Abstract
The endocannabinoid 2-arachidonoylglycerol (2-AG) regulates neurotransmission and neuroinflammation by activating CB1 cannabinoid receptors on neurons and CB2 cannabinoid receptors on microglia. Enzymes that hydrolyze 2-AG, such as monoacylglycerol lipase, regulate the accumulation and efficacy of 2-AG at cannabinoid receptors. We found that the recently described serine hydrolase α-β-hydrolase domain 6 (ABHD6) also controls the accumulation and efficacy of 2-AG at cannabinoid receptors. In cells from the BV-2 microglia cell line, ABHD6 knockdown reduced hydrolysis of 2-AG and increased the efficacy with which 2-AG can stimulate CB2-mediated cell migration. ABHD6 was expressed by neurons in primary culture and its inhibition led to activity-dependent accumulation of 2-AG. In adult mouse cortex, ABHD6 was located postsynaptically and its selective inhibition allowed the induction of CB1-dependent long-term depression by otherwise subthreshold stimulation. Our results indicate that ABHD6 is a rate-limiting step of 2-AG signaling and is therefore a bona fide member of the endocannabinoid signaling system.
In the nervous system, the endocannabinoids (eCBs) arachidonoylethanolamide (anandamide) and 2-AG are produced and inactivated by neurons and glia1,2. The production of eCBs increases in response to specific stimuli, including membrane receptor activation, ion channel opening and calcium influx2. eCBs are inactivated by cellular uptake followed by intracellular enzymatic hydrolysis3,4. The balance between this production and inactivation dictates the levels of extracellular eCB accumulation and the ensuing activation of CB1 receptors expressed by neurons (regulating neurotransmitter release) and CB2 receptors expressed by microglia (regulating their motility and ability to produce immunomodulators)4–7. Thus, the enzymatic steps that control the production and inactivation of eCBs constitute promising molecular targets for indirectly modulating CB1 and CB2 receptor activity, and thereby controlling neurotransmission and neuroinflammation.
Of all the steps that control the accumulation of eCBs, the hydrolytic enzymes that inactivate anandamide and 2-AG represent the most promising pharmacological and genetic targets for fine-tuning the local accumulation of these lipid transmitters. Inhibition of fatty acid amide hydrolase (FAAH) increases anandamide levels in the brain and leads to CB1-mediated analgesia, and also has anxiolytic and antidepressant effects8–10. The inhibition of monoacylglycerol lipase (MAGL) increases 2-AG levels in the brain and leads to CB1-mediated analgesia and hypomotility11–13. Notably, these therapeutic outcomes are achieved without eliciting the broad spectrum of cognitive effects that are typically associated with direct CB1 receptor agonists8–12. Conversely, concomitant inhibition of FAAH and MAGL leads to an increase in the levels of both anandamide and 2-AG in the brain, recapitulating many of the effects produced by direct CB1 receptor agonists14. Together, these results suggest that the selective inhibition of distinct eCB-hydrolyzing enzymes allows differential control of eCB signaling, with each hydrolase likely to provide unique therapeutic opportunities. This idea has led to the search for novel enzymes responsible for eCB hydrolysis.
There is evidence that, in addition to MAGL, the brain expresses other enzymes that can hydrolyze 2-AG. Pharmacological inhibition of MAGL in crude brain homogenates does not fully eliminate 2-AG hydrolysis, leaving ∼20% of this activity intact15,16. Furthermore, immunoprecipitation of MAGL from brain cytosolic fractions reduces 2-AG hydrolysis by only ∼50%17. BV-2 cells hydrolyze 2-AG even though they do not express MAGL mRNA18. Two new enzymes that are expressed in the brain and can hydrolyze 2-AG (ABHD6 and ABHD12) were recently identified using a functional proteomics approach15 (activity-based protein profiling–multidimensional protein identification technology: ABPP-MudPIT19). Whether these enzymes control the accumulation and efficacy of 2-AG at cannabinoid receptors in intact brain cells still needs to be directly tested. Here we used ABPP-MudPIT to identify the unknown 2-AG-hydrolyzing activity expressed by BV-2 cells, and found that it is ABHD6. We then tested whether ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors in several models: intact BV-2 cells, mouse microglia in primary culture, mouse neurons in primary culture, and slices prepared from adult mouse brain.
Results
ABHD6 hydrolyzes 2-AG in BV-2 cells
It has been shown that the unknown 2-AG-hydrolyzing activity expressed by BV-2 cells is sensitive to methyl arachidonyl fluorophosphonate (MAFP), a non-selective serine hydrolase inhibitor, and enriched in the mitochondrial fraction of these cells18. These results suggest that this enzyme can be isolated from BV-2 cell fractions by using a fluorophosphonate-based probe that targets serine hydrolases (FP-biotin)20, and subsequently identified by the functional proteomics platform ABPP-MudPIT19,21 (Fig. 1a). As an initial proof of concept, we found that the FP-biotin probe inhibited ∼90% of the [3H]-2-AG hydrolysis in BV-2 mitochondrial fractions (Supplementary Fig. 1). We carried out FP-biotin pull-down and subsequent ABPP-MudPIT analysis on both BV-2 mitochondrial and BV-2 cytosolic fractions (which show a ∼10-fold difference in their amount of 2-AG-hydrolyzing activity18) with the goal of identifying serine hydrolases that are differentially expressed between these two fractions. Of the ten enzymes that were most highly enriched in the mitochondrial fraction (Table 1), four have been shown to hydrolyze 2-AG: ABHD12, NTE, FAAH and ABHD6 (ref. 15). MAGL was not detected in either of the BV-2 cell fractions, consistent with the previous finding that BV-2 cells do not express MAGL mRNA18. Thus, using FP-biotin probes and ABPP-MudPIT analysis, we identified ABHD12, NTE, FAAH and ABHD6 as viable candidates for the unknown 2-AG-hydrolyzing activity expressed by BV-2 cells.
Figure 1.
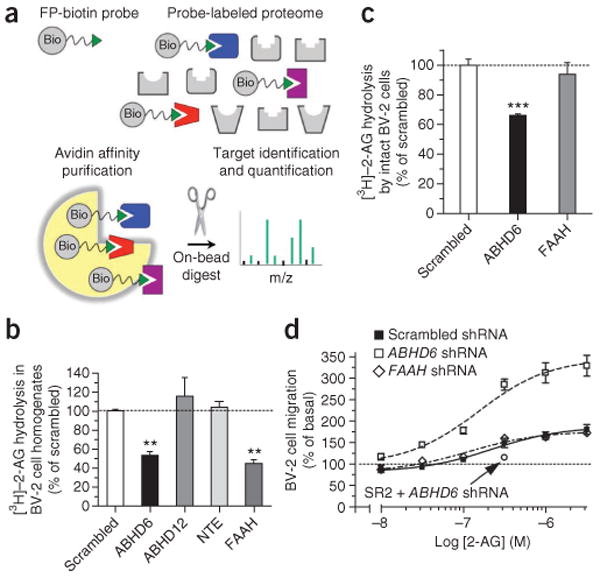
ABHD6 hydrolyzes 2-AG in BV-2 cells and controls the efficacy of 2-AG at CB2 receptors. (a) Cartoon scheme of the ABPP-MudPIT procedure used to identify serine hydrolases expressed by BV-2 cells. (b) [3H]–2-AG hydrolysis in homogenates prepared from BV-2 cells infected with shRNAs targeting ABHD6, ABHD12, NTE or FAAH, compared to scrambled shRNA. **P < 0.01 compared to scrambled response (ANOVA one-way, Dunnett's post test). (c) [3H]–2-AG hydrolysis in intact BV-2 cells infected with shRNA targeting either ABHD6 or FAAH, compared to scrambled shRNA. ***P < 0.0001 compared to scrambled response (Student's t test). (d) 2-AG-induced cell migration of BV-2 cells infected with shRNA targeting ABHD6 or FAAH, compared to scrambled shRNA. Data are expressed as percentage of basal migration (migration of the shRNA-treated clones in the presence of 0.1% DMSO). The 2-AG response is inhibited by pretreatment with the CB2 receptor antagonist SR144528 (SR2, 200 nM). Data are shown as mean ± s.e.m. of 3–5 independent experiments, each performed in triplicate. The percentage knockdown was systematically verified for each infection and reported in Supplementary Table 1.
Table 1. Four serine hydrolases were identified as candidates for the 2-AG–hydrolyzing activity measured in BV-2 cells.
| IPI number | Common name | Abbreviation | 2-AG hydrolase activity | Spectral counts (mitochondrial fraction) |
Spectral counts (cytosolic fraction) |
|---|---|---|---|---|---|
| IPI00331610 | Patatin-like phospholipase domain 7 (NTE-related esterase) | PNPLA7 | No | 163 | 0 |
| IPI00165731* | α-β hydrolase 12 | ABHD12 | Yes | 44 | 0 |
| IPI00128034* | Neuropathy target esterase | NTE | Yes | 31 | 0 |
| IPI00117176* | Fatty acid amide hydrolase | FAAH | Yes | 24 | 0 |
| IPI00321386* | α-β hydrolase 6 | ABHD6 | Yes | 23 | 0 |
| IPI00108883 | FAM108B | FAM108B | No | 23 | 0 |
| IPI00403586 | Arylacetamide deacetylase-like 1 (KIAA1363) | AADACL1 | No | 260 | 1 |
| IPI00170213 | α-β hydrolase 11 | ABHD11 | No | 64 | 2 |
| IPI00319188 | Lipoprotein lipase | LPL | No | 37 | 2 |
| IPI00136683 | Acyl coenzyme A thioester hydrolase 2 | ACOT2 | No | 35 | 3 |
ABPP was performed on subcellular fractions from BV-2 cells to identify functional serine hydrolases. Listed are the ten enzymes that were the most abundant in the mitochondrial fraction relative to the cytosolic fraction.
indicates enzymes that are known to hydrolyze 2-AG when transfected and their activity assessed in homogenates.
We then used short hairpin RNA (shRNA) constructs to selectively knockdown the expression of each candidate enzyme in BV-2 cells to determine the relative contribution of each enzyme to the 2-AG hydrolysis measured in these cells. To obtain reliable knockdowns of each enzyme, we tested 25 shRNA constructs (Supplementary Table 1). Using the most efficient shRNAs, we found that knocking down the expression of ABHD6 or FAAH each reduced [3H]-2-AG hydrolysis in BV-2 cell homogenates by ∼50%, whereas knocking down the expression of ABHD12 or NTE had no significant effect (Fig. 1b). These data provide two important results. First, they confirm genetically that FAAH is responsible for ∼50% of the [3H]-2-AG hydrolysis measured in BV-2 cell homogenates (consistent with the finding that ∼50% of the [3H]-2-AG hydrolysis in BV-2 cell homogenates is sensitive to the FAAH inhibitor URB59718). Second, they suggest that ABHD6 (rather than ABHD12 or NTE) is responsible for the remaining 50% of the 2-AG hydrolysis in BV-2 cell homogenates, and thus lead us to conclude that ABHD6 is the novel enzyme for which we have been searching.
ABHD6 controls 2-AG's efficacy at CB2 receptors
Results showing that an enzyme hydrolyzes a specific lipid in cell homogenates do not necessarily mean that the enzyme also hydrolyzes this lipid in intact cells. For example, FAAH hydrolyzes both anandamide and 2-AG in cell homogenates, but often only hydrolyzes anandamide in intact cells18,22. To determine whether ABHD6 hydrolyzes 2-AG in intact cells, we incubated intact BV-2 cells in culture with [3H]-2-AG and measured the production of free [3H]-glycerol. Intact BV-2 cells efficiently hydrolyzed [3H]-2-AG, and this activity was significantly reduced in BV-2 cells infected with shRNA targeting ABHD6, whereas this activity was unaffected in BV-2 cells infected with shRNA targeting FAAH (Fig. 1c). These data show that, unlike FAAH, ABHD6 hydrolyzes 2-AG in intact BV-2 cells.
BV-2 cell migration is stimulated by 2-AG, and this response is blocked by the CB2 receptor antagonist SR144528 (ref. 23). Here, to determine whether ABHD6 controls the efficacy of 2-AG at cannabinoid receptors, we tested whether ABHD6 knockdown in BV-2 cells increases this response. Using an unbiased method that quantifies cell migration with a near-infrared-emitting dye24, we found that 2-AG increased the migration of BV-2 cells infected with either scrambled shRNA or shRNA targeting FAAH ∼2-fold, but stimulated by ∼3-fold the migration of BV-2 cells infected with shRNA targeting ABHD6 (Fig. 1d). This result supports the notion that less 2-AG is hydrolyzed when ABHD6 is knocked down, and thus more 2-AG reaches and activates CB2 receptors. Three controls are worth noting here. First, the stimulatory effect of 2-AG on the migration of ABHD6-knockdown BV-2 cells was blocked by the CB2 receptor antagonist SR144528 (Fig. 1d). Second, CB2 receptor expression (as determined by radioligand binding using [3H]-CP55940 and SR144528 at 100 nM) did not differ between BV-2 cells infected with scrambled shRNA or shRNA targeting ABHD6 (data not shown), indicating that these infections did not affect CB2 receptor expression. Third, the half-maximum effective concentration (EC50) of 2-AG to stimulate BV-2 cell migration did not differ between BV-2 cells infected with scrambled shRNA or shRNA targeting ABHD6, suggesting that the coupling of CB2 receptors to second messenger signaling was not affected by shRNA treatment (Fig. 1d). Together, these results indicate that ABHD6 hydrolyzes 2-AG in intact cells and controls its efficacy at CB receptors, making this enzyme a rate-limiting step in controlling the bioactivity of this lipid transmitter.
Neurons express ABHD6
To explore the expression of ABHD6 in primary brain cells, we quantified both ABHD6 mRNA and ABHD6 enzymatic activity in various mouse brain preparations. Using quantitative PCR (qPCR), we found that ABHD6 mRNA was abundant in adult mouse brain and neurons in primary culture, and low in mouse microglia in primary culture (Table 2). To measure the enzymatic activity of ABHD6, we determined the amount of [3H]-2-AG hydrolysis that is blocked by WWL70, a highly selective ABHD6 inhibitor25. This study provided three further pieces of evidence that WWL70 is an effective and specific inhibitor of mouse ABHD6 activity. First, WWL70 dose-dependently inhibited [3H]-2-AG hydrolysis in homogenates prepared from ABHD6-transfected COS-7 cells (Fig. 2a); by contrast, the selective MAGL inhibitor JZL18411 had no significant effect on [3H]-2-AG hydrolysis in these homogenates (Fig. 2b). Second, WWL70 inhibited ∼50% of the [3H]-2-AG hydrolysis in BV-2 cell homogenates (Fig. 2c). Third, WWL70 did not directly interact with CB1 or CB2 receptors (Supplementary Fig. 2).
Table 2. ABHD6 is abundantly expressed in brain and more is found in neurons than microglia.
| HPRT CT | δCT (ABHD6) | Relative (%) | |
|---|---|---|---|
| Brain | 21.5–23.7 | 1.8 | 100 |
| BV-2 | 22.9–23.2 | 3.9 | 23 |
| Microglia | 20.8–23.0 | 4.7 | 13 |
| Neurons | 23.2–24.2 | 3 | 44 |
Levels of ABHD6 mRNA were quantifed by qPCR and normalized to the housekeeping gene HPRT. Here the range of threshold cycles (CT) that we obtained in our measurements is provided, showing that HPRT can be used to compare ABHD6 mRNA levels between different cells and tissue. Relative expression levels of ABHD6 (calculated using δ-δCT) were assessed in BV-2 cells, microglia and neurons, and compared to total brain (which was set at 100%). Independent qPCR measurements were performed in independent cell cultures and brain tissue samples (N = 3 to 4).
Figure 2.
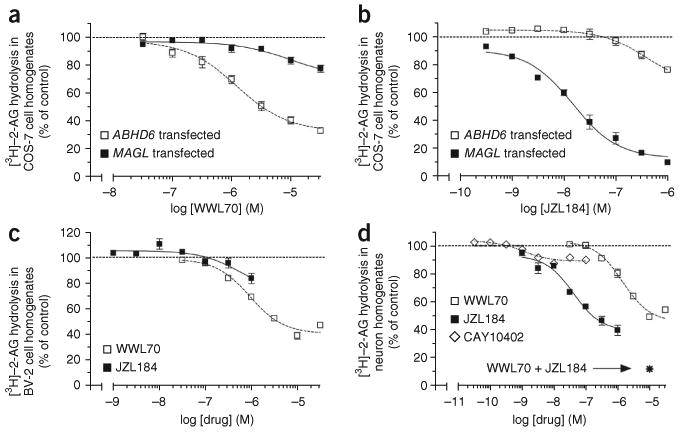
Effect of the ABHD6 inhibitor WWL70 and the MAGL inhibitor JZL184 on [3H]–2-AG hydrolysis in cell homogenates. [3H]–2-AG hydrolysis in homogenates prepared from (a,b) COS-7 cells transfected with mouse ABHD6 (5 μg of protein per reaction) or with mouse MAGL (0.25 μg of protein per reaction); (c) BV-2 cells (10 μg of protein per reaction); and (d) mouse neurons in primary culture (1 μg of protein per reaction). For the combination of the two inhibitors in d, WWL70 was used at 10 μM and JZL184 at 1 μM. Data are mean ± s.e.m. of 3 to 4 independent experiments. Each experiment was performed in triplicate with homogenates or cells from independent transfections and cell preparations. Transfection of COS-7 cells with ABHD6 increased [3H]–2-AG hydrolysis by about twofold compared to COS-7 cells transfected with empty vector, whereas transfection of COS-7 cells with MAGL increased [3H]–2-AG hydrolysis by 17-fold compared to COS-7 cells transfected with empty vector (data not shown).
WWL70 inhibited ∼20% of the [3H]-2-AG hydrolysis in homogenates prepared from adult mouse brain (data not shown), which is in line with a previous report showing that ABHD6 accounts for a small portion of the overall 2-AG hydrolysis measured in whole brain homogenates15. In homogenates prepared from neurons in primary culture, WWL70 inhibited ∼50% of the total [3H]-2-AG hydrolysis (Fig. 2d). These results reveal that whereas adult mouse brain shows a higher overall 2-AG hydrolysis activity than neurons in primary culture (13.2 versus 3.8 pmol of 2-AG per mg of protein per min), the relative contribution of ABHD6 is greater in neurons in primary culture than in adult mouse brain. In homogenates prepared from microglia in primary culture, WWL70 had no significant effect on [3H]-2-AG hydrolysis (Supplementary Fig. 3), consistent with the lower level of ABHD6 mRNA in these cells. Thus, we focused our next set of experiments on the functional significance of ABHD6 in neurons.
ABHD6 and MAGL control 2-AG accumulation in neurons
Previous results indicate that 2-AG hydrolysis in neurons is mediated by MAGL26, and we now show that ABHD6 is also likely to be involved. We investigated the relative contributions of MAGL and ABHD6 to 2-AG hydrolysis in two neuronal preparations: (i) Homogenates prepared from neurons in primary culture, and (ii) intact neurons in primary culture. We used the ABHD6 inhibitor WWL70 and the MAGL inhibitor JZL184. We confirmed the efficacy of JZL184 at inhibiting mouse MAGL activity, as it inhibited [3H]-2-AG hydrolysis in homogenates prepared from MAGL-transfected COS-7 cells (Fig. 2b). Note that these inhibitors can be used to discriminate the activities of these two enzymes, as 1 μM JZL184 did not significantly affect 2-AG hydrolysis in homogenates prepared from ABHD6-transfected COS-7 cells and 10 μM WWL70 did not significantly affect 2-AG hydrolysis in homogenates prepared from MAGL-transfected COS-7 cells (Fig. 2a,b). When tested in homogenates prepared from primary neurons, JZL184 inhibited ∼50% of the total 2-AG hydrolysis (Fig. 2d). Furthermore, the combination of the maximally effective concentrations of WWL70 and JZL184 blocked almost all of the 2-AG hydrolysis in these homogenates (Fig. 2d). These results show that ABHD6 and MAGL are each responsible for about half of the total 2-AG hydrolysis in primary neuron homogenates. The remaining 2-AG hydrolysis in neuron homogenates (∼10%) is probably mediated by FAAH, as its selective inhibitor CAY10402 blocks 10% of this activity (Fig. 2d).
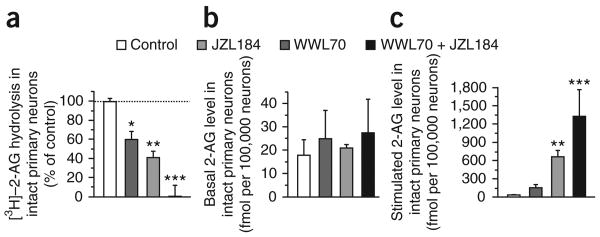
We then tested the relative contributions of ABHD6 and MAGL to 2-AG hydrolysis in intact neurons. Here we performed two sets of experiments. First, we incubated intact neurons in primary culture with [3H]-2-AG and measured the production of [3H]-glycerol. These cells efficiently hydrolyzed [3H]-2-AG and this activity was significantly reduced by treatment with either WWL70 or JZL184, with JZL184 being more efficacious (Fig. 3a). The combination of WWL70 and JZL184 (10 and 1 μM, respectively) blocked all of the 2-AG hydrolysis in these cells (Fig. 3a), which supports the idea that FAAH does not hydrolyze 2-AG in intact cells. In the second set of experiments, we pretreated neurons in primary culture with WWL70 and JZL184 (alone and in combination), and then quantified eCB levels under either basal or stimulated conditions by using gas chromatography-mass spectrometry (GC-MS). The stimulation we chose was a combination of glutamate and carbachol because this treatment has been shown to increase 2-AG production in these cells27. Neither WWL70 nor JZL184 affected basal 2-AG levels (Fig. 3b). Conversely, WWL70 induced a ∼2-fold potentiation of the stimulated response, and JZL184 induced a ∼7-fold potentiation of the stimulated response (Fig. 3c). Combining the two inhibitors resulted in a more significant potentiation of the stimulated response than JZL184 alone (Fig. 3c), recapitulating what was seen with our intact cell hydrolysis assay (Fig. 3a). Neither of these inhibitors had a significant effect on anandamide levels (Supplementary Fig. 4). These results show that both ABHD6 and MAGL regulate stimulus-dependent accumulation of 2-AG without affecting the basal level of this eCB in neurons in primary culture. Although it is clear that MAGL is much more efficient at controlling the stimulated accumulation of 2-AG in these cells, it seems that ABHD6 and MAGL might have non-overlapping functions. Specifically, WWL70 increased by ∼2-fold the stimulated accumulation of 2-AG in both the presence and absence of JZL184 (Supplementary Fig. 5). This suggests that ABHD6 and MAGL independently control the stimulated accumulation of 2-AG in intact neurons.
Figure 3.
Effect of WWL70 and JZL184 on [3H]–2-AG hydrolysis and 2-AG accumulation in intact neurons in primary culture. (a) [3H]–2-AG hydrolysis by intact primary neurons after 30 min pretreatment with WWL70 (10 μM) or JZL184 (1 μM). The data are expressed as % of control hydrolysis (pretreatment with 0.1% DMSO). (b,c) Levels of 2-AG in intact primary neurons pretreated for 30 min with WWL70 (10 μM), JZL184 (1 μM) or vehicle (0.1% DMSO, control; b) and stimulated with glutamate (100 μM) and carbachol (1 mM) for 2.5 min, after which lipids were extracted and 2-AG amounts measured by GC-MS (c). Treatment with glutamate plus carbachol led to a 2.5-fold increase in 2-AG amounts (in fmol per 100,000 cells: 18 to 44). Data are shown as mean ± s.e.m. of 3–6 independent experiments. Experiments were performed in triplicate for the hydrolysis assay and in duplicate for 2-AG quantification by GC-MS, using cells from independent cell culture preparations. *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA one-way, Bonferroni's post test).
ABHD6 regulates eCB-LTD in adult mouse prefrontal cortex
Both CB1 receptors and MAGL are expressed presynaptically in neurons28. To visualize ABHD6 expression, we generated a rabbit polyclonal antibody that recognizes a 38-amino-acid sequence in the middle of the mouse ABHD6 protein (Supplementary Fig. 6). This antibody recognizes ABHD6, as it labels ABHD6-transfected COS-7 cells (using GFP as a transfection marker; Fig. 4a–c and Supplementary Fig. 7), whereas it does not label ABHD6-transfected COS-7 cells when the antibody is pre-absorbed with the immunizing peptide (Fig. 4a–c) or when the antibody is tested on pcDNA-transfected COS-7 cells (Supplementary Fig. 7). Using this antibody, we confirmed ABHD6 expression in BV-2 cells (Fig. 4d) and a lack of detectable ABHD6 expression in primary microglia (data not shown). In neurons in primary culture, this antibody showed that ABHD6 is mainly expressed in the cell soma and dendrites, where it co-localizes with MAP2 and not with axonal CB1 receptors (Fig. 4e–h). In adult mouse brain, ABHD6 is abundantly expressed in cortical areas (Supplementary Fig. 8). More specifically, in the prefrontal cortex, ABHD6 often juxtaposes, but does not overlap, with presynaptic CB1 receptors (Fig. 5a–f). Accordingly, using electron microscopy, we found that most of the ABHD6 immunoreactivity was detected in postsynaptic structures (Fig. 5g,h). Using various cellular markers, we confirmed that ABHD6 is expressed by many principal glutamatergic neurons, as well as by some GABAergic interneurons and astrocytes, but not by microglia (Supplementary Fig. 9). Together, these results show that neurons express ABHD6, and that this enzyme is prevalent in the postsynaptic spines of glutamatergic neurons. This finding raises the question of whether ABHD6 participates in the control of 2-AG's efficacy at neuronal CB1 receptors in the adult brain.
Figure 4.
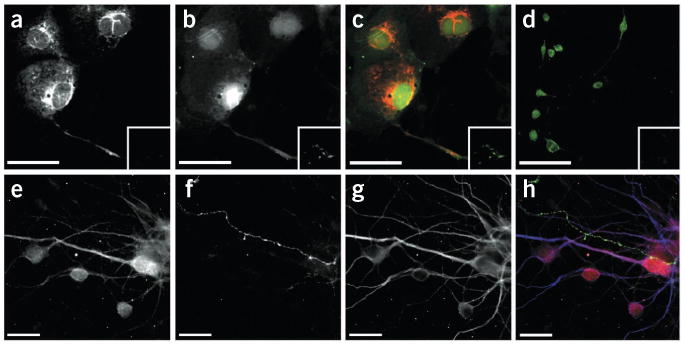
Visualization of ABHD6 protein in different cell types. (a–h) Representative images of COS-7 cells transfected with plasmids encoding ABHD6 and GFP (a–c; scale bar, 50 μm), BV-2 cells (d; scale bar, 100 μm) and mouse neurons in primary culture (e–h; scale bar, 20 μm). Fluorescence detection of primary antibodies recognizing ABHD6 (a, d, e; red in c, h), CB1 receptor (f, green in h), or MAP2 (g, blue in h). Fluorescence detection of GFP (b, green in c). Insets show pre-incubation with 5 μg per ml of the ABHD6 immunizing peptide (a–d).
Figure 5.
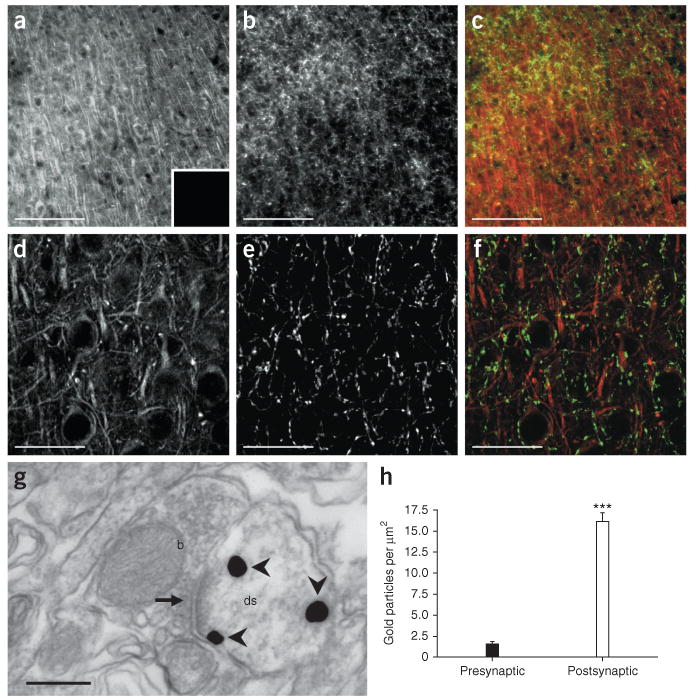
Localization of ABHD6 protein in mouse prefrontal cortex. (a–f) Fluorescent immunohistochemical staining of adult mouse prefrontal cortex (scale bars: a–c, 100 μm; d–f, 20 μm) using antibodies that recognize ABHD6 (a,d; red in c,f) and CB1 receptors (b,e; green in c,f). Inset in a shows pre-incubation with 5 μg per ml of the ABHD6 immunizing peptide. (g) Electron micrograph of antibodies recognizing ABHD6 (arrowheads) in adult mouse prefrontal cortex, labeled with silver-enhanced gold particles (scale bar, 200 nm). Note that ABHD6 is predominantly found on the postsynaptic side of the synapse (arrow) in dendritic spines (ds), and not in presynaptic boutons (b). (h) Quantitative analysis of all electron microscopy images using ImageJ software. The number of gold particles on pre- and postsynaptic sides of identifiable synapses were manually counted and then normalized to the area of the respective structures for comparison. Data are mean ± s.e.m. of 154 identifiable synapses, in two animals. ***P < 0.001 (unpaired two-tailed t-test).
By activating presynaptic CB1 receptors, 2-AG induces several forms of synaptic plasticity29–31. To test whether ABHD6 controls 2-AG's ability to induce synaptic plasticity, we tested long-term depression (LTD) in prefrontal cortical slices prepared from adult mouse. Sub-threshold stimulation of pyramidal neurons in layer 2 of this brain area induces eCB-dependent LTD (eCB-LTD) in layer 5 when a non-specific inhibitor of 2-AG hydrolysis, URB602, is added32. Importantly, the FAAH inhibitor URB597 has no effect on this response32. Thus, we sought to determine which 2-AG hydrolase(s), MAGL or ABHD6, is responsible for regulating this cortical eCB-LTD. First, we confirmed that sub-threshold stimulation (5 min at 10 Hz) in layer 2 does not induce LTD in layer 5 (Fig. 6a). Using this sub-threshold stimulation protocol, we found that application of WWL70 allowed the induction of robust LTD (a 22% reduction in synaptic transmission for at least 40 min after the stimulation). This response was mediated by CB1 receptors, as it was fully blocked by the CB1 antagonist AM251 (Fig. 6b). The MAGL inhibitor JZL184 also facilitated LTD induction at these synapses (14%), and this response was also CB1-dependent (Fig. 6b). Surprisingly, the combination of WWL70 and JZL184 in this paradigm did not induce significant LTD, which might reflect an increase in the baseline level (before electrical stimulation) of 2-AG in these brain slices after pre-treatment with both inhibitors. Together, these results indicate that, like MAGL, ABHD6 is a rate-limiting enzyme for 2-AG inactivation in adult mouse brain, and that its postsynaptic expression allows it to regulate the efficacy of 2-AG at presynaptic CB1 receptors.
Figure 6.
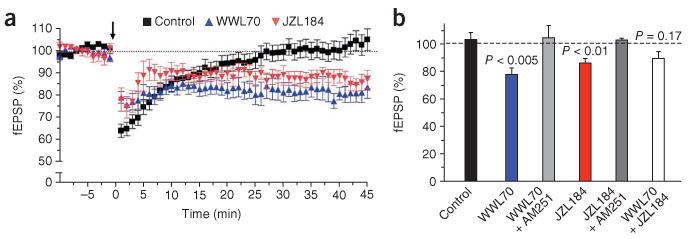
Effect of WWL70 and JZL184 on CB1-dependent LTD in mouse prefrontal cortex. Field potentials measured in layer 5 after stimulation in layer 2 (arrow, 5 min at 10 Hz). Both the area and amplitude of the field excitatory postsynaptic potential (fEPSP) were measured (graphs depict area). Slices were preincubated for 10 min with WWL70 (10 μM), JZL184 (1 μM) or vehicle (0.01% DMSO, control), and the inhibitors remained present in the superfusion medium throughout the experiment. (a) Time course and (b) summary of the 40-min time point. Data are mean ± s.e.m. of at least ten different brain slices. Statistical significance (above bars) was calculated relative to control (Mann Whitney).
To further characterize the roles of ABHD6 and MAGL in regulating synaptic plasticity in cortex, we used two relevant short-term synaptic plasticity paradigms, namely depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Using standardized induction protocols, we found that neither DSI nor DSE could be induced in cortical layer 5 (Supplementary Fig. 10). In contrast, we could induce DSI in cortical layer 2; however, the decay rate of this effect was not altered by treatment with either WWL70 or JZL184 (Supplementary Fig. 10), suggesting that ABHD6 and MAGL are not involved in the regulation of this form of short-term synaptic plasticity in cortical layer 2.
Discussion
BV-2 cells do not express MAGL and yet they efficiently hydrolyze 2-AG, providing evidence for the existence of another enzyme that can hydrolyze this eCB18. The serine hydrolases ABHD6 and ABHD12, which were identified in adult mouse brain, can hydrolyze 2-AG when they are heterologously expressed in COS-7 cells and their activity is assayed in cell homogenates15. We now report that ABHD6 is responsible for the previously uncharacterized endogenous 2-AG-hydrolyzing activity in BV-2 cells, and that this enzyme controls the accumulation and efficacy of 2-AG at cannabinoid receptors in intact BV-2 cells, neurons, and adult mouse brain slices, but not in primary microglia.
Although BV-2 cells are widely used as a surrogate model for studying the immune function of microglia, many laboratories have reported that their phenotype is quite different from that of microglia in primary culture and adult brain. Adding to this list of differences, we found that BV-2 cells express functional ABHD6, whereas microglia, whether they are in primary culture or in adult mouse brain, do not. It is worth noting that the phenotype of microglia is extremely plastic, and thus it is possible that specific pathogens or immunomodulators might induce ABHD6 expression in these cells. In line with this notion, Epstein-Barr virus antigens induce ABHD6 expression in B cells33. A question that remains unanswered at this point is: What is the enzyme responsible for the 2-AG-hydrolyzing activity measured in homogenates prepared from primary microglia, as this activity is unaffected by the ABHD6 inhibitor WWL70 and the MAGL inhibitor JZL184 (Supplementary Fig. 3)?
In neurons in primary culture, 2-AG hydrolysis is mediated by both MAGL and ABHD6; however, when assessing their relative contributions to this process, we found slight differences depending on the assay that was used and the conditions that were tested. Specifically, when measuring 2-AG hydrolysis in neuron homogenates, we found that ABHD6 and MAGL contributed about equally (Fig. 2d). Here, in a homogenous environment, the relative contributions of ABHD6 and MAGL to the hydrolysis of 2-AG are dictated by both their intrinsic enzymatic activity and their expression. Because ABHD6 has a lower intrinsic 2-AG hydrolysis activity than MAGL15, our results suggest that ABHD6 is expressed at a higher level than MAGL in primary neurons. When determining the relative contributions of ABHD6 and MAGL to 2-AG hydrolysis in intact neurons in primary culture, we found that the less abundant enzyme, MAGL, has a greater role in controlling 2-AG levels than the more abundant enzyme, ABHD6 (Fig. 3). This predominant role for MAGL over ABHD6 in intact neurons in primary culture was found in two different experimental conditions: when [3H]-2-AG was provided from the extracellular space, and when endogenous 2-AG production was stimulated by receptors expressed at the plasma membrane (glutamatergic and cholinergic receptors). These results indicate that MAGL and ABHD6 are found in distinct sub-cellular locations, and that MAGL is strategically positioned within intact neurons in primary culture to hydrolyze 2-AG when this lipid reaches the cells from the extracellular space, as well as when it is endogenously produced.
Three independent sets of data further support the notion that MAGL and ABHD6 have distinct sub-cellular locations in neurons, resulting in the independent control of 2-AG accumulation. First, dual inhibition of MAGL and ABHD6 activities in intact neurons in primary culture had an additive effect on both the hydrolysis of exogenously applied 2-AG and the stimulated production of endogenous 2-AG (Fig. 3). Second, an analysis of their amino acid sequences indicates that ABHD6 is likely to be an integral membrane enzyme, whereas MAGL is a cytosolic enzyme that peripherally associates with membranes15,34. Western blot analysis of sub-cellular fractions from mouse brain supports this prediction, as ABHD6 immunoreactivity was found only in the membrane fraction, whereas MAGL immunoreactivity was distributed between both membrane and cytosolic fractions (Supplementary Fig. 11). Third, our immunofluorescence and electron microscopy images revealed that ABHD6 is expressed in postsynaptic dendrites in the adult brain, whereas there is evidence that MAGL is localized to presynaptic axon terminals28. Thus, the relative contributions of MAGL and ABHD6 to the control of 2-AG levels in intact neurons in primary culture and adult brain are probably dictated by their distinct sub-cellular localizations.
Inhibition of either ABHD6 or MAGL had a similar effect on synaptic plasticity at cortical layer 5 excitatory synapses (that is, inhibition of either enzyme allowed the induction of CB1-dependent LTD by an otherwise sub-threshold stimulation; Fig. 6). This suggests that the amount of 2-AG that reaches presynaptic CB1 receptors is controlled both at the site of 2-AG production (postsynaptic) by ABHD6 and at the site of the CB1 receptor targets (presynaptic) by MAGL. This sort of redundancy is likely to offer the system greater versatility to finetune the magnitude and duration of 2-AG signaling. Furthermore, 2-AG produced by other cell types found in the nervous system, such as glia and invading immune cells, is also likely to be regulated at the source of production by these cells' own 2-AG–hydrolyzing enzyme(s)35,36. Thus, whereas ABHD6 is strategically positioned to regulate neuronal—and perhaps also astroglial—production of 2-AG, MAGL is well positioned to control the amount of 2-AG that reaches presynaptic CB1 receptors regardless of the source of the 2-AG. We were surprised to find that the co-inhibition of both MAGL and ABHD6 did not allow subthreshold stimulation to produce significant LTD of cortical layer 5 glutamatergic synapses (Fig. 6b). Although it is counterintuitive, this result could reflect a saturation effect in which basal 2-AG levels in the brain slice are elevated by the inhibitors before the electrical stimulation, thus inducing LTD prematurely and reducing the measured difference between synaptic efficacy before and after the electrical stimulation. Accordingly, basal neuronal activity is much higher in brain slices than in dissociated neurons in primary culture, and thus inhibition of 2-AG hydrolysis would be expected to have a greater effect on basal 2-AG levels in brain slices (Fig. 6) than in neurons in primary culture (Fig. 3). Together, our results suggest that the relative contributions of MAGL, ABHD6 and any other 2-AG-hydrolyzing enzymes to the regulation of 2-AG signaling will vary depending on the cellular origin of this eCB and the type of stimulation that increases its production. This arrangement could offer an additional modality in the control of 2-AG signaling in various pathophysiological conditions.
The identification and characterization of enzymes that hydrolyze eCBs is necessary to obtain a mechanistic understanding of this signaling system and to exploit these pathways for therapeutic gain. The criteria that a hydrolytic enzyme needs to fulfill to be considered a bona fide member of the eCB signaling system include showing that it hydrolyzes eCBs in intact cells and tissue, and that this enzymatic activity controls the efficacy of eCB signaling at cannabinoid receptors. Thus, the results provided by our study suggest that ABHD6 should be added to the list of members of the eCB signaling system.
Methods
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (DA14486 and DA26430 to N.S., DA017259, DA009789 and DA025285 to B.F.C. and DA026161 to J.L.B.) and from the National Institute of General Medical Sciences (PHS NRSA 2T32 GM007270 to W.R.M.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Author Contributions: W.R.M. prepared the cell cultures, performed the hydrolysis experiments, conducted the data analysis and wrote the manuscript. J.L.B. performed the ABPP experiments and contributed to the data analysis. E.A.H. performed the GC-MS and immunofluoresence experiments and contributed to the electron microscopy experiments and data analysis. A.T. and M.L. performed the electrophysiology experiments. Y.H.L. prepared the cell culture transfections and the shRNA constructs, and performed the qPCR experiments. J.C. contributed to the immunofluorescence experiments. A.L.B. performed the electron microscopy experiments. G.G.M. contributed to the hydrolysis experiments. S.S.-J.H. contributed to antibody production. G.W. and S.F. performed the cell migration experiments. J.P.A. contributed to the ABPP experiments. J.Z.L. and W.L. produced the hydrolase inhibitors. C.X. contributed to the cell culture experiments. T.M. provided the transgenic mice. K.M. provided antibodies. O.J.M. supervised the electrophysiology experiments. B.F.C. supervised the ABPP experiments and the development of hydrolase inhibitors. N.S. supervised the project and wrote the manuscript.
Competing Financial Interests: The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprintsandpermissions/.
References
- 1.Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56(Suppl. 1):244–253. doi: 10.1016/j.neuropharm.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horne E, Stella N. The ins and outs of endocannabinoid signaling in healthy and diseased brain. Future Lipidol. 2008;3:435–452. [Google Scholar]
- 3.Marrs W, Stella N. Measuring endocannabinoid hydrolysis: refning our tools and understanding. AAPS J. 2009;11:307–311. doi: 10.1208/s12248-009-9109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 5.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 6.Straiker A, Mackie K. Cannabinoids, electrophysiology, and retrograde messengers: challenges for the next 5 years. AAPS J. 2006;8:E272–E276. doi: 10.1007/BF02854897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabral GA, Griffn-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinfammation. Expert Rev Mol Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobbi G, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fegley D, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 10.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 11.Long JZ, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burston JJ, et al. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–553. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisogno T, et al. Development of a potent inhibitor of 2-arachidonoylglycerol hydrolysis with antinociceptive activity in vivo. Biochim Biophys Acta. 2009;1791:53–60. doi: 10.1016/j.bbalip.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Long JZ, et al. Dual blockade of FAAH and MAGL identifes behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blankman JL, Simon GM, Cravatt BF. A comprehensive profle of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saario SM, et al. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- 18.Muccioli GG, et al. Identifcation of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J Neurosci. 2007;27:2883–2889. doi: 10.1523/JNEUROSCI.4830-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessani N, et al. A streamlined platform for high-content functional proteomics of primary human specimens. Nat Methods. 2005;2:691–697. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profling: the serine hydrolases. Proc Natl Acad Sci USA. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yates JR, III, McCormack AL, Eng J. Mining genomes with MS. Anal Chem. 1996;68:534A–540A. doi: 10.1021/ac962050l. [DOI] [PubMed] [Google Scholar]
- 22.Goparaju SK, Natsuo U, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 23.Walter L, et al. Non-psychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller AM, Stella N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantifcation of migration by a novel near-infrared method. Glia. 2008;57:875–883. doi: 10.1002/glia.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Blankman JL, Cravatt BF. A functional proteomic strategy to discover inhibitors for uncharacterized hydrolases. J Am Chem Soc. 2007;129:9594–9595. doi: 10.1021/ja073650c. [DOI] [PubMed] [Google Scholar]
- 26.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- 28.Gulyas AI, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 30.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 31.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 32.Lafourcade M, et al. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maier S, et al. Cellular target genes of Epstein-Barr virus nuclear antigen 2. J Virol. 2006;80:9761–9771. doi: 10.1128/JVI.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrs W, Stella N. 2-AG + 2 new players = forecast for therapeutic advances. Chem Biol. 2007;14:1309–1311. doi: 10.1016/j.chembiol.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Walter L, Dinh T, Stella N. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J Neurosci. 2004;24:8068–8074. doi: 10.1523/JNEUROSCI.2419-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witting A, Walter L, Wacker J, Moller T, Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci USA. 2004;101:3214–3219. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.