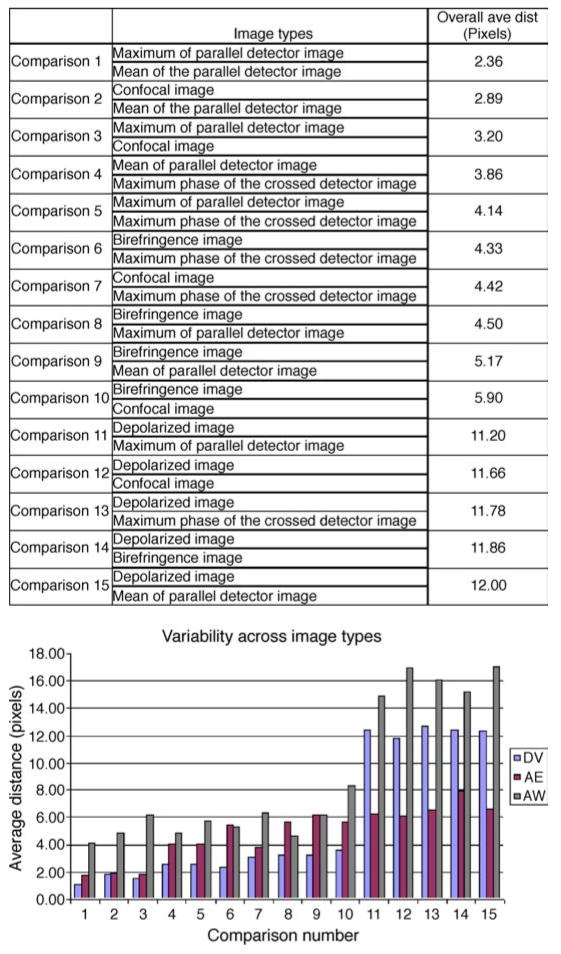
Figure 7 shows the average distance in foveal location between image types, separated by grader. The comparisons are arranged from smallest overall discrepancy between image type comparisons to largest. When averaged across the 3 graders, the difference in foveal location was least between the Maximum of the Parallel Detector Image and the Mean of the Parallel Detector Image, with an overall average distance of 2.36 pixels (41.5 microns). The greatest discrepancy in foveal position was seen between the Depolarized Image and the Mean of the Parallel Detector Image, with an overall average distance of 12.00 pixels (211 microns). ANOVA indicated a significant difference in foveal locations based on image type for each of the graders (p < 0.001).
Figure 7.
(Top) Table of graders’ average values for inter-image distances. (Bottom) Inter-image variability demonstrating the difference in average Time 1 and Time 2 foveal locations between different image types for each of the 3 graders.
Birefringence image comparisons
We tested whether the fovea could be more precisely located when information about the birefringence orientation is available. The graded foveal locations from the images containing birefringence information, the Birefringence Image and the Maximum Phase of the Crossed Detector Image, were compared to the graded foveal locations in the Maximum of the Parallel Detector Image. When examining the 10 subjects with the poorest intra-grader repeatability, ANOVA testing showed a statistically significant smaller distance in graded foveal location between the Maximum of the Parallel Detector Image and the Maximum Phase of the Crossed Detector Image than between the Maximum of the Parallel Detector Image and the Birefringence Image (p = 0.0023). The average distance for the Maximum Phase of the Crossed Detector Image in those 10 images was 1.92 pixels (33.8 microns), while the average distance for the Birefringence Image was 6.98 pixels (123 microns). This difference is demonstrated in Figure 4 (top), which shows the subject with the largest overall variability, and has the largest variability in graded foveal positions for the Birefringence Image.
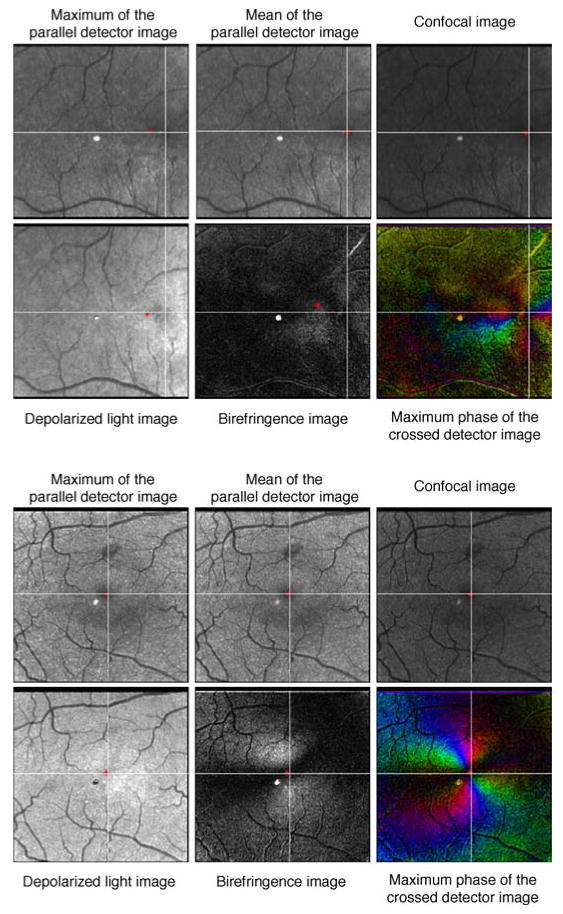
The image type with the overall best repeatability in this study was the Mean of the Parallel Detector Image. Figure 8 demonstrates the variability among the other image types when compared to the Mean of the Parallel Detector using the same subjects as Figure 4. Figure 8 shows the data set with the most variability and the least variability with the average of the foveal location coordinates marked with an asterisk. The graded foveal coordinates from the most consistent image type as determined by the data from this study, the Mean of the Parallel Detector, are marked by the intersection of gray lines.
Figure 8.
The overall average graded foveal location in the subject with the most overall variability (top) and least overall variability (bottom) marked with an asterisk. The coordinates of the overall average foveal location of the most consistent image type, the Mean of the Parallel Detector Image, is marked in each of the 6 images with gray lines. The light or dark spot in the center of the images is a reflection artifact inherent in the GDx instrument.
Discussion
There are many techniques used to determine the foveal location, but most methods previously used have significant limitations, even in the presence of normal ocular structure. These limitations are compounded in the presence of macular disease, where tissue is disrupted, and normal landmarks typically used to locate the fovea may be altered or undetectable. Currently, ophthalmoscopy, fundus photography, scanning laser ophthalmoscopy, fundus reflectometry, OCT, fluorescein angiography, entoptic methods, and fixation stability measured with fundus imaging devices are the major techniques used to determine the foveal position, but these techniques are limited by normal variations in the retina, retinal vasculature, differences in macular pigment distribution, and landmark positions relative to the fovea.
There is substantial variability in foveal location among individuals with respect to landmarks such as the optic disc (Williams & Wilkinson, 1992). Use of a Scanning Laser Ophthalmoscope (SLO) with near infrared light more clearly demarcates the fovea and optic nerve head border (Chen et al., 1992), but normal variability in the size of the optic disc (Quigley, Brown, Morrison, & Drance, 1990), as well as the shape (Jonas, Gusek, Guggenmoos-Holzmann, & Naumann, 1988; Jonas, Gusek, & Naumann, 1988a), cause the optic disc to be an unreliable reference point for foveal localization. This is even more difficult in common eye diseases, such as high myopia and glaucoma, where extensive atrophy and hyperpigmentation in the peripapillary region and tilting of the optic disk can cause the border of the optic disk to be difficult to recognize (Jonas, Gusek, & Naumann, 1988b; Xu, Wang, Yang, & Jonas, 2007).
The foveal light reflex is not a reliable landmark, as it is not present in all normal individuals (Newcomb & Potter, 1981) and becomes less visible as the foveal depression shallows and as the surface of the internal limiting membrane becomes less reflective (Gorrand & Delori, 1999), often causing the foveal light reflex to be absent. In normal subjects, the fovea can be localized accurately with macular thickness measurements using Spectral-Domain Optical Coherence Tomography (SDOCT; Legarreta et al., 2008), but in some patients, the foveal pit is absent, even in the presence of good visual acuities ranging from 20/20 to 20/50 (Marmor, Choi, Zawadzki, & Werner, 2008). Additionally, the foveal pit and foveal light reflex may be affected by fluid accumulation in central macular edema, or other pathological changes affecting the central macula, which disrupt the concavity of the foveal depression (Campbell, Coupland, Buhrmann, & Kertes, 2007; Massin et al., 2001; Polito et al., 2002; Pons & Garcia-Valenzuela, 2005).
Optical density of the ocular fundus among individuals also varies greatly (Van Norren & Tiemeijer, 1986), in the region of the fovea due to macular pigment (Wyszecki & Stiles, 1982) or melanin (Feeney-Burns, Hilderbrand, & Eldridge, 1984; Weiter, Delori, Wing, & Fitch, 1986), and elsewhere due to the retinal pigment epithelium (RPE) and choroidal melanin (Weiter et al., 1986). The macular pigments, lutein and zeaxanthin, have the highest density at the foveal center in young healthy subjects (Handelman, Dratz, Reay, & van Kuijk, 1988; Snodderly, Brown, Delori, & Auran, 1984) and decline rapidly with increasing eccentricity to low, relatively constant levels within 1-mm retinal eccentricity (Bone, Landrum, & Tarsis, 1985; Snodderly, Auran, & Delori, 1984). Macular pigment is not always a good indication of foveal position because it may show an irregular or annual pattern, often noted with increasing age (Delori, Goger, Keilhauer, Salvetti, & Staurenghi, 2006; Elsner, Burns, Beausencourt, & Weiter, 1998) or disease (Aleman et al., 2001; Duncan et al., 2002).
More advanced imaging techniques including cone photopigment density have also been used to determine the foveal location. Similar to macular pigment, cone photopigment density is highest in this area in young healthy subjects and measurements of the cone photopigment density can be used to determine the foveal location. Even though cone photopigment density maps readily pinpoint the fovea in normal subjects, there are significant changes in aging and disease, making these techniques impractical for accurate determination of the fovea (Elsner et al., 1998; Elsner, Burns, Hughes, & Webb, 1992; Zagers et al., 2002). Optical constraints for imaging the central fovea are also found when using ocular speckle interferometry (Marcos, Navarro, & Artal, 1996) or high-resolution mapping of light returned by cone photo-receptors (Burns, Tumbar, Elsner, Ferguson, & Hammer, 2007; Miller, Williams, Morris, & Liang, 1996; Roorda et al., 2002; Wade & Fitzke, 1998). All of these methods can be used to indicate a general region of highest density of pigments or photoreceptors but do not necessarily indicate the center of the anatomic fovea.
The fovea is often assumed to be at the center of the foveal avascular zone (FAZ), which can be demarcated using fluorescein angiography (FA), high contrast imaging with moderate to high magnification and short wavelengths, or entoptic methods (Bradley et al., 1998; Martin & Roorda, 2005; Zeffren, Applegate, Bradley, & van Heuven, 1990). There is large variability in the size of the FAZ, even in normal individuals (Bird & Weale, 1974). The size of the FAZ increases in patients with retinal vascular pathology (Applegate, Bradley, van Heuven, Lee, & Garcia, 1997; Arend et al., 1991; Conrath, Giorgi, Raccah, & Ridings, 2005; Hilmantel et al., 1999; Mansour, 1990; Yap, Gilchrist, & Weatherill, 1987), and with capillary dropout, there can be the appearance of several avascular regions. Similarly, there is inter-individual variability in the extent and distribution of the vessel arcade pattern, making precise foveal localization difficult (Bradley et al., 1998; Zeffren et al., 1990). Due to the invasive nature and potential risks of FA (Kwan, Barry, McAllister, & Constable, 2006; Yannuzzi et al., 1986), it is not used to locate the fovea in the absence of retinal disease. Non-invasive, entoptic methods use short wavelength light to enable subjects to visualize the FAZ by the absence of leukocyte activity in the retinal circulation (Bradley, Applegate, Zeffren, & van Heuven, 1992; Riva & Petrig, 1980; Yap et al., 1987) but are subjective and require a patient to have sufficient visual function to detect small blood vessels or small, moving leukocytes. Similarly, there is inter-individual variability in the extent and distribution of the vessel arcade pattern, making precise foveal localization difficult (Bradley et al., 1998). Furthermore, FA and entoptic methods show an increase in the FAZ individuals with retinal vascular pathology (Applegate et al., 1997; Arend et al., 1991; Conrath et al., 2005; Hilmantel et al., 1999; Mansour, 1990; Yap et al., 1987), and with capillary dropout, there can be the appearance of several avascular regions.
Fixation stability is examined non-invasively with a variety of instruments that image the retina or infer retinal position (Crossland, Culham, & Rubin, 2004; Lei & Schuchard, 1997; Midena et al., 2004; Okada et al., 2005; Remky, Beausencourt, & Elsner, 1996; Rohrschneider, 2004; Rohrschneider, Becker, Kruse, Fendrich, & Völcker, 1995; Timberlake et al., 1986; Trauzettel-Klosinski & Reinhard, 1998), but the Preferred Retinal Locus of fixation differs in size and shape among subjects and does not always correspond to the foveal center (Putnam et al., 2005). The standard deviation of the locus of fixation for normal subjects exceeds 10 microns in the horizontal direction for over half the normal subjects tested, i.e., the fixation area covering a diameter >40 microns (Timberlake et al., 2005).
Algorithms have been developed to automate the localization of the fovea based on traditional imaging techniques including fundus photographs (Fleming, Goatman, Philip, Olson, & Sharp, 2007; Li & Chutatape, 2004; Sinthanayothin, Boyce, Cook, & Williamson, 1999), scanning laser ophthalmoscopy (Pinz, Bernögger, Datlinger, & Kruger, 1998), and fluorescein angiography (Cree, Olson, McHardy, Sharp, & Forrester, 1999; Gutierrez, Epifanio, Ves, & Ferri, 2000). Fleming et al. reported automated foveal localization accuracy better than 1/2 of an optic disc diameter for 96.5% of subjects evaluated, which corresponds to about 750 microns. Variation is introduced for methods that require relative locations of either the retinal vasculature, the optic disc boundary, macular pigment changes, or areas of brightness changes in fluorescein angiography because these features can be highly variable in both normal and diseased eyes.
We demonstrated that the fovea can be identified repeatably and accurately in most of the near infrared image types with differing polarization content used in this study. All three of the graders participating in this study were able to locate the fovea in the 15 × 15 degree near infrared images, despite the lack of some of the typical retinal landmarks, which are absent in some near infrared image types. There was little intra-grader, intergrader, and inter-image variabilities in the graded foveal position in 5 of the 6 image types examined. The image type that was least reliable in determining the foveal position was the Depolarized Light Image, which was nevertheless better than with the methods described above and did not require bright lights, mydriasis, or injection of dye. Graders were able to judge the foveal location in the remaining 5 image types with accuracy more than sufficient for clinical purposes.
For most subjects, particularly those with a foveal light reflex, the image with the least intra-grader and inter-grader variabilities was the Mean of the Parallel Detector Image, with an overall average intra-grader foveal location distance between Time 1 and Time 2 of 1.89 pixels (33.3 microns) and an overall average inter-grader distance of 3.27 pixels (57.6 microns). The Mean of the Parallel Detector Image contains light that retains the properties of the linearly polarized input light and contains retinal features that are easily recognizable. This is also one of the images likely to contain the foveal light reflex, which, when present, is one of the most accurate markers of foveal location. In cases where retinal disease is present in the central macula, landmark features will be less regular and accuracy in determining the foveal location could decrease in the Mean of the Parallel Detector Image. The other image types used in this study, with the exception of the depolarized light image, showed slightly more intra-grader and inter-grader variabilities in the foveal locations than did the Mean of the Parallel Detector Image, but this difference was small and not statistically significant. One surprising finding was that the Maximum of the Parallel Detector Image did not yield the most consistent foveal locations, i.e., was not statistically different from the Mean of the Parallel Detector Image. Initially, we predicted that the Maximum of the Parallel Detector Image would be the most repeatable due to the stronger emphasis on light returning to the parallel detector. Given the assumption that the Maximum of the Parallel Detector Image would be the most consistent, prior to grading we chose this image as the “gold standard” with which to judge the accuracy of the birefringence images. The small difference that would have been created by using the Mean of the Parallel Detector Image instead of the Maximum of the Parallel Detector Image would not have significantly influenced any of our comparisons.
Inter-image comparisons demonstrated good agreement in foveal location for the majority of image types used in this study, emphasizing that in healthy individuals, 5 of the 6 image types could be used to accurately and interchangeably locate the fovea within a narrow range. The Depolarized Image was the only exception, and the most variable inter-image comparisons all included the Depolarized Image. The high intra-grader, inter-grader, and inter-image variabilities in the depolarized light image locations were not surprising. The depolarized image typically includes light that is returning from relatively deeper retinal layers, does not include specularly reflected light, and therefore would not include a foveal light reflex. The Depolarized Light Image also shows less distinct changes due to the geometry of the foveal crest, making determination of the macular boundary and fovea more difficult. The vessel arcades and the limited amount of macular pigment or foveal depression that could be visualized were the best signs used by the graders to determine the foveal location in the depolarized image type. These landmarks leave a larger margin for error, do not isolate the foveal location to a single point, and are variable among individuals.
The only images in this study that that did not require the use of typical retinal landmarks for localization of the fovea were images that included birefringence information, the Birefringence Image and the Maximum Phase of the Crossed Detector Image. These images use the structural properties of the radially symmetric Henle fiber layer surrounding the fovea to produce the macular cross, an image of the interaction between the retinal birefringence and corneal birefringence. Measurements from the Birefringence Image and the Maximum Phase of the Crossed Detector Image demonstrated that birefringence information can be used to detect the foveal position accurately based on intra-grader, inter-grader, and inter-image comparisons. When using the Maximum of the Parallel Detector Image as the standard and comparing repeatability in the Birefringence Image and the Maximum Phase of the Crossed Detector Image, the Maximum Phase of the Crossed Detector Image showed a significant advantage. This was expected, as the Maximum Phase of the Crossed Detector Image displays information regarding both the magnitude and orientation of the Henle fiber layer birefringence, giving a more precise marker of foveal location, while the Birefringence Image contains only birefringence magnitude information.
Retinal imaging using scanning laser polarimetry is multidimensional, in that it can be used to localize the fovea as well as detect retinal pathology. The polarized light/tissue interaction of retinal pathology has been characterized in patients with central serous chorioretin-opathy (Miura et al., 2005) and neovascular age-related macular degeneration (Elsner et al., 2007; Weber et al., 2007). Pixel locations in images taken from the same raw data series correspond to the same retinal location. Multiple image types with custom polarization content, i.e., the Depolarized Light Image and the Maximum Phase of the Crossed Detector Image, can be used in combination to detect pathological retinal features exhibiting differing polarization characteristics and determine the relative distance from the fovea. The Depolarized Light Image was shown in this study to be relatively less useful in determining the foveal location but has been used to delineate deep retinal pathological changes (Elsner et al., 2007; Mellem-Kairala et al., 2005; Miura et al., 2005). The Maximum Phase of the Crossed Detector Image is potentially most useful in instances where macular disease disrupts the deeper retinal structure, while the more superficial Henle fiber layer remains relatively intact. This is demonstrated in Figure 9, which shows the 6 image types used in this study in a patient with non-exudative (dry) age-related macular degeneration (AMD). The foveal location is difficult to reliably determine using image types without birefringence information. Despite the limitations in foveal localization, the image types that do not include birefringence information more accurately outline the extent of retinal pathology.
Figure 9.
Six images used in this study in a patient with non-exudative (dry) age-related macular degeneration. The fovea is not easily localizable in images that do not contain birefringence information but can easily be localized within a small area in both the Birefringence Image and the Maximum Phase of the Crossed Detector Image. The bright spot in the center of the images is a reflection artifact inherent in the GDx instrument.
The macular cross pattern can be used to locate the foveal position directly, based on the foveal anatomy, and does not require additional retinal landmarks, which can be irregular and differ greatly among normal individuals. There is little inter-individual variability in the structural regularity of the normal Henle fiber layer, which does not appreciably change with normal aging, and can persist in the presence of moderate macular disease (Weber et al., 2007) and glaucoma (Bagga, Greenfield, & Feuer, 2005). This makes scanning laser polarimetry a potentially robust method for foveal localization and a measure of structure in the central macula that can then be used to also evaluate function. Additional studies are required to determine the extent of pathology necessary to degrade scanning laser polar-imetry images to a degree where the fovea can no longer be localized.
Acknowledgments
This publication was made possible by the National Institutes of Health Grants EY017886 (DAV), EY007624 (AEE), and EB002346 (AEE). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NEI or NIBIB.
Footnotes
Commercial relationships: none.
Contributor Information
Dean A. VanNasdale, School of Optometry, Indiana University, Bloomington, IN, USA
Ann E. Elsner, School of Optometry, Indiana University, Bloomington, IN, USA
Anke Weber, Department of Ophthalmology, RWTH Aachen, University Hospital, Aachen, Germany.
Masahiro Miura, Department of Ophthalmology, Tokyo Medical University, Tokyo, Japan.
Bryan P. Haggerty, School of Optometry, Indiana University, Bloomington, IN, USA
References
- Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, et al. Macular pigment and lutein supplementation in retinitis pig-mentosa and Usher syndrome. Investigative Ophthalmology & Visual Science. 2001;42:1873–1881. [PubMed] [Google Scholar]
- Applegate RA, Bradley A, van Heuven WA, Lee BL, Garcia CA. Entoptic evaluation of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 1997;38:783–791. [PubMed] [Google Scholar]
- Arend O, Wolf S, Jung F, Bertram B, Pöstgens H, Toonen H, et al. Retinal microcirculation in patients with diabetes mellitus: Dynamic and morphological analysis of perifoveal capillary network. British Journal of Ophthalmology. 1991;75:514–518. doi: 10.1136/bjo.75.9.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga H, Greenfield DS, Feuer WJ. Quantitative assessment of atypical birefringence images using scanning laser polarimetry with variable corneal compensation. American Journal of Ophthalmology. 2005;139:437–446. doi: 10.1016/j.ajo.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Bird AC, Weale RA. On the retinal vasculature of the human fovea. Experimental Eye Research. 1974;19:409–417. doi: 10.1016/0014-4835(74)90050-5. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Research. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- Bradley A, Applegate RA, Zeffren BS, van Heuven WA. Psychophysical measurement of the size and shape of the human foveal avascular zone. Ophthalmic & Physiological Optics. 1992;12:18–23. [PubMed] [Google Scholar]
- Bradley A, Zhang H, Applegate RA, Thibos LN, Elsner AE. Entoptic image quality of the retinal vasculature. Vision Research. 1998;38:2685–2696. doi: 10.1016/s0042-6989(97)00345-3. [DOI] [PubMed] [Google Scholar]
- Burns SA, Elsner AE, Mellem-Kairala MB, Simmons RB. Improved contrast of subretinal structures using polarization analysis. Investigative Ophthalmology & Visual Science. 2003;44:4061–4068. doi: 10.1167/iovs.03-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SA, Tumbar R, Elsner AE, Ferguson D, Hammer DX. Large-field-of-view, modular, stabilized, adaptive-optics-based scanning laser ophthalmoscope. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2007;24:1313–1326. doi: 10.1364/josaa.24.001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RJ, Coupland SG, Buhrmann RR, Kertes PJ. Effect of eccentric and inconsistent fixation on retinal optical coherence tomography measures. Archives of Ophthalmology. 2007;125:624–627. doi: 10.1001/archopht.125.5.624. [DOI] [PubMed] [Google Scholar]
- Chen JF, Elsner AE, Burns SB, Hansen RM, Lou PL, Kwong KK, et al. The effect of eye shape on retinal responses. Clinical Vision Sciences. 1992;7:521–530. [Google Scholar]
- Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: Quantitative vs. qualitative assessment. Eye. 2005;19:322–326. doi: 10.1038/sj.eye.6701456. [DOI] [PubMed] [Google Scholar]
- Cree MJ, Olson JA, McHardy KC, Sharp PF, Forrester JV. The preprocessing of retinal images for the detection of fluorescein leakage. Physics in Medicine and Biology. 1999;44:293–308. doi: 10.1088/0031-9155/44/1/021. [DOI] [PubMed] [Google Scholar]
- Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic & Physiological Optics. 2004;24:327–333. doi: 10.1111/j.1475-1313.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: Evidence of a gender relationship. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2006;23:521–538. doi: 10.1364/josaa.23.000521. [DOI] [PubMed] [Google Scholar]
- Dreher AW, Reiter K, Weinreb RN. Spatially resolved birefringence of the retinal nerve-fiber layer assessed with a retinal laser ellipsometer. Applied Optics. 1992;31:3730–3735. doi: 10.1364/AO.31.003730. [DOI] [PubMed] [Google Scholar]
- Duncan JL, Aleman TS, Gardner LM, De Castro E, Marks DA, Emmons JM, et al. Macular pigment and lutein supplementation in choroidere-mia. Experimental Eye Research. 2002;74:371–381. doi: 10.1006/exer.2001.1126. [DOI] [PubMed] [Google Scholar]
- Early Treatment for Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology. 1991;98:807–822. [PubMed] [Google Scholar]
- Elsner AE, Burns SA, Beausencourt E, Weiter JJ. Foveal cone photopigment distribution: Small alterations associated with macular pigment distribution. Investigative Ophthalmology & Visual Science. 1998;39:2394–2404. [PubMed] [Google Scholar]
- Elsner AE, Burns SA, Hughes GW, Webb RH. Reflectometry with a scanning laser ophthalmoscope. Applied Optics. 1992;31:3697–3710. doi: 10.1364/AO.31.003697. [DOI] [PubMed] [Google Scholar]
- Elsner AE, Miura M, Stewart JB, Kairala MB, Burns SA. Novel algorithms for polarization imaging resulting in improved quantification of retinal blood vessels. Studies in Health Technology and Informatics. 2003;94:59–61. [PubMed] [Google Scholar]
- Elsner AE, Weber A, Cheney MC, VanNasdale DA. Spatial distribution of macular birefringence associated with the Henle fibers. Vision Research. 2008;48:2578–2585. doi: 10.1016/j.visres.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner AE, Weber A, Cheney MC, VanNasdale DA, Miura M. Imaging polarimetry in patients with neovascular age-related macular degeneration. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2007;24:1468–1480. doi: 10.1364/josaa.24.001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investigative Ophthalmology & Visual Science. 1984;25:195–200. [PubMed] [Google Scholar]
- Fleming AD, Goatman KA, Philip S, Olson JA, Sharp PF. Automatic detection of retinal anatomy to assist diabetic retinopathy screening. Physics in Medicine and Biology. 2007;52:331–345. doi: 10.1088/0031-9155/52/2/002. [DOI] [PubMed] [Google Scholar]
- Gorrand JM, Delori FC. Reflectance and curvature of the inner limiting membrane at the foveola. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1999;16:1229–1237. doi: 10.1364/josaa.16.001229. [DOI] [PubMed] [Google Scholar]
- Gramatikov BI, Zalloum OH, Wu YK, Hunter DG, Guyton DL. Birefringence-based eye fixation monitor with no moving parts. Journal of Biomedical Optics. 2006;11:34025. doi: 10.1117/1.2209003. [DOI] [PubMed] [Google Scholar]
- Gramatikov BI, Zalloum OH, Wu YK, Hunter DG, Guyton DL. Directional eye fixation sensor using birefringence-based foveal detection. Applied Optics. 2007;46:1809–1818. doi: 10.1364/ao.46.001809. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Epifanio I, Ves ED, Ferri FJ. Proceedings of the International Conference on Pattern Recognition. Barcelona, Spain: IEEE; 2000. An active contour model for the automatic detection of the fovea in fluorescein angiographies; pp. 4312–4315. [Google Scholar]
- Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Investigative Ophthalmology & Visual Science. 1988;29:850–855. [PubMed] [Google Scholar]
- Hemenger RP. Birefringence of a medium of tenuous parallel cylinders. Applied Optics. 1989;28:4030–4034. doi: 10.1364/AO.28.004030. [DOI] [PubMed] [Google Scholar]
- Hilmantel G, Applegate RA, van Heuven WA, Stowers SP, Bradley A, Lee BL. Entoptic foveal avascular zone measurement and diabetic retinopathy. Optometry and Vision Science. 1999;76:826–831. doi: 10.1097/00006324-199912000-00017. [DOI] [PubMed] [Google Scholar]
- Huang XR, Knighton RW. Linear birefringence of retinal nerve fiber layer measured in vitro with a multispectral imaging micropolarimeter. Journal of Biomedical Optics. 2002;7:199–204. doi: 10.1117/1.1463050. [DOI] [PubMed] [Google Scholar]
- Hunter DG, Nassif DS, Piskun NV, Winsor R, Gramatikov BI, Guyton DL. Pediatric Vision Screener 1: Instrument design and operation. Journal of Biomedical Optics. 2004;9:1363–1368. doi: 10.1117/1.1805560. [DOI] [PubMed] [Google Scholar]
- Hunter DG, Patel SN, Guyton DL. Automated detection of foveal fixation by use of retinal birefringence scanning. Applied Optics. 1999;38:1273–1279. doi: 10.1364/ao.38.001273. [DOI] [PubMed] [Google Scholar]
- Hunter DG, Shah AS, Sau S, Nassif D, Guyton DL. Automated detection of ocular alignment with binocular retinal birefringence scanning. Applied Optics. 2003;42:3047–3053. doi: 10.1364/ao.42.003047. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Gusek GC, Guggenmoos-Holzmann I, Naumann GO. Variability of the real dimensions of normal human optic discs. Graefes Archive for Clinical & Experimental Ophthalmology. 1988;226:332–336. doi: 10.1007/BF02172962. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Gusek GC, Naumann GO. Optic disk morphometry in high myopia. Graefes Archive for Clinical & Experimental Ophthalmology. 1988a;226:587–590. doi: 10.1007/BF02169209. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configu-ration and correlations in normal eyes. Investigative Ophthalmology & Visual Science. 1988b;29:1151–1158. [PubMed] [Google Scholar]
- Kwan AS, Barry C, McAllister IL, Constable I. Fluorescein angiography and adverse drug reactions revisited: The Lions Eye experience. Clinical & Experimental Ophthalmology. 2006;34:33–38. doi: 10.1111/j.1442-9071.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Legarreta JE, Gregori G, Punjabi OS, Knighton RW, Lalwani GA, Puliafito CA. Macular thickness measurements in normal eyes using spectral domain optical coherence tomography. Ophthalmic Surgery, Lasers Imaging. 2008;39:S43–S49. doi: 10.3928/15428877-20080715-02. [DOI] [PubMed] [Google Scholar]
- Lei H, Schuchard RA. Using two preferred retinal loci for different lighting conditions in patients with central scotomas. Investigative Ophthalmology & Visual Science. 1997;38:1812–1818. [PubMed] [Google Scholar]
- Li H, Chutatape O. Automated feature extraction in color retinal images by a model based approach. IEEE Transactions on Biomedical Engineering. 2004;51:246–254. doi: 10.1109/TBME.2003.820400. [DOI] [PubMed] [Google Scholar]
- Mansour AM. Measuring fundus landmarks. Investigative Ophthalmology & Visual Science. 1990;31:41–42. [PubMed] [Google Scholar]
- Marcos S, Navarro R, Artal P. Coherent imaging of the cone mosaic in the living human eye. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1996;13:897–905. doi: 10.1364/josaa.13.000897. [DOI] [PubMed] [Google Scholar]
- Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: Reassessment of foveal hypoplasia as fovea plana. Archives of Ophthalmology. 2008;126:907–913. doi: 10.1001/archopht.126.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Roorda A. Direct and non-invasive assessment of parafoveal capillary leukocyte velocity. Ophthalmology. 2005;112:2219–2224. doi: 10.1016/j.ophtha.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A. Reproducibility of retinal mapping using optical coherence tomography. Archives of Ophthalmology. 2001;119:1135–1142. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- Mellem-Kairala MB, Elsner AE, Weber A, Simmons RB, Burns SA. Improved contrast of peripapillary hyperpigmentation using polarization analysis. Investigative Ophthalmology & Visual Science. 2005;46:1099–1106. doi: 10.1167/iovs.04-0574. [DOI] [PubMed] [Google Scholar]
- Midena E, Radin PP, Pilotto E, Ghirlando A, Convento E, Varano M. Fixation pattern and macular sensitivity in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. A microperimetry study. Seminars in Ophthalmology. 2004;19:55–61. doi: 10.1080/08820530490882896. [DOI] [PubMed] [Google Scholar]
- Miller DT, Williams DR, Morris GM, Liang J. Images of cone photoreceptors in the living human eye. Vision Research. 1996;36:1067–1079. doi: 10.1016/0042-6989(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Miura M, Elsner AE, Cheney MC, Usui M, Iwasaki T. Imaging polarimetry and retinal blood vessel quantification at the epiretinal membrane. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2007;24:1431–1437. doi: 10.1364/josaa.24.001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Elsner AE, Weber A, Cheney MC, Osako M, Usui M, et al. Imaging polarimetry in central serous chorioretinopathy. American Journal of Ophthalmology. 2005;140:1014–1019. doi: 10.1016/j.ajo.2005.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif D, Gramatikov B, Guyton D, Hunter D. Pediatric vision screening using binocular retinal birefringence scanning. Ophthalmic Technologies XIII, Proceedings of SPIE. 2003;4951:9–20. [Google Scholar]
- Newcomb RD, Potter JW. Clinical investigation of the foveal light reflex. American Journal of Optometry and Physiological Optics. 1981;58:1110–1119. doi: 10.1097/00006324-198112000-00007. [DOI] [PubMed] [Google Scholar]
- Okada K, Yamamoto S, Mizunoya S, Hoshino A, Arai M, Takatsuna Y. Correlation of retinal sensitivity measured with fundus-related micro-perimetry to visual acuity and retinal thickness in eyes with diabetic macular edema. Eye. 2005;20:805–809. doi: 10.1038/sj.eye.6702014. [DOI] [PubMed] [Google Scholar]
- Pinz A, Bernögger S, Datlinger P, Kruger A. Mapping the human retina. IEEE Transactions on Medical Imaging. 1998;17:606–619. doi: 10.1109/42.730405. [DOI] [PubMed] [Google Scholar]
- Polito A, Shah SM, Haller JA, Zimmer-Galler I, Zeimer R, Campochiaro PA, et al. Comparison between retinal thickness analyzer and optical coherence tomography for assessment of foveal thickness in eyes with macular disease. American Journal of Ophthalmology. 2002;134:240–251. doi: 10.1016/s0002-9394(02)01528-3. [DOI] [PubMed] [Google Scholar]
- Pons ME, Garcia-Valenzuela E. Redefining the limit of the outer retina in optical coherence tomography scans. Ophthalmology. 2005;112:1079–1085. doi: 10.1016/j.ophtha.2004.11.061. [DOI] [PubMed] [Google Scholar]
- Putnam NM, Hofer HJ, Doble N, Chen L, Carroll J, Williams DR. The locus of fixation and the foveal cone mosaic. Journal of Vision. 2005;5(7):3,632–639. doi: 10.1167/5.7.3. http://journalofvision.org/5/7/3/ [DOI] [PubMed]
- Quigley HA, Brown AE, Morrison JD, Drance SM. The size and shape of the optic disc in normal human eyes. Archives of Ophthalmology. 1990;108:51–57. doi: 10.1001/archopht.1990.01070030057028. [DOI] [PubMed] [Google Scholar]
- Remky A, Beausencourt E, Elsner AE. Angioscotometry with the scanning laser ophthalmoscope. Comparison of the effect of different wavelengths. Investigative Ophthalmology & Visual Science. 1996;37:2350–2355. [PubMed] [Google Scholar]
- Riva CE, Petrig B. Blue field entoptic phenomenon and blood velocity in the retinal capillaries. Journal of the Optical Society of America. 1980;70:1234–1238. doi: 10.1364/josa.70.001234. [DOI] [PubMed] [Google Scholar]
- Rohrschneider K. Determination of the location of the fovea on the fundus. Investigative Ophthalmology & Visual Science. 2004;45:3257–3258. doi: 10.1167/iovs.03-1157. [DOI] [PubMed] [Google Scholar]
- Rohrschneider K, Becker M, Kruse FE, Fendrich T, Völcker HE. Stability of fixation: Results of fundus-controlled examination using the scanning laser ophthalmoscope. German Journal of Ophthalmology. 1995;4:197–202. [PubMed] [Google Scholar]
- Roorda A, Romero-Borja F, Donnelly WJ, III, Queener H, Hebert TJ, Campbell MCW. Adaptive optics scanning laser ophthalmo-scopy. Optics Express. 2002;10:405–412. doi: 10.1364/oe.10.000405. [DOI] [PubMed] [Google Scholar]
- Sinthanayothin C, Boyce JF, Cook HL, Williamson TH. Automated localisation of the optic disc, fovea, and retinal blood vessels from digital colour fundus images. British Journal of Ophthalmology. 1999;83:902–910. doi: 10.1136/bjo.83.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Investigative Ophthalmology & Visual Science. 1984;25:674–685. [PubMed] [Google Scholar]
- Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investigative Ophthalmology & Visual Science. 1984;25:660–673. [PubMed] [Google Scholar]
- Timberlake GT, Mainster MA, Peli E, Augliere RA, Essock EA, Arend LE. Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investigative Ophthalmology & Visual Science. 1986;27:1137–1147. [PubMed] [Google Scholar]
- Timberlake GT, Sharma MK, Grose SA, Gobert DV, Gauch JM, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optometry and Vision Science. 2005;82:177–185. doi: 10.1097/01.opx.0000156311.49058.c8. [DOI] [PubMed] [Google Scholar]
- Trauzettel-Klosinski S, Reinhard J. The vertical field border in hemianopia and its significance for fixation and reading. Investigative Ophthalmology & Visual Science. 1998;39:2177–2186. [PubMed] [Google Scholar]
- Van Norren D, Tiemeijer LF. Spectral reflectance of the human eye. Vision Research. 1986;26:313–320. doi: 10.1016/0042-6989(86)90028-3. [DOI] [PubMed] [Google Scholar]
- Wade AR, Fitzke FW. A fast, robust pattern recognition system for low light level image registration and its application to retinal imaging. Optics Express. 1998;3:190–197. doi: 10.1364/oe.3.000190. [DOI] [PubMed] [Google Scholar]
- Weber A, Cheney MC, Smithwick QYJ, Elsner AE. Polarimetric imaging and blood vessel quantification. Optics Express. 2004;12:5178–5190. doi: 10.1364/opex.12.005178. [DOI] [PubMed] [Google Scholar]
- Weber A, Elsner AE, Miura M, Kompa S, Cheney MC. Relationship between foveal birefringence and visual acuity in neovascular age-related macular degeneration. Eye. 2007;21:353–361. doi: 10.1038/sj.eye.6702203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Investigative Ophthalmology & Visual Science. 1986;27:145–152. [PubMed] [Google Scholar]
- Williams TD, Wilkinson JM. Position of the fovea centralis with respect to the optic nerve head. Optometry and Vision Science. 1992;69:369–377. doi: 10.1097/00006324-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Wolf S, Toonen H, Arend O, Jung F, Kaupp A, Kiesewetter H, et al. Quantifying retinal capillary circulation using the scanning laser ophthalmoscope. Biomedical Technology. 1990;35:131–134. [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. Color science: Concepts and methods, quantitative data and formulae. New York: Wiley; 1982. [Google Scholar]
- Xu L, Wang Y, Yang H, Jonas JB. Differences in parapapillary atrophy between glaucomatous and normal eyes: The Beijing Eye Study. American Journal of Ophthalmology. 2007;144:541–546. doi: 10.1016/j.ajo.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93:611–617. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- Yap M, Gilchrist J, Weatherill J. Psychophysical measurement of the foveal avascular zone. Ophthalmic & Physiological Optics. 1987;7:405–410. [PubMed] [Google Scholar]
- Zagers NP, van de Kraats J, Berendschot TT, van Norren D. Simultaneous measurement of foveal spectral reflectance and cone-photoreceptor directionality. Applied Optics. 2002;41:4686–4696. doi: 10.1364/ao.41.004686. [DOI] [PubMed] [Google Scholar]
- Zeffren BS, Applegate RA, Bradley A, van Heuven WA. Retinal fixation point location in the foveal avascular zone. Investigative Ophthalmology & Visual Science. 1990;31:2099–2105. [PubMed] [Google Scholar]
- Zhou Q, Knighton RW. Light scattering and form birefringence of parallel cylindrical arrays that represent cellular organelles of the retinal nerve fiber layer. Applied Optics. 1997;36:2273–2285. doi: 10.1364/ao.36.002273. [DOI] [PubMed] [Google Scholar]