Abstract
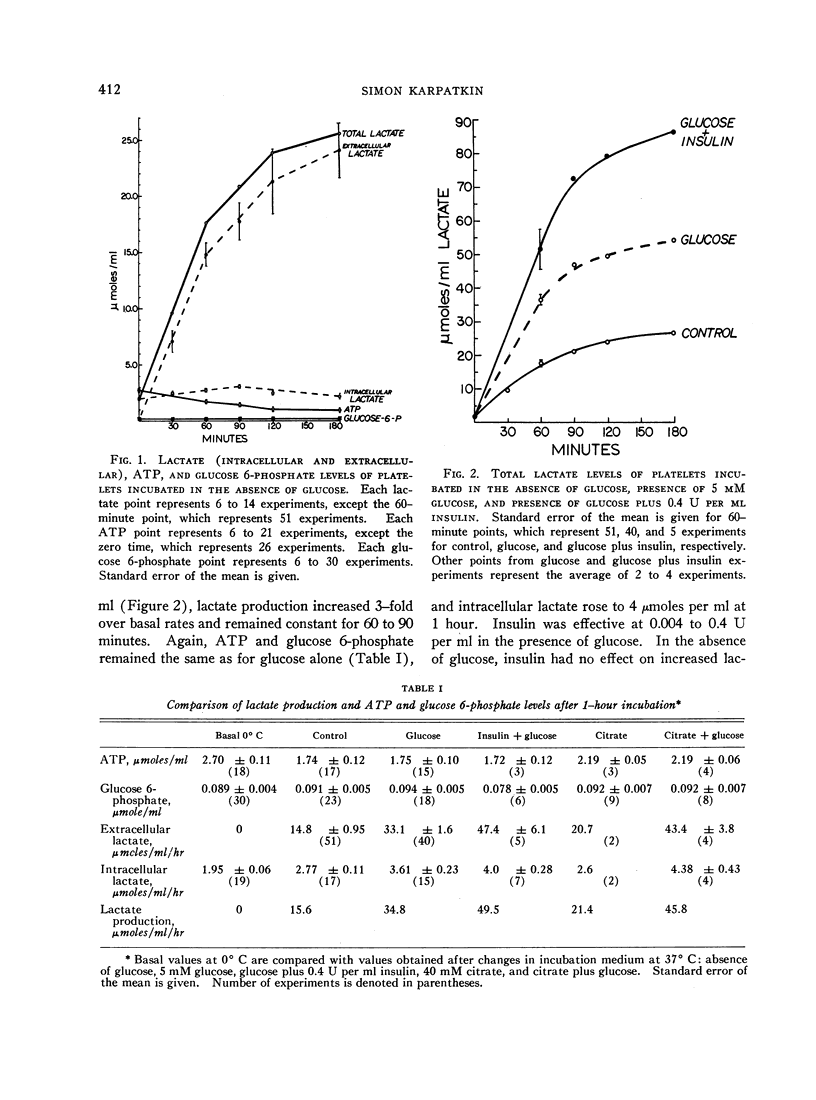
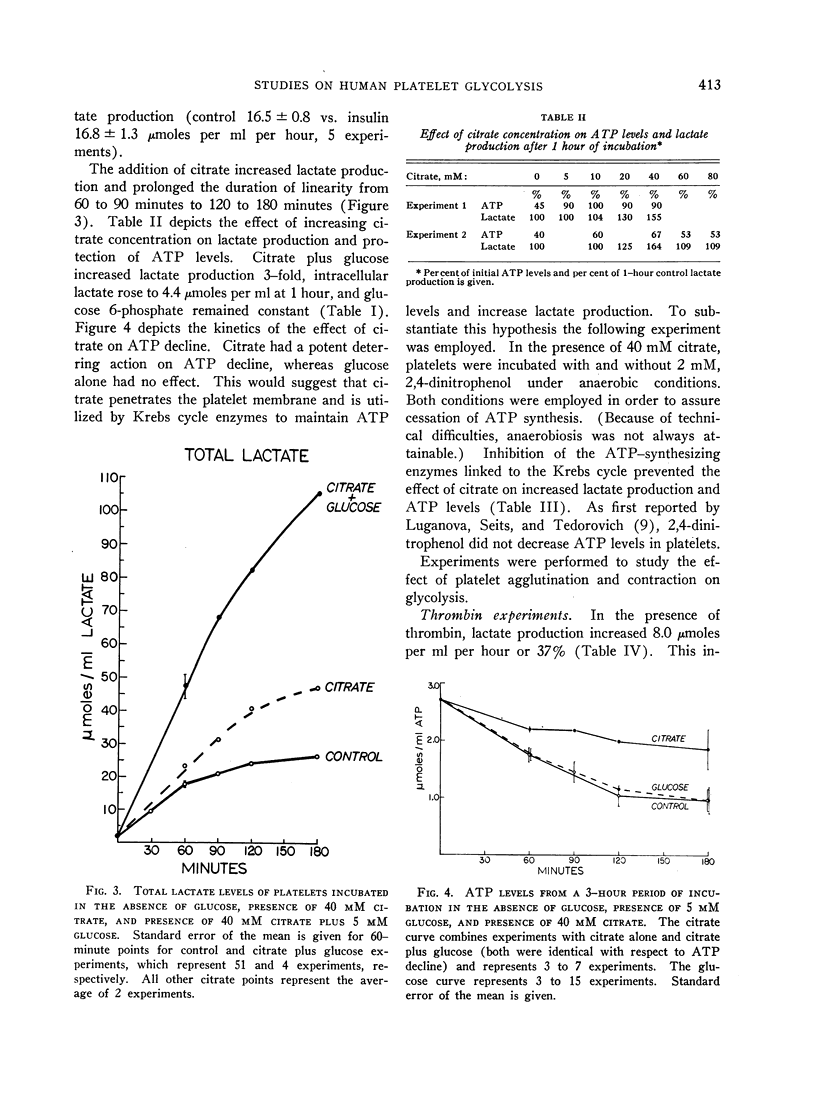
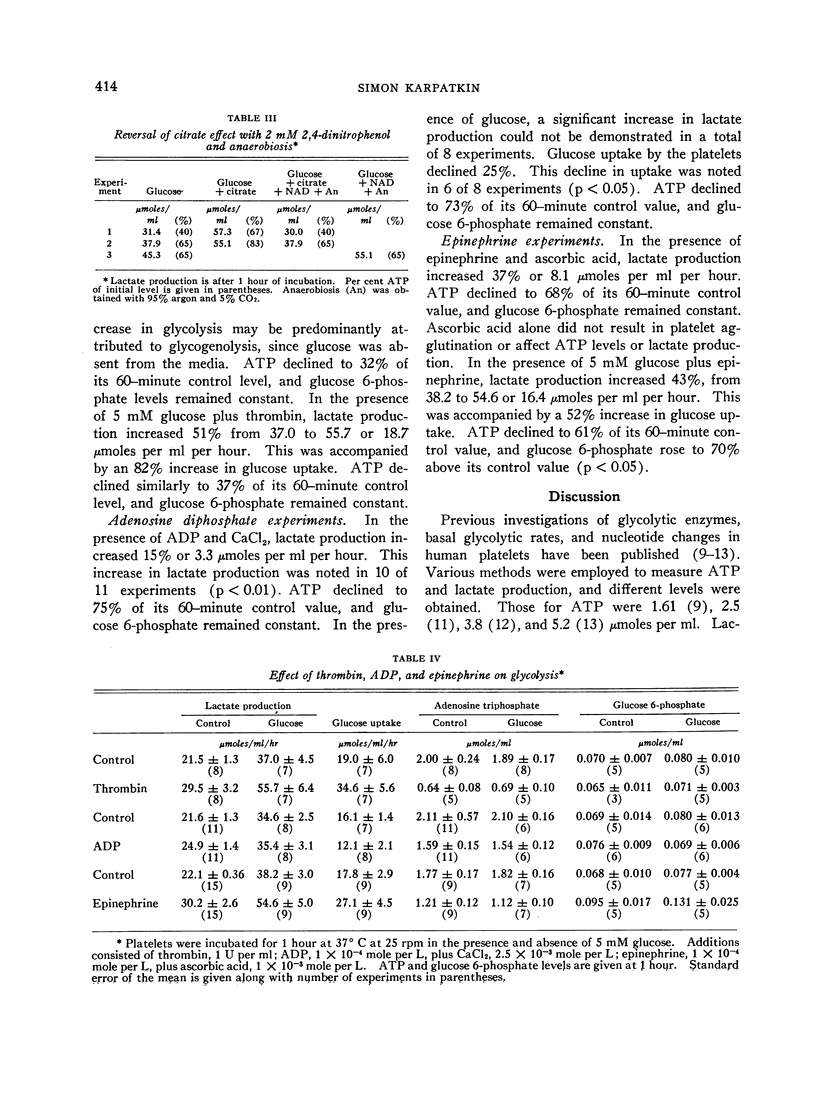
Evidence for a metabolically active plasma-free platelet system is presented. Glycogenolysis was found to be a potent pathway for lactate production. Aerobic glycolysis constituted a major fraction of glucose metabolized. Both insulin and cyanide increased lactate production in the presence of glucose. The Krebs cycle appeared to be operative for ATP synthesis when citrate was used as substrate. The first stages of gluconeogenesis were noted to be present. Glucose uptake contributed to increased lactate production.
Thrombin, epinephrine, and ADP resulted in platelet agglutination and contraction and increased platelet glycogenolysis (phosphorylase activity). Epinephrine appeared to be a more potent activator of phosphorylase, resulting in a 70% increase in glucose 6-phosphate levels. Thrombin and epinephrine both increased glucose uptake and lactate production. Glucose uptake decreased in the presence of ADP. Except for incubations with epinephrine, glucose 6-phosphate remained constant under conditions in which lactate flux increased 3-fold and glucose uptake increased 2-fold. Thrombin, epinephrine, and ADP decreased ATP levels in the presence or absence of glucose.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETTEX-GALLAND M., LUSCHER E. F. Studies on the metabolism of human blood platelets in relation to clot retraction. Thromb Diath Haemorrh. 1960 Mar 1;4:178–195. [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. EFFECTS OF INORGANIC IONS AND OF PLASMA PROTEINS ON THE AGGREGATION OF BLOOD PLATELETS BY ADENOSINE DIPHOSPHATE. J Physiol. 1964 Mar;170:397–414. doi: 10.1113/jphysiol.1964.sp007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V. Changes in the distribution of phosphorus in platelet-rich plasma during clotting. Biochem J. 1958 Apr;68(4):695–704. doi: 10.1042/bj0680695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUNAMEAUX Y. Recherches sur le mécanisme de la rétraction du caillot et de la métamorphose visqueuse des plaquettes. Experientia. 1956 Sep 15;12(9):355–356. doi: 10.1007/BF02165352. [DOI] [PubMed] [Google Scholar]
- Beatty C. H., Peterson R. D., Basinger G. M., Bocek R. M. Major metabolic pathways for carbohydrate metabolism of voluntary skeletal muscle. Am J Physiol. 1966 Feb;210(2):404–410. doi: 10.1152/ajplegacy.1966.210.2.404. [DOI] [PubMed] [Google Scholar]
- CONN H. L., Jr, WOOD J. C., MORALES G. S. Rate of change in myocardial glycogen and lactic acid following arrest of coronary circulation. Circ Res. 1959 Sep;7:721–727. doi: 10.1161/01.res.7.5.721. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E., CORICF The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1191–1199. doi: 10.1073/pnas.48.7.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAARDER A., JONSEN J., LALAND S., HELLEM A., OWREN P. A. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature. 1961 Nov 11;192:531–532. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- HAZELWOOD R. L., ULLRICK W. C. Glycogen mobilization and work in the rat heart. Am J Physiol. 1961 May;200:999–1003. doi: 10.1152/ajplegacy.1961.200.5.999. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Narahara H. T. Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem. 1965 Sep;240(9):3493–3500. [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MITCHELL J. R., SHARP A. A. PLATELET CLUMPING IN VITRO. Br J Haematol. 1964 Jan;10:78–93. doi: 10.1111/j.1365-2141.1964.tb00681.x. [DOI] [PubMed] [Google Scholar]
- REEVES R. B. Control of glycogen utilization and glucose uptake in the anaerobic turtle heart. Am J Physiol. 1963 Jul;205:23–29. doi: 10.1152/ajplegacy.1963.205.1.23. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. THE ROLE OF GLUCOSE 6-PHOSPHATE IN THE REGULATION OF GLUCOSE METABOLISM IN HUMAN ERYTHROCYTES. J Biol Chem. 1964 Jan;239:12–17. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V., O'Connell E. L. Role of inorganic phosphate in stimulating the glucose utilization of human red blood cells. Biochem Biophys Res Commun. 1964 Feb 18;15(1):33–37. doi: 10.1016/0006-291x(64)90098-1. [DOI] [PubMed] [Google Scholar]


