Abstract
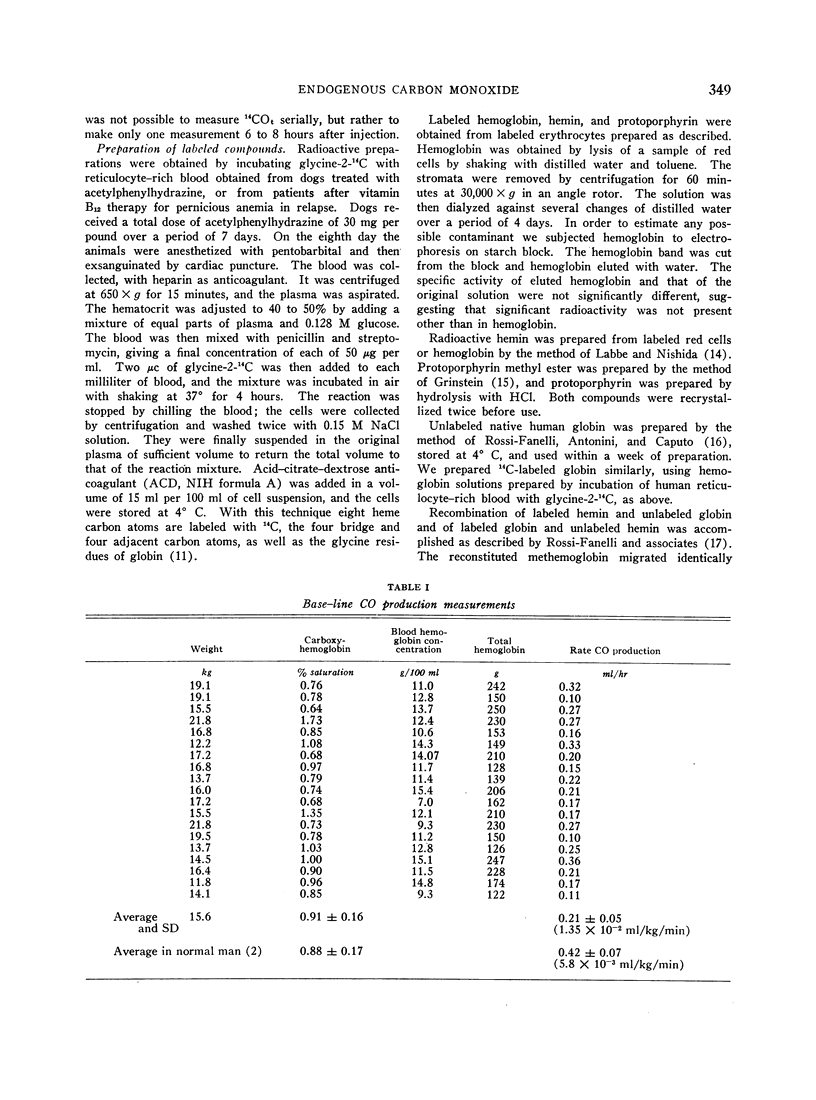
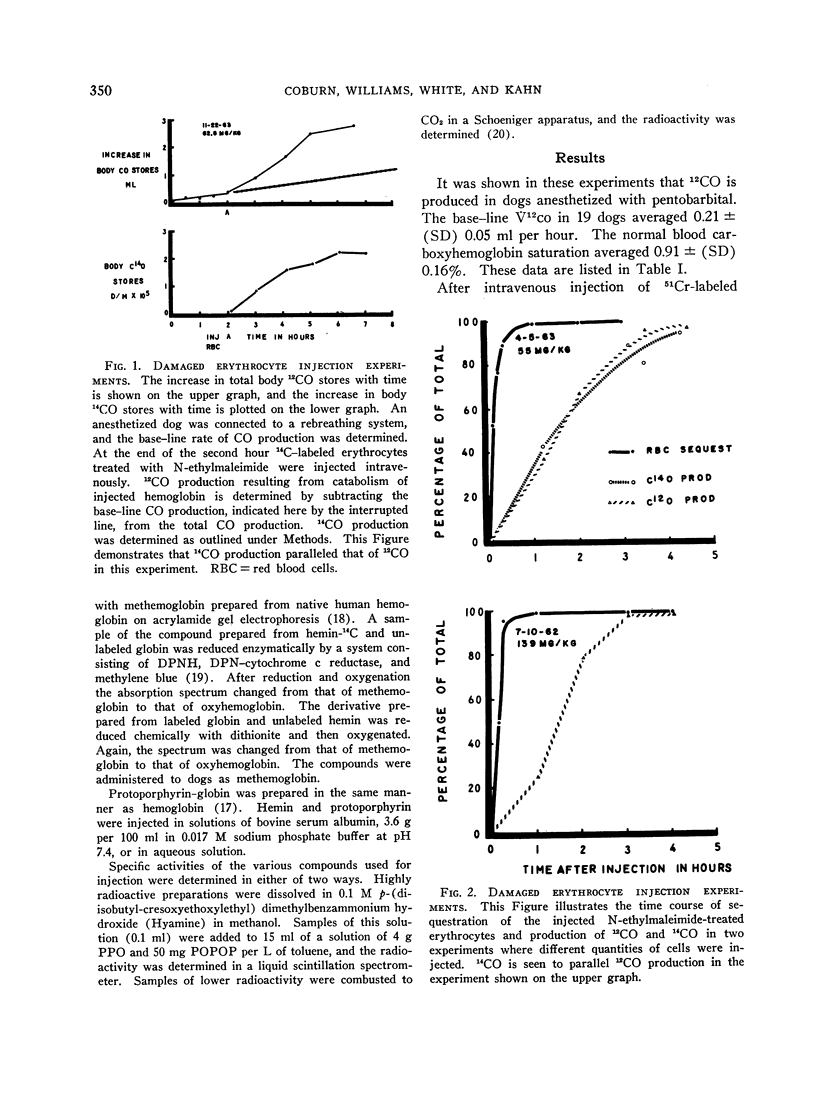
Dogs anesthetized with pentobarbital were shown to produce carbon monoxide at an average rate of 0.21 ± (SD) 0.05 ml per hour. After intravenous injection of erythrocytes damaged by incubation with N-ethylmaleimide, CO was produced in excess of base-line production for 3 to 4 hours with an average yield of 0.89 ± (SE) 0.046 μmole of carbon monoxide to 1 μmole of heme degraded.
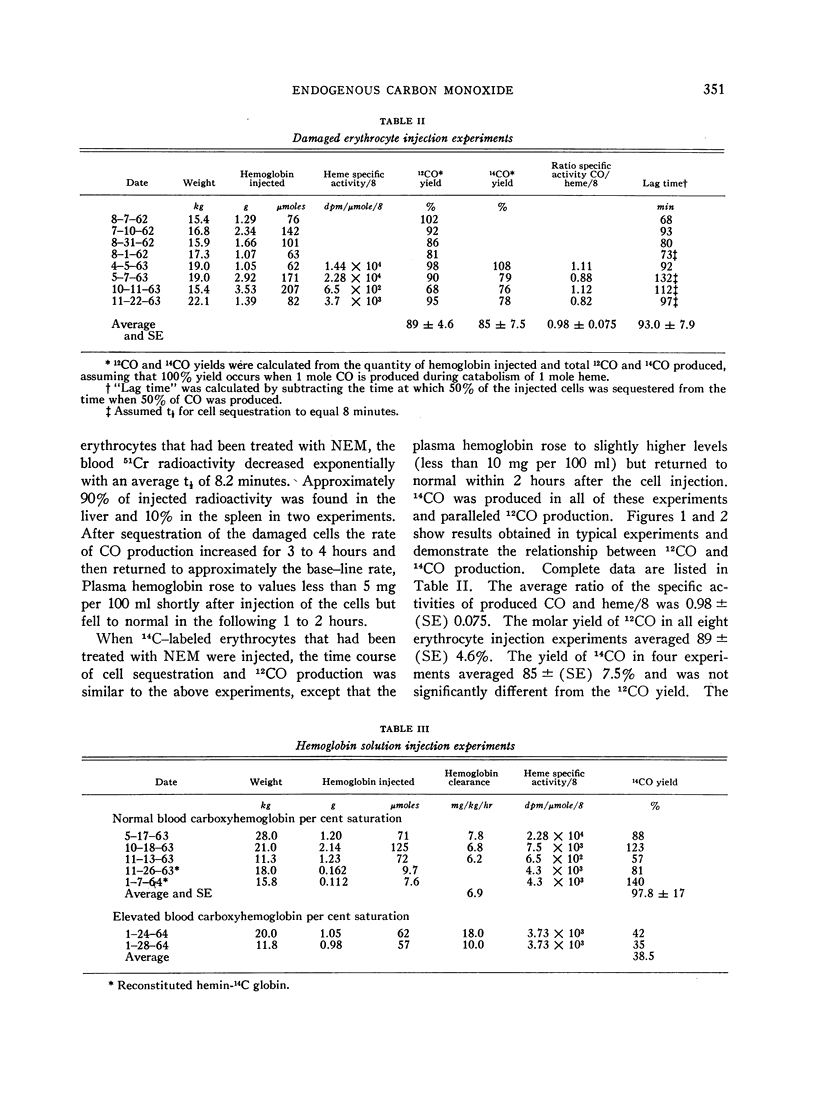
After intravenous injection of N-ethylmaleimide (NEM)-treated erythrocytes containing hemoglobin labeled with 14carbon, 14CO was produced. Its specific activity was approximately one-eighth that of the injected heme. It was also produced after intravenous injection of solutions of hemoglobin-14C and of reconstituted methemoglobin containing hemin-14C, but not after injections of methemoglobin containing globin-14C. The average yields of 14CO from metabolized heme in the experiments with damaged erythrocytes and hemoglobin solutions were 89 ± (SE) 4.6 and 97 ± (SE) 17.0%, respectively. These results demonstrate that the CO produced during hemoglobin degradation arises from the heme moiety.
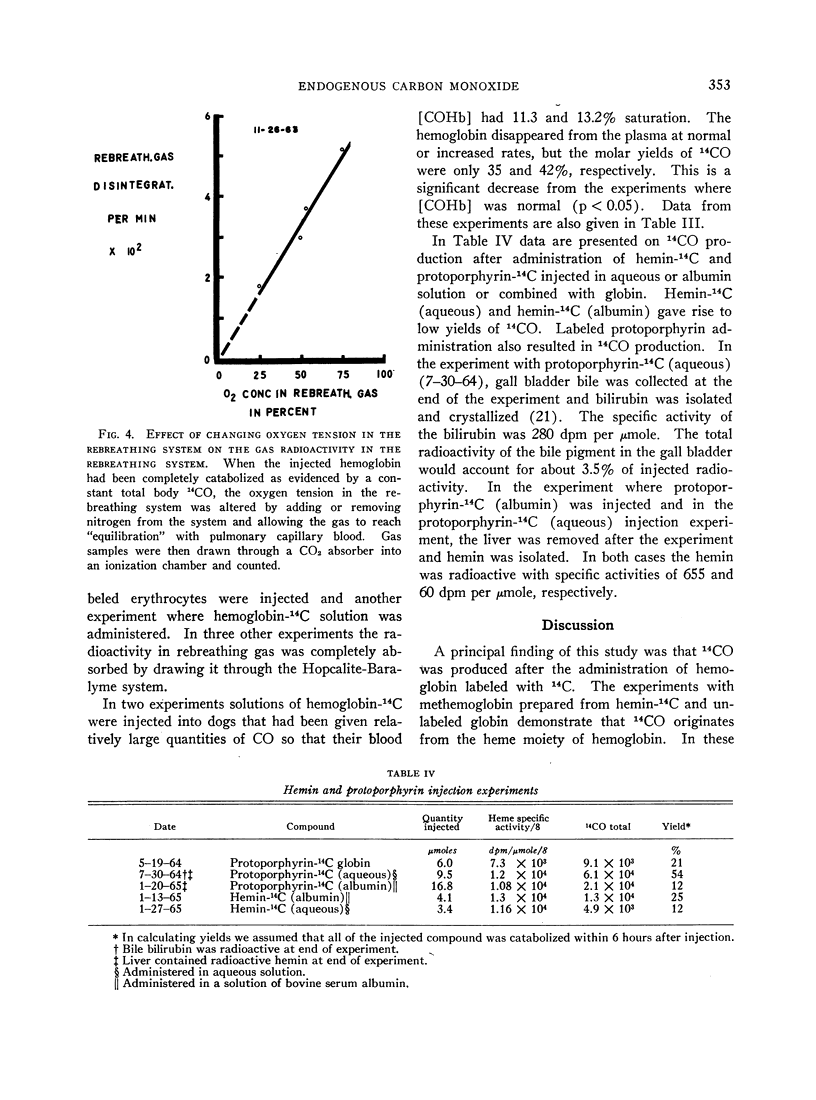
The yield of 14CO after injection of hemoglobin-14C solutions decreased significantly to values of 35 and 42% in two experiments when exogenous CO was added to the body stores, resulting in blood carboxyhemoglobin levels of 11.3 and 13.2% saturation. This finding suggests that oxidative metabolism is required during catabolism of hemoglobin to CO and that carboxy-hemoglobin levels in this range are sufficient to cause inhibition.
After intravenous injection of either hemin-14C or protoporphyrin-14C, 14CO was also produced. After injection of protoporphyrin-14C labeled bilirubin was isolated from gall bladder bile, and labeled hemin was isolated from the liver. It is thus very likely that protoporphyrin is converted to heme before the formation of CO.
There was a large difference between the maximal rates of catabolism of hemoglobin to CO observed after injection of damaged erythrocytes and hemoglobin solutions. The limiting parameters in these processes are not yet clear.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL E. G., STRITTMATTER C. F., COOPER O. The reaction of cytochrome oxidase with carbon monoxide. J Biol Chem. 1951 Dec;193(2):635–647. [PubMed] [Google Scholar]
- COBURN R. F., BLAKEMORE W. S., FORSTER R. E. Endogenous carbon monoxide production in man. J Clin Invest. 1963 Jul;42:1172–1178. doi: 10.1172/JCI104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COBURN R. F., WILLIAMS W. J., FORSTER R. E. EFFECT OF ERYTHROCYTE DESTRUCTION ON CARBON MONOXIDE PRODUCTION IN MAN. J Clin Invest. 1964 Jun;43:1098–1103. doi: 10.1172/JCI104994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSBY W. H., FURTH F. W. A modification of the benzidine method for measurement of hemoglobin in plasma and urine. Blood. 1956 Apr;11(4):380–383. [PubMed] [Google Scholar]
- Coburn R. F., Forster R. E., Kane P. B. Considerations of the physiological variables that determine the blood carboxyhemoglobin concentration in man. J Clin Invest. 1965 Nov;44(11):1899–1910. doi: 10.1172/JCI105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Williams W. J., Kahn S. B. Endogenous carbon monoxide production in patients with hemolytic anemia. J Clin Invest. 1966 Apr;45(4):460–468. doi: 10.1172/JCI105360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- GRAY C. H., NICHOLSON D. C., NICOLAUS R. A. The IX-alpha structure of the common bile pigments. Nature. 1958 Jan 18;181(4603):183–185. doi: 10.1038/181183b0. [DOI] [PubMed] [Google Scholar]
- Haldane J., Smith J. L. The Absorption of Oxygen by the Lungs. J Physiol. 1897 Nov 20;22(3):231–258. doi: 10.1113/jphysiol.1897.sp000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATHEM W., WORLEY W. E. The distribution of extracorpuscular hemoglobin in circulating plasma. J Clin Invest. 1959 Mar;38(3):474–483. doi: 10.1172/JCI103823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONDON I. M. The conversion of hematin to bile pigment. J Biol Chem. 1950 May;184(1):373–376. [PubMed] [Google Scholar]
- NAKAMICHI M., RAYMOND S. Acrylamide-gel electrophoresis of hemoglobins. Clin Chem. 1963 Apr;9:135–145. [PubMed] [Google Scholar]
- NATHAN D. G., GABUZDA T. G., GARDNER F. H. LIQUID SCINTILLATION COUNTING OF C14-LABELED HEMOGLOBIN AND HEMIN BY A MODIFIED SCHOENIGER TECHNIQUE. J Lab Clin Med. 1963 Sep;62:511–516. [PubMed] [Google Scholar]
- OSTROW J. D., HAMMAKER L., SCHMID R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961 Aug;40:1442–1452. doi: 10.1172/JCI104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTROW J. D., JANDL J. H., SCHMID R. The formation of bilirubin from hemoglobin in vivo. J Clin Invest. 1962 Aug;41:1628–1637. doi: 10.1172/JCI104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRYKA Z., NICHOLSON D. C., GRAY C. H. Isomeric bile pigments as products of the in vitro fission of haemin. Nature. 1962 Jun 16;194:1047–1048. doi: 10.1038/1941047a0. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. II. Properties of reconstituted protohemoglobin and protoporphyrin-globin. Biochim Biophys Acta. 1959 Sep;35:93–101. doi: 10.1016/0006-3002(59)90338-5. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., MONDOVI B. Enzymic reduction of ferrimyoglobin. Arch Biochem Biophys. 1957 Jun;68(2):341–354. doi: 10.1016/0003-9861(57)90366-1. [DOI] [PubMed] [Google Scholar]
- SNYDER A. L., SCHMID R. THE CONVERSION OF HEMATIN TO BILE PIGMENT IN THE RAT. J Lab Clin Med. 1965 May;65:817–824. [PubMed] [Google Scholar]
- YOUNG R. C., Jr, NAGANO H., VAUGHAN T. R., Jr, STAUB N. C. Pulmonary capillary blood volume in dog: effects of 5-hydroxytryptamine. J Appl Physiol. 1963 Mar;18:264–268. doi: 10.1152/jappl.1963.18.2.264. [DOI] [PubMed] [Google Scholar]


