Abstract
Insulin secretory responses to paired intravenous and oral glucose loads were determined in 38 nonobese individuals classified as normal (nondiabetic) subjects, “mild” diabetics (fasting blood glucose below 105 mg per 100 ml), or “moderate” diabetics (fasting glucose below 192 mg per 100 ml). Studies were also performed in 29 obese persons who were similarly grouped. The intravenous load was given to assess the alacrity of hormonal release after glycemic stimulus, and the oral glucose to determine how the speed of initial insulinogenesis modifies the disposition of ingested carbohydrate.
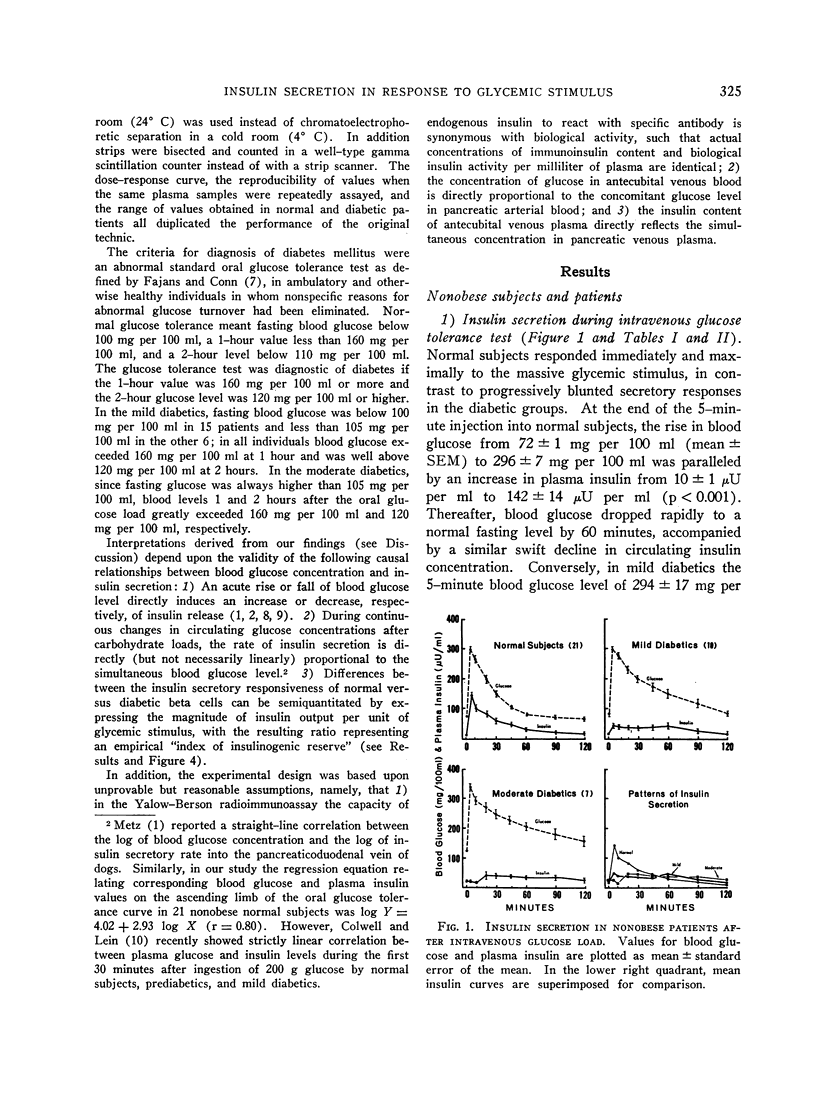
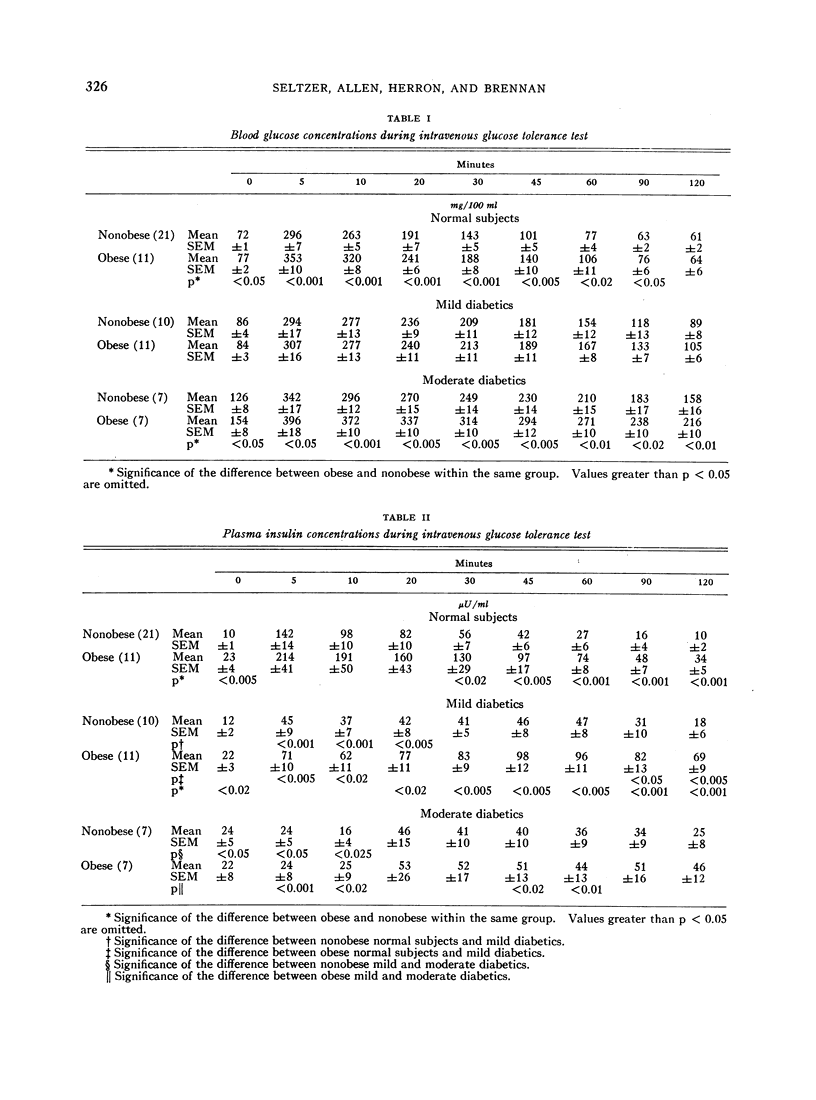
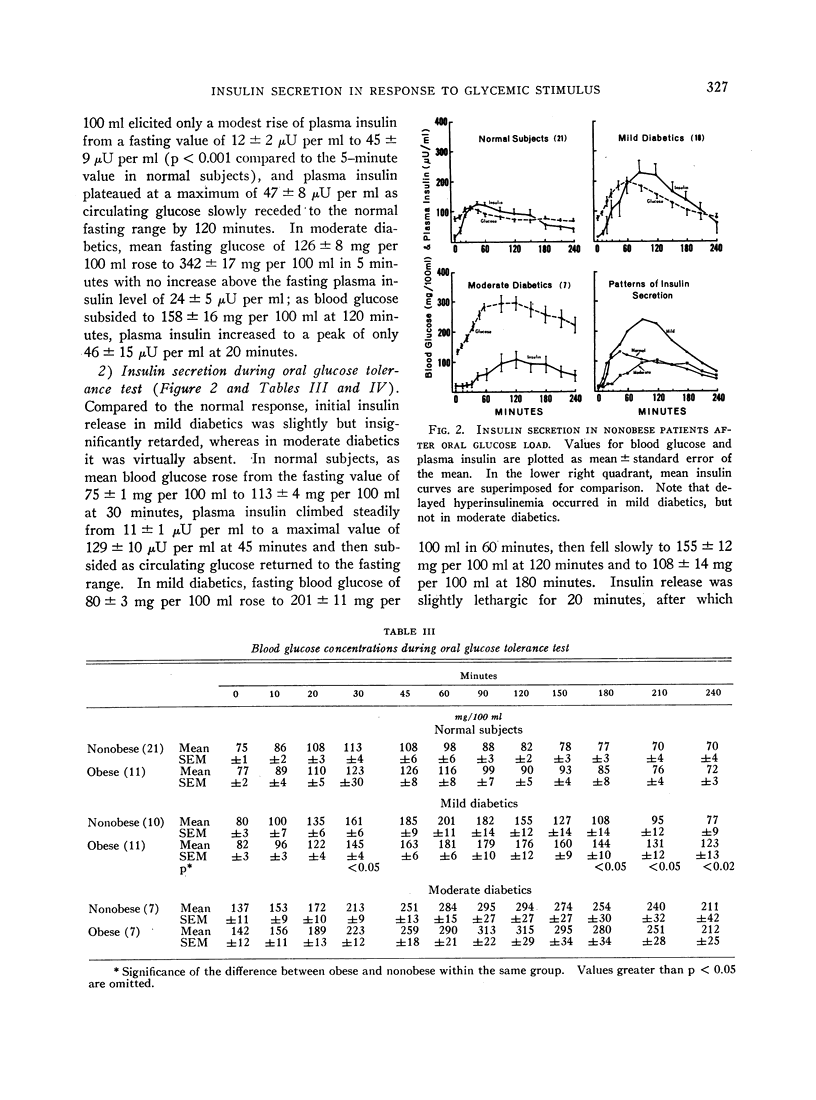
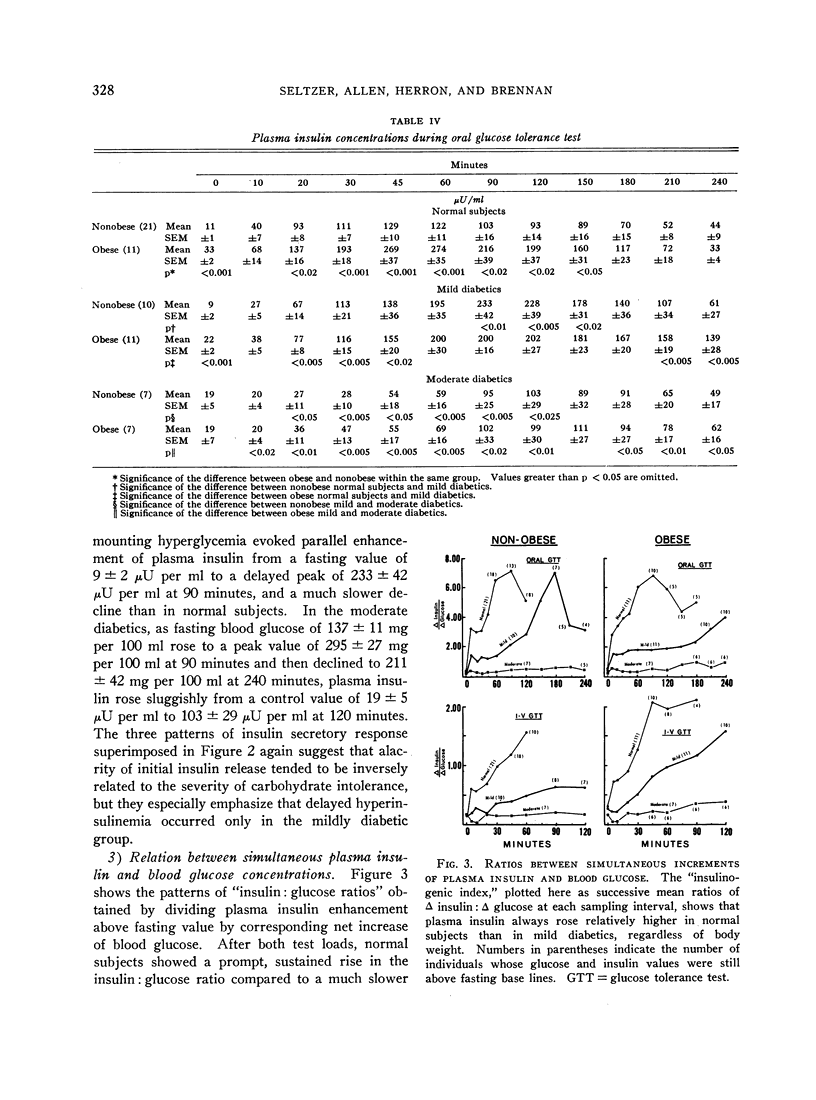
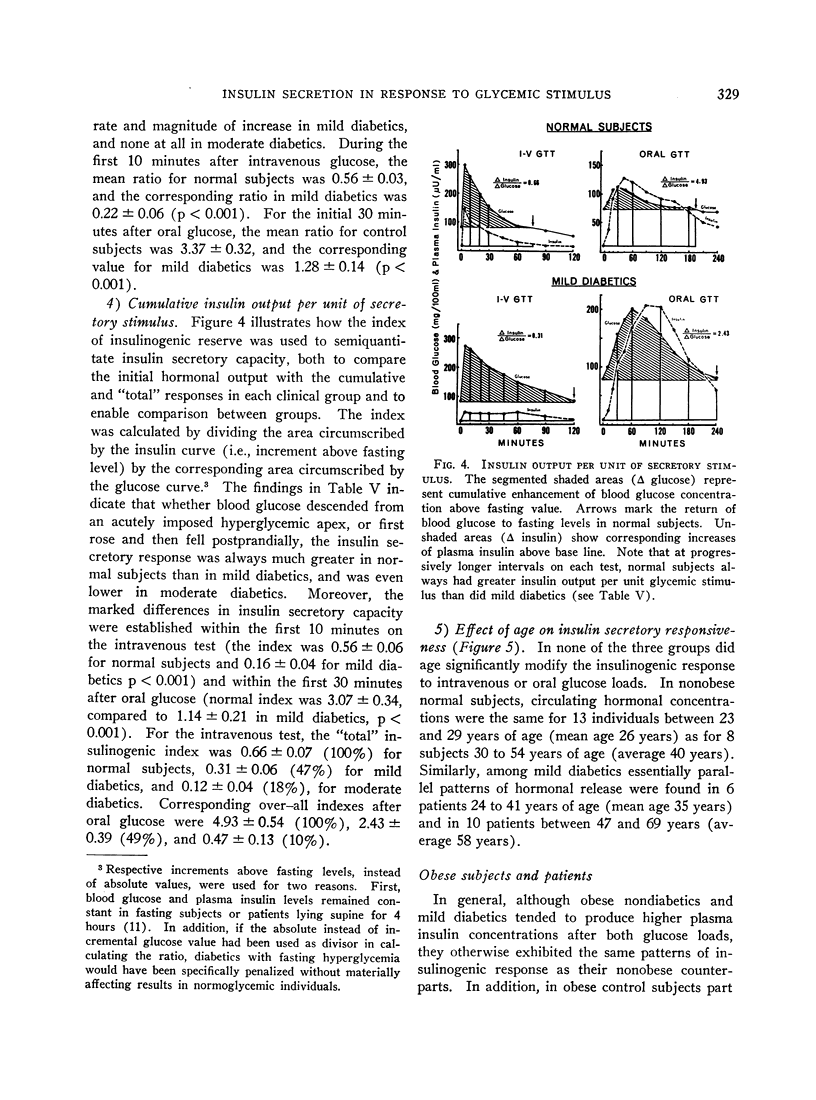
In the nonobese group, normal subjects responded to massive hyperglycemia after rapid injection of glucose with immediate and maximal outpouring of insulin, in contrast to a desultory insulinogenic response in patients with mild diabetes, and no initial response at all in moderate diabetics. During oral glucose tolerance tests, the much faster clearance of blood sugar in nondiabetic subjects was actually associated with lower absolute insulin output than was found in mildly diabetic patients, since the latter exhibited delayed hyperinsulinemia in concert with prolonged hyperglycemia. Moderate diabetics never showed excessive insulin release despite even greater hyperglycemia. An empirical “insulinogenic index,” the ratio relating enhancement of circulating insulin to magnitude of corresponding glycemic stimulus, was used to compare the secretory capacities of respective groups. Despite the higher absolute hormonal output after oral glucose in mild diabetics, the index revealed that insulin release in normal subjects was proportionally more than twice as great. This relatively greater normal secretory response declared itself shortly after the administration of glucose by either route, and was maintained throughout both tests.
In the 29 obese individuals, differences among groups were essentially the same as in persons of normal weight. Obese nondiabetics did show much larger absolute insulinogenic responses during both tests than did nonobese controls. Since corresponding glucose tolerance curves were also higher, the mean insulinogenic indexes for obese subjects were not statistically greater. Moreover, when comparable glucose curves of obese and nonobese controls
Full text
PDF

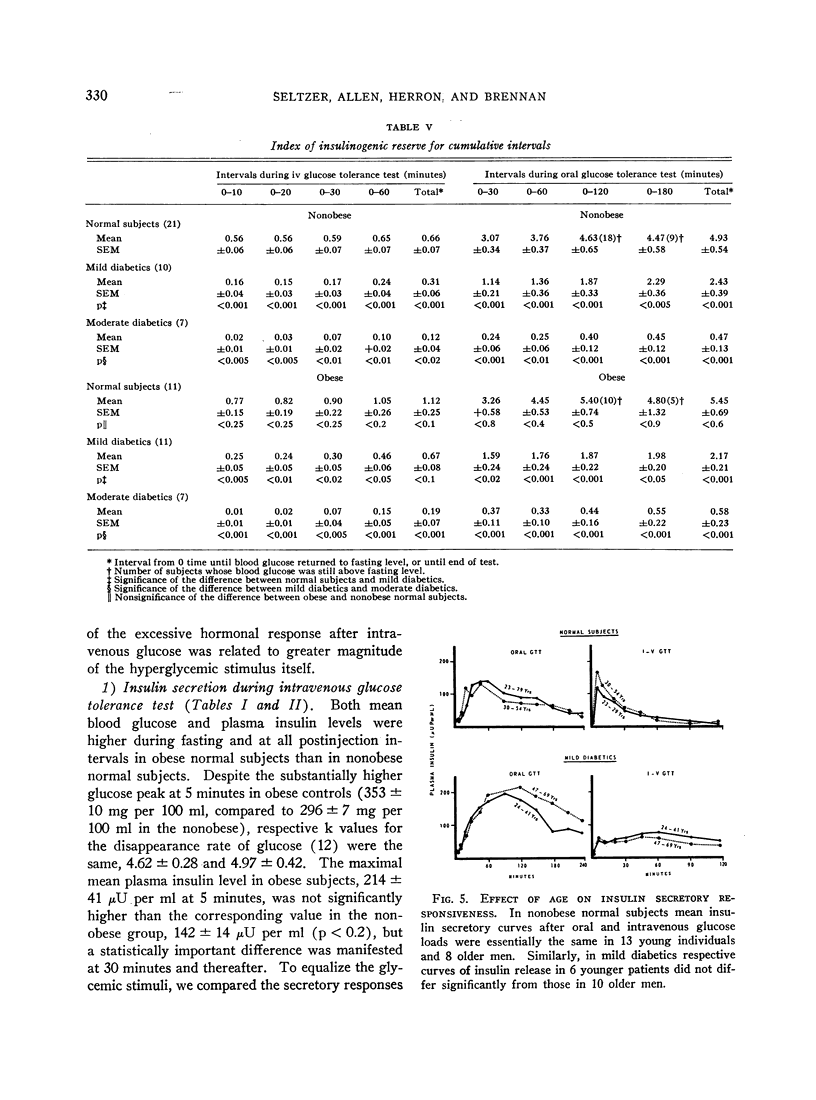




Selected References
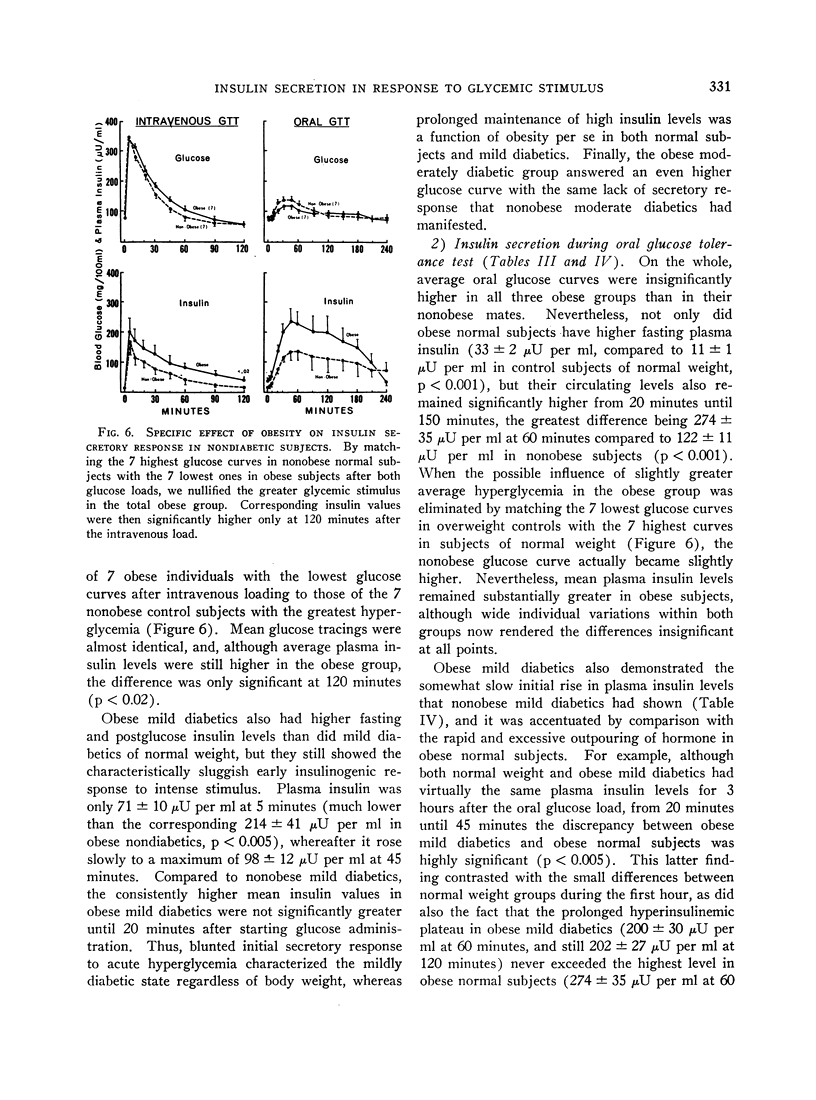
These references are in PubMed. This may not be the complete list of references from this article.
- AMATUZIO D. S., STUTZMAN F. L., VANDERBILT M. J., NESBITT S. Interpretation of the rapid intravenous glucose tolerance test in normal individuals and in mild diabetes mellitus. J Clin Invest. 1953 May;32(5):428–435. doi: 10.1172/JCI102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONN J. W., FAJANS S. S., SELTZER H. S. Spontaneous hypoglycemia as an early manifestation of diabetes mellitus. Diabetes. 1956 Nov-Dec;5(6):437–442. doi: 10.2337/diab.5.6.437. [DOI] [PubMed] [Google Scholar]
- Conn J. W. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med. 1965 Nov 18;273(21):1135–1143. doi: 10.1056/NEJM196511182732106. [DOI] [PubMed] [Google Scholar]
- DUPRE J. AN INTESTINAL HORMONE AFFECTING GLUCOSE DISPOSAL IN MAN. Lancet. 1964 Sep 26;2(7361):672–673. doi: 10.1016/s0140-6736(64)92481-x. [DOI] [PubMed] [Google Scholar]
- ELRICK H., STIMMLER L., HLAD C. J., Jr, ARAI Y. PLASMA INSULIN RESPONSE TO ORAL AND INTRAVENOUS GLUCOSE ADMINISTRATION. J Clin Endocrinol Metab. 1964 Oct;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- Karam J. H., Grasso S. G., Wegienka L. C., Grodsky G. M., Forsham P. H. Effect of selected hexoses, of epinephrine and of glucagon on insulin secretion in man. Diabetes. 1966 Aug;15(8):571–578. doi: 10.2337/diab.15.8.571. [DOI] [PubMed] [Google Scholar]
- McIntyre N., Holdsworth C. D., Turner D. S. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab. 1965 Oct;25(10):1317–1324. doi: 10.1210/jcem-25-10-1317. [DOI] [PubMed] [Google Scholar]
- Perley M., Kipnis D. M. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966 Dec;15(12):867–874. doi: 10.2337/diab.15.12.867. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMOLS E., MARRI G., MARKS V. PROMOTION OF INSULIN SECRETION BY GLUCAGON. Lancet. 1965 Aug 28;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- SELTZER H. S., ALLEN E. W., BRENNAN M. T. FAILURE OF PROLONGED SULFONYLUREA ADMINISTRATION TO ENHANCE INSULOGENIC RESPONSE TO GLYCEMIC STIMULUS. Diabetes. 1965 Jul;14:392–395. doi: 10.2337/diab.14.7.392. [DOI] [PubMed] [Google Scholar]
- SELTZER H. S., HARRIS V. L. EXHAUSTION OF INSULOGENIC RESERVE IN MATURITY-ONSET DIABETIC PATIENTS DURING PROLONGED AND CONTINUOUS HYPERGLYCEMIC STRESS. Diabetes. 1964 Jan-Feb;13:6–13. doi: 10.2337/diab.13.1.6. [DOI] [PubMed] [Google Scholar]
- SELTZER H. S. Quantitative effects of glucose, sulfonylureas, salicylate, and indole-3-acetic acid on the secretion of insulin activity into pancreatic venous blood. J Clin Invest. 1962 Feb;41:289–300. doi: 10.1172/JCI104482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELTZER H. S., SMITH W. L. Plasma insulin activity after glucose: an index of insulogenic reserve in normal and diabetic man. Diabetes. 1959 Nov-Dec;8:417–424. doi: 10.2337/diab.8.6.417. [DOI] [PubMed] [Google Scholar]
- Schalch D. S., Kipnis D. M. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest. 1965 Dec;44(12):2010–2020. doi: 10.1172/JCI105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman K. E., Vaughan G. D., Timmer R. F. An in vitro method for studying insulin secretion in the perfused isolated rat pancreas. Metabolism. 1966 May;15(5):466–476. doi: 10.1016/0026-0495(66)90089-8. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Eisentraut A., Dupré J. Effect of secretin on insulin secretion. Lancet. 1966 Jul 2;2(7453):24–26. doi: 10.1016/s0140-6736(66)91749-1. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Glick S. M., Roth J., Berson S. A. Plasma insulin and growth hormone levels in obesity and diabetes. Ann N Y Acad Sci. 1965 Oct 8;131(1):357–373. doi: 10.1111/j.1749-6632.1965.tb34803.x. [DOI] [PubMed] [Google Scholar]



