Abstract
Myofibroblast numbers and α-smooth muscle actin expression are increased in anterior joint capsules of patients with posttraumatic elbow contractures. The purpose of our study was to determine whether these changes occur regionally or throughout the entire joint capsule. We hypothesized that the α-smooth muscle actin mRNA expression and the myofibroblast numbers in posterior joint capsules would be elevated in elbows obtained from patients with posttraumatic joint contractures compared with joint capsules obtained from organ donor elbows without contractures. Semiquantitative reverse transcription-polymerase chain reaction was used to evaluate relative mRNA levels of α-smooth muscle actin. Glyceraldehyde-3-phosphate dehydrogenase was used to normalize the levels. Immunohistochemical analysis was used to determine the myofibroblast cell numbers. Higher α-smooth muscle actin mRNA levels were observed in elbows of patients with contractures compared with organ donor elbows without contractures. Immunohistochemical studies determined that myofibroblast numbers and the percentage of total cells that were myofibroblasts were elevated (2–2.5-fold) in the joint capsules from patients with posttraumatic joint contractures compared with similar tissue obtained from organ donor elbows without contractures. These results suggest elevated myofibroblast numbers occur throughout the whole joint capsule in posttraumatic elbow contractures, although there is some regional variation.
Human elbows are synovial hinge joints that are stabilized by ligaments and an articular capsule.18 Contracture formation can result after an injury such as a dislocation and/or fracture.14,16 Efforts to mobilize joint injuries in their early stages have been beneficial in preventing or decreasing the severity of posttraumatic joint contractures; however, they remain a problem.14,16 There is no apparent solution for joint contractures, and contractures in elbows are a common complication after injury.15 Changes in the joint capsule are key elements that limit joint motion and are poorly understood.3,7
From the perspective of surgical approach, the human elbow capsule is divided into two separated regions: the anterior and the posterior capsules. Studies involving other joint capsules, such as the hip and shoulder, have shown the structural and material properties vary throughout the hip capsule, and this often is associated with observed differences in thickness.1,4,17 Biochemical regional variation in joint capsules has been described, with evidence of collagen type II near insertions to bone and in areas of joint capsules under compressive loads.2,8 These regional variations in normal joint capsules imply functional differences in the capsule and would suggest a pathologic capsule from posttraumatic contractures may have regional variation. Work in our laboratory evaluating anterior capsules of the human elbow has shown suggestions of elevated myofibroblast numbers and an increase in the expression of the myofibroblast marker α-smooth muscle actin (α-SMA) in patients with chronic posttraumatic elbow contractures when compared with similar tissues obtained from age-matched organ donors free of elbow contractures.7
We hypothesized that α-SMA mRNA levels and myofibroblast numbers are elevated in posterior joint capsules obtained from patients with posttraumatic contractures compared with similar tissue obtained from joints without contractures. In addition, we hypothesized the total extension-flexion arc of motion and elbow flexion have an inverse correlation with myofibroblast numbers in posterior joint capsules.
MATERIALS AND METHODS
Experimental posterior joint capsules of human elbows were obtained from eight patients, five women and three men, with contractures (mean ± standard deviation [SD], 37 ± 15 years) at the time of surgery (Table 1). Four patients fractured the distal humerus, three patients fractured the radial head, and one fractured the proximal radius and ulna. The patients with contractures all had decreased range of motion (ROM) of the elbow with an average preoperative extension-flexion arc of motion of 63° ± 22° (normal, > 130°). Six elbows from organ donors without contractures were used as the control posterior joint capsules (range, 26 ± 15 years). There was no difference in the ages of the two groups. The organ donors consisted of four males and two females (Table 1). For organ donors with rigor mortis, the clinical history was used to determine whether the joints were free of contractures. The tissues from the organ donors were collected within 2 to 18 hours of death (body stored at 4°C). It has been reported mRNA levels in periarticular tissue samples are unaffected for at least 96 hours after death.11 Institutional review board approval was obtained from our institution and donor program.
TABLE 1.
Data Summary
| Contracture | Total Motion (°) | Preoperative Extension (°) | Preoperative Flexion (°) | Myofibroblasts | Percent Myofibroblasts | Total Cells | α-SMA mRNA |
|---|---|---|---|---|---|---|---|
| Experimental | 60 | 40 | 100 | 54 | 26 | 203 | 2.3 |
| 58 | 32 | 90 | 52 | 22 | 242 | 2.4 | |
| 73 | 44 | 117 | 44 | 21 | 206 | 4.2 | |
| 85 | 38 | 123 | 27 | 20 | 134 | 3.4 | |
| 57 | 28 | 85 | 47 | 19 | 253 | 7.2 | |
| 15 | 75 | 90 | 57 | 20 | 285 | 5.5 | |
| 81 | 45 | 126 | 58 | 21 | 273 | 3.1 | |
| 74 | 34 | 108 | 29 | 10 | 279 | 2.0 | |
| Mean | 63 | 42 | 105 | 46* | 20‡ | 234 | 3.8† |
| SD | 22 | 14 | 15 | 12 | 5 | 51 | 1.8 |
| Control | > 130 | — | — | 18 | 10 | 182 | 2.0 |
| > 130 | — | — | 15 | 10 | 155 | 1.9 | |
| > 130 | — | — | 18 | 10 | 191 | 3.2 | |
| > 130 | — | — | 18 | 9 | 199 | 2.5 | |
| > 130 | — | — | 21 | 11 | 188 | 1.8 | |
| > 130 | — | — | 25 | 10 | 249 | 2.9 | |
| Mean | > 130 | — | — | 19* | 10‡ | 194 | 2.4† |
| SD | — | — | — | 3 | 1 | 31 | 0.6 |
Total motion = extension-flexion arc of motion; myofibroblasts = number of cells/field; percent myofibroblasts = myofibroblast numbers/total cell numbers; total cells = number of cells/field; α-SMA mRNA = optical density of scanned semiquantitative RT-PCR α-SMA/GAPDH mRNA expression; SD = standard deviation;
Significantly different at p < 0.0002;
Significantly different at p < 0.05;
Significantly different at p < 0.05
Immediately after collection, the capsules were stored at −80°C. For immunohistochemical analysis, the tissue samples were placed in optimal cutting temperature embedding material (Sakura Finetek, Torrance, CA), snap frozen in liquid nitrogen, and stored at −80°C.
Total RNA from each tissue sample was extracted using the TRIspin method.7 The frozen tissues were powdered at liquid nitrogen temperatures and TRIzol reagent was added (1 mL TRIzol/~30 mg tissue). The mixture was thawed to room temperature and then was kept at −20°C until further processing. Chloroform was added to the samples once they were thawed and then centrifuged. The aqueous top layer that contained the RNA was removed and 70% ethanol was added. The RNase-Free DNase Set Protocol (Qiagen, Chatsworth, CA) was used to extract and purify the total RNA. This was accomplished with the RNeasy Total RNA kit (Qiagen). Total RNA was quantified using Sybr® Green II (FMC BioProducts, Rockland, MN) reagent and was compared with the standards obtained from calf liver. The samples were stored at −80°C until analyzed.
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed as follows. Reverse transcription (RT) of the total RNA was accomplished with 1 μg of total RNA and the Qiagen Omniscript Reverse Transcriptase Kit (Qiagen). RNase inhibitor, RNAse-free water, 10× RT buffer, random primers, Omniscript RT, and deoxynucleoside triphosphates were added to the template RNA. The mixtures were incubated for 60 minutes at 37°C, heated for 5 minutes at 93°C, and then cooled on ice. Aliquots of cDNA were amplified with 10× PCR buffer, forward and reverse primers, deoxynucleoside triphosphates, and Taq DNA polymerase. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control gene. Each RT sample was analyzed for GAPDH cDNA level and the volumes were normalized. The polymerase chain reaction (PCR) was repeated until the integrated density values for GAPDH were similar. Polymerase chain reactions were performed with human primers at optimized conditions (Table 2). The integrated density values obtained from the assays performed with the human primers were compared with an average of the normalized GAPDH values. The PCR profile consisted of 23 to 35 cycles of 1 minute at 94°C, 1 minute at the annealing temperature, and 1 minute at 72°C. All assays were performed at least in duplicate for each sample. The PCR products were obtained, mixed with loading buffer, and loaded to a 2% agarose gel in which electrophoresis occurred. Immediately after, the gels were stained with ethidium bromide and destained with distilled water. A photograph of the gel was taken with Polaroid film number 55 (Technicare Inc, Calgary, AB, Canada). The standard 1-kb DNA Ladder (Life Technologies, Rockville, MD) was used to confirm the proper size of the PCR products. The approximate optical densities of the bands were determined by densitometric scanning of negatives (Masterscan Interpretive Densitometer, CSPI, Billerica, MA). The scanned images were analyzed with restriction fragment length polymorphism scanalytics (Scanalytics, CSPI).
TABLE 2.
List of Primers
| Primer | Sequence | Size (bp) | TA (°C) | TM (°C) | Optimal Cycles |
|---|---|---|---|---|---|
| GAPDH | 5′-TCACCATCTTCCAGGAGCGA-3′ (forward) 5′-AGTGATGGCATGGACTGTGG-3′ (reverse) |
326 | 65 | 62 | 23 |
| α-SMA | 5′-GCTCACGGAGGCACCCCTGAA-3′ (forward) 5′-CTGATAGGACATTGTTGACAT-3′ (reverse) |
591 | 60 | 58 | 35 |
TA = annealing temperature; TM = melting temperature; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; α-SMA = alpha-smooth muscle actin
We used the following immunohistochemical protocol established in our laboratory.7 A double labeling technique was applied for α-smooth muscle actin and laminin. Alpha-smooth muscle actin was used as the marker for myofibroblasts; however, it also detected smooth muscle cells in blood vessels. Because laminin is a marker of blood vessels, it is able to distinguish α-SMA in vascular tissue from α-SMA in myofibroblasts.7 Specific antibodies were used to detect α-SMA and laminin (Table 3).
TABLE 3.
Antibodies
| Protein | Primary Antibody |
Secondary Antibody |
||
|---|---|---|---|---|
| Antibody | Dilution | Antibody | Dilution | |
| α-SMA | Anti α-SMA monoclonal antibody | 5 μg/mL | Sheep anti-mouse IgG horseradish conjugate | 1:100 |
| Laminin | Rabbit anti-laminin | 1:25 | Goat anti-rabbit IgG antibody conjugated with alexa fluor 488 | 1:1000 |
α-SMA = alpha-smooth muscle actin
Frozen sections were cut at 8 to 10 μm with a cryostat at −25°C and mounted on precoated glass slides. The tissues were fixed with acetone at −20°C for 10 minutes and stored at −80°C until further processing. Frozen slides were thawed at room temperature and washed three times for 5 minutes with 0.1 mol/L phosphate buffered saline (PBS). Endogenous peroxidase activity was quenched by incubation in 0.3% hydrogen peroxide in methanol for 20 minutes. Sections were rinsed three times in PBS for 5 minutes, then nonspecific binding was reduced with 10% normal goat serum in PBS applied for 20 minutes at 37°C. The monoclonal α-SMA antibody (clone asm-1, Röche Molecular Biochemicals, Laval, QC, Canada) was applied (5 μg/mL) and incubated for 60 minutes at 37°C, followed by three 5-minute washes with PBS. The secondary antibody, a sheep anti-mouse immunoglobulin (IgG) horseradish conjugate (Röche), was applied to the sections (dilution 1:100) for 60 minutes at room temperature followed by three 5-minute washes with PBS. Diaminobenzidine (DAB)/peroxide buffer was applied to each section until the end point was reached which took approximately 5 to 10 minutes. The slides were rinsed with PBS three times for 5 minutes each. Incubation with the second primary antibody, rabbit anti-laminin (dilution 1:25; rabbit affinity purified antibodies, Sigma, St Louis, MO), was for 24 hours at 4°C. Sections then were washed with PBS three times for 5 minutes each. The secondary antibody, a goat anti-rabbit IgG antibody conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR), was applied (dilution 1:1000) for 60 minutes in the dark at room temperature. Each section was rinsed three times for 5 minutes in PBS. The tissues were treated with 4′-6-diamidine-2-phenyl indole (DAPI, Vector Laboratories, Burlington, ON, Canada) and cover slips were placed on each section. The slides were stored in the dark at 4°C.
A light microscope (Zeiss, Axioskop 2 Plus, Toronto, ON, Canada) was used to view the sections. Three randomly selected areas (×200) from each of the six serial sections were photographed with a digital camera (Zeiss, Axiocam). Total cell counts were analyzed with Image-Pro Plus (Media Cybernetics, Silver Spring, MD). Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) was used to determine the myofibroblast numbers. Cell nuclei associated with α-SMA and not laminin were counted as myofibroblasts. These data were collected as absolute myofibroblast numbers and expressed as a ratio of myofibroblast cells to total cells.
The data are presented as mean ± SD. Analysis of the mean data was accomplished by using a Student’s t test for two samples assuming unequal variance. Regression analysis was used to examine the relationships between myofibroblast numbers or α-SMA mRNA levels and total ROM and flexion combining the myofibroblast numbers and elbow motion numbers of the two groups. Probability values of 0.05 or less were considered significant.
RESULTS
The mRNA levels for α-SMA were elevated (p < 0.05) in the joint capsules of patients with contractures compared with similar tissues from the organ donors without contractures (Table 1; Fig 1). The normalized α-SMA/GAPDH ratio for the patients with contractures was 3.8 ± 1.8 whereas the value for the organ donors was 2.4 ± 0.6 The mRNA levels of GAPDH were greater (p < 0.05) for patients with contractures (0.80 ± 0.08) than for donors without contractures (0.59 ± 0.04).
Fig 1.
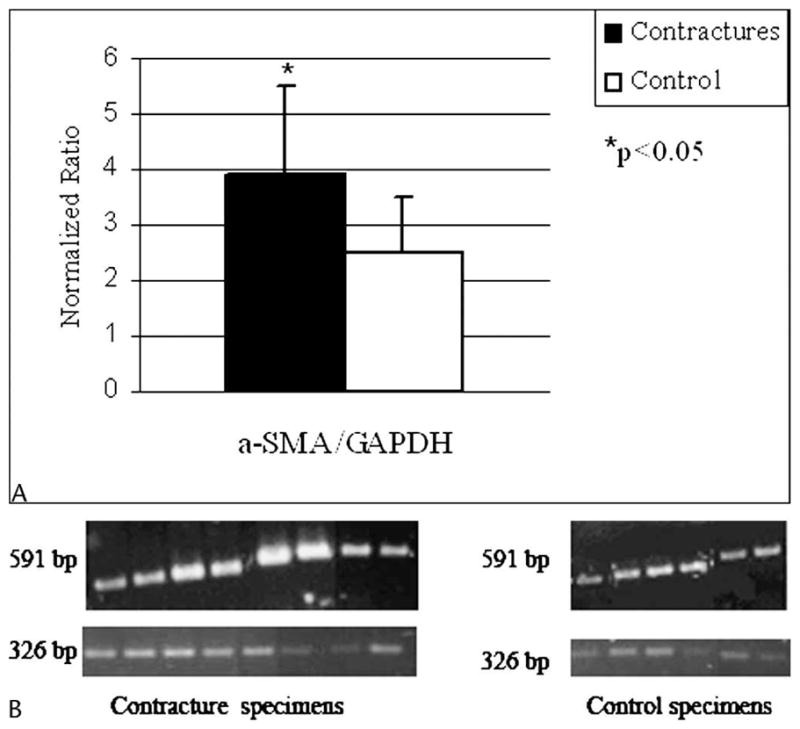
A–B. (A) A graph shows the optical density measures for the contracture (n = 8) and control (n = 6) specimens. (B) A representative RT-PCR analysis shows α-SMA mRNA expression and the housekeeping gene, GAPDH. The α-SMA appears at 591 bp and GAPDH appears at 326 bp. There are eight contracture specimens and six control specimens.
Myofibroblast numbers were greater (p < 0.0002) in the joint capsules of patients with contractures compared with the donors without contractures (Table 1; Fig 2). The absolute number of myofibroblasts was elevated (p < 0.0002) in the joint capsules of patients with posttraumatic contractures (46 ± 12 cells per field) compared with the joint capsules of the donors without joint contractures (19 ± 3 cells per field) (Table 1). The percentage of total cells that were myofibroblasts was elevated (p < 0.05) in the joint capsules from patients with posttraumatic contractures (20% ± 5%) compared with the capsules of the donors without contractures (10% ± 1%) (Table 1). The total number of cells per field was similar in the groups (Table 1).
Fig 2.
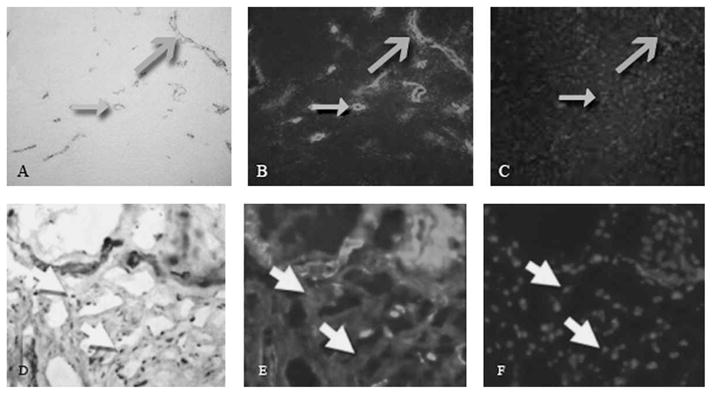
A–F. Immunohistochemical analyses were done of the posterior joint capsule from an organ donor free of contractures (A–C), and a patient with a contracture (D–F). The images are from the same area of the same section for each specimen. Images A and D represent α-SMA (α-SMA antibody (original magnification, ×200). Images B and E represent laminin (Stain, laminin antibody; original magnification, ×200). Images C and F represent cell nuclei (Stain, DAPI; original magnification ×200). The closed grey arrows indicate the vascular structures in cross section, and the open grey arrows indicate the vascular structures in longitudinal section. The white arrows show areas where there is positive α-SMA staining without laminin indicating myofibroblasts. Myofibroblasts were seen in the contracture group, but were not seen in the control group.
Regression analysis of the data indicated an inverse correlation between total extension-flexion arc of motion and myofibroblast numbers (r2 = 0.77; p < 0.0001). This inverse relationship was also seen when comparing the degree of flexion and the number of myofibroblasts (r2 = 0.56; p = 0.0001).
DISCUSSION
Regional variation has been reported in biomechanical and biochemical evaluations of normal capsules obtained from hips, shoulders, knees, and facet joints.1,4,13,17 There is no information on regional variation in the elbow capsule, or pathologic joint capsule in posttraumatic contractures. Previously, we found an increase in myofibroblasts in the anterior joint capsule of the human elbow with posttraumatic contractures when compared with similar tissue obtained from organ donors free of contractures.7 We found inverse correlations between myofibroblast numbers of the posterior joint capsule and total arc of extension-flexion and flexion of the elbow. Total cellularity in the posterior joint capsule was similar in the two groups.
Our study has several limitations. The gene product detected in the gel was not sequenced to confirm it is α-SMA. However, the increased numbers of myofibroblasts shown by immunohistochemical techniques detecting α-SMA protein are consistent with the elevated mRNA levels of the gene product that was detected at the expected base-pair site for the α-SMA primers. Only one evaluator (M.Z.) determined the myofibroblast and cell numbers in the immunohistochemistry studies. Although this enhances reliability, it may introduce systematic bias. However, this same evaluator counted cells and myofibroblasts in previous studies of the anterior joint capsule done in our laboratory, which allows comparisons to be made between the studies on the anterior and posterior capsules of human elbows.5,7 Another possible limitation to the current study is that regional variation may exist in the posterior (or anterior) joint capsule. Although defining anterior versus posterior joint capsule is easy anatomically and surgically, it is technically difficult to consistently sample the same parts or region of the anterior or posterior joint capsule surgically. Therefore, it is difficult to pool regional results in an anterior or posterior elbow capsule.
The RT-PCR results suggested the two groups differed in the GAPDH. However, since the alterations of the α-SMA mRNA levels in the two groups were greater than the alterations of the GAPDH mRNA levels, the difference in GAPDH is insignificant and did not make enough of an effect on the overall trend observed. A significant difference between the groups for the α-SMA/GAPDH ratios was still seen. Thirty-five cycles were used to detect the α-SMA which is consistent with results of studies of matrix and cellular molecules from joint capsules and ligaments.6,7,9,10
We found differences in the cellularity and the number of myofibroblasts between the posterior and anterior joint capsules of these two groups (Table 4). The anterior capsule of normal and pathologic joints had increased cellularity with respect to the posterior joint capsule. The total cell counts were approximately four times greater in the anterior capsule compared with the posterior capsule. Myofibroblast numbers also were dependent on location (anterior versus posterior). When considering donor (control) elbows, myofibroblast numbers were approximately 3.5 times greater in the anterior capsule than in the posterior capsule. However, the percentage of total cells that were myofibroblasts was similar; 10% in the posterior control capsules and 9% in the anterior control capsules. When considering myofibroblast numbers in elbows with contractures, there seemed to be a selectively greater increase of myofibroblasts in the anterior capsule compared with the posterior capsule. The myofibroblast numbers in the anterior capsule of patients with contractures was at least seven times greater than in the posterior capsule (Table 4). The control donors used for the posterior capsule analysis were the same donors used in our previous study.7 For the control capsule, age, gender, and genetics were matched. Myofibroblast proliferation occurs throughout the entire joint capsule in patients with post-traumatic joint contractures. The differences in cellularity and myofibroblast numbers between anterior and posterior capsules were not likely the result of laboratory methods. The experimental techniques used for the anterior capsule were identical to those used for the posterior capsule.
TABLE 4.
Comparison of Cellularity and Myofibroblasts in Elbow Capsules
| Specimen | Total Cells | Myofibroblasts | Percent Myofibroblasts |
|---|---|---|---|
| Control | |||
| Anterior | 845 ± 335 | 69 ± 41 | 9 ± 0.04 |
| Posterior | 194 ± 31 | 19 ± 3 | 10 ± 1 |
| Contracture | |||
| Anterior | 919 ± 187 | 326 ± 61 | 36 ± 0.04 |
| Posterior | 234 ± 51 | 46 ± 12 | 20 ± 5 |
Reports of comparisons of biomechanical variations in human hip and shoulder capsules are similar to the cellular comparisons made in this study.13,17 The human hip capsule differs in thickness and tangent structural stiffness, therefore heterogeneity exists throughout the hip capsule.17 Similar work describing regional variation has been done on the proximal interphalangeal joint of the human finger and the dorsal capsule of the lumbar and thoracic facet joints.2,8 Some studies13,17 have focused on functional differences rather than cellular differences, although one histologic study compared normal shoulder capsules with unstable shoulders and shoulders after failed thermal capsulorrhaphy.12 The investigators did not discuss regional variation, but confirmed a difference in cellularity between injured and uninjured capsules.12 Being able to characterize and understand the entire capsule is important because each component of a joint has a certain purpose in joint function. In addition, strategies to modify cellular factors with techniques such as gene therapy, may be different depending on where the cells are located to be modified.
We determined that myofibroblast numbers and normalized α-SMA mRNA levels are elevated in posterior joint capsules of patients with posttraumatic joint contractures when compared with similar tissues obtained from age-matched organ donor elbows that were free of contractures. The myofibroblast numbers in the posterior joint capsules are inversely related to the total extension-flexion arc of motion and the maximum elbow flexion. We found an increase in the total cellularity of the anterior joint capsule when compared with the posterior joint capsule in normal organ donor elbows.7 Anterior and posterior joint capsules have increased numbers of myofibroblasts over the organ donor control capsules, but there is a selectively greater increase of myofibroblast numbers in the anterior capsule when compared with the posterior capsule.
Acknowledgments
We thank Mei Zhang (MZ) for technical support and expert assistance with the immunohistochemistry and RT-PCR.
One or more of the authors (KAH and NMG) has received funding from the Canadian Institute of Health Research, the Alberta Heritage Foundation for Medical Research, the Health Research Foundation, and the Markin-Flanagan Research Grant from the Undergraduate Student Research Program in bone and joint research at the University of Calgary.
Footnotes
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Bey MJ, Hunter SA, Kilambi N, Butler DL, Lindenfeld TN. Structural and mechanical properties of the glenohumeral joint posterior capsule. J Shoulder Elbow Surg. 2005;14:201–206. doi: 10.1016/j.jse.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Boszczyk BM, Boszczyk AA, Putz R, Büttner A, Benjamin M, Milz S. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine. 2001;26:E338–E343. doi: 10.1097/00007632-200108010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cooney WP., III . Contractures of the elbow. In: Morrey BF, editor. The Elbow and Its Disorders. 2. 1993. pp. 464–475. [Google Scholar]
- 4.Hewitt J, Guilak F, Glisson R, Vail TP. Regional material properties of the human hip joint capsule ligaments. J Orthop Res. 2001;19:359–364. doi: 10.1016/S0736-0266(00)00035-8. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand KA, Zhang M, Hart DA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop Relat Res. 2005;439:228–234. doi: 10.1097/01.blo.0000177718.78028.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrand KA, Zhang M, van Snellenberg W, King GJ, Hart DA. Myofibroblast numbers are elevated in human elbow capsules after trauma. Clin Orthop Relat Res. 2004;419:189–197. doi: 10.1097/00003086-200402000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Lewis AR, Ralphs JR, Kneafsey B, Benjamin M. Distribution of collagens and glycosaminoglycans in the joint capsule of the proximal interphalangeal joint of the human finger. Anat Rec. 1998;250:281–291. doi: 10.1002/(SICI)1097-0185(199803)250:3<281::AID-AR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Lo IK, Boorman R, Marchuk L, Hollinshead R, Hart DA, Frank CB. Matrix molecule mRNA levels in the bursa and rotator cuff of patients with full-thickness rotator cuff tears. Arthroscopy. 2005;21:645–651. doi: 10.1016/j.arthro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Lo IK, Marchuk L, Hart DA, Frank CB. Messenger ribonucleic acid levels in disrupted human anterior cruciate ligaments. Clin Orthop Relat Res. 2003;407:249–258. doi: 10.1097/00003086-200302000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Marchuk L, Sciore P, Reno C, Frank CB, Hart DA. Postmortem stability of total RNA isolated from rabbit ligament, tendon, and cartilage. Biochim Biophys Acta. 1998;1379:171–177. doi: 10.1016/s0304-4165(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 12.McFarland EG, Kim TK, Banchasuek P, McCarthy EF. Histologic evaluation of the shoulder capsule in normal shoulders, unstable shoulders, and after failed thermal capsulorrhaphy. Am J Sports Med. 2002;30:636–642. doi: 10.1177/03635465020300050201. [DOI] [PubMed] [Google Scholar]
- 13.Moore SM, McMahon PJ, Azemi E, Debski RE. Bi-directional mechanical properties of the posterior region of the glenohumeral capsule. J Biomech. 2005;38:1365–1369. doi: 10.1016/j.jbiomech.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Morrey BF. The posttraumatic still elbow. Clin Orthop Relat Res. 2005;431:26–35. [PubMed] [Google Scholar]
- 15.Ohtera K, Zobitz ME, Luo ZP, Morrey BF, O’Driscoll SW, Ramin KD, An KN. Effect of pregnancy on joint contracture in the rat knee. J Appl Physiol. 2002;92:1494–1498. doi: 10.1152/japplphysiol.00614.2001. [DOI] [PubMed] [Google Scholar]
- 16.Perry J. A historical perspective. Clin Orthop Relat Res. 1987;219:8–14. [PubMed] [Google Scholar]
- 17.Stewart KJ, Edmonds-Wilson RH, Brand RA, Brown TD. Spatial distribution of hip capsule structural and material properties. J Biomech. 2002;35:1491–1498. doi: 10.1016/s0021-9290(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 18.Tortora GJ. Principles of Human Anatomy. 8. Menlo Park, CA: Benjamin/Cummings Science Publishing; 1999. [Google Scholar]


