Abstract
Aims
We investigated whether chronic fetal anaemia affects myocardial infarct in adulthood and elicits functional modifications in adult coronary vasoreactivity.
Methods
Seven-month-old sheep that were made anaemic in utero and transfused to normal haematocrit before birth were studied. Infarct size was determined by tetrazolium after 1-h ischaemia (occlusion of the mid of left anterior descending artery) and 2-h reperfusion. The dose–response to vasoconstrictors and vasodilators was assessed in small resistance coronary arteries.
Results
There were no significant differences between the animals previously subjected to in utero anaemia and the control animals regarding the percentage infarct size and the area-at-risk to the left ventricle. The ventricular function (dP/dt) was preserved. The percentage infarct size of the area-at-risk (70.7 ± 3.5%) was larger than that in the controls (49.8 ± 4.5%) (P = 0.006). The vascular responses were not altered. Endothelium-dependent relaxation to bradykinin (96.0 ± 2.6% vs. 98.8 ± 1.0%) was not affected by PGI2 inhibitor (94.6 ± 2.6% vs. 98.5 ± 1.0%) but significantly reduced by the inhibition of nitric oxide (NO) in both anaemic (P < 0.05) and control (P < 0.001) groups with a significant right shift of EC50 (P < 0.01). The non-NO–non-PGI2-mediated relaxation was slightly potentiated in anaemic animals.
Conclusions
Exposing fetal sheep to in utero anaemia in late gestation for 3 weeks may increase the susceptibility of adult hearts to ischaemia–reperfusion injury without major alterations in coronary vasomotor responsiveness. The impact of in utero anaemia at earlier period of pregnancy and on the earlier or later life of the adult is yet to be further investigated.
Keywords: coronary circulation, endothelial function, fetal anaemia, ischaemia, myocardial infarct, reperfusion
Chronic fetal anaemia in sheep initiates multiple adaptations in the cardiovascular system to maintain myocardial and systemic oxygen supply that include a 30% increase in heart-to-body weight ratio, a 50% increase in stroke volume and cardiac output, a sixfold increase in coronary blood flow and a doubling of maximal coronary conductance with preservation of coronary reserve (Davis et al. 1999). Further studies demonstrated that even the anaemia is corrected prior to birth, maximal coronary conductance is twice normal in adult sheep that were anaemic in utero, although the resting coronary flow is normal (Davis et al. 2003). This functional adaptation may have contributed to our subsequent observation that young adult sheep subjected to in utero anaemia displayed augmented contractile function in response to acute hypoxic stress when compared with their non-anaemic twins (Broberg et al. 2003). In contrast, Li et al. (2003) demonstrated that adult rats with hypoxia exposure before birth showed greater infarct size and exacerbated left ventricular dysfunction after global ischaemia and reperfusion.
Thus, an adverse prenatal environment, such as maternal undernutrition (Cheema et al. 2005), placental insufficiency (Gagnon 2003) and fetal hypoxia (Zhang 2005), is strongly associated with an increased risk of cardiovascular diseases in later adult life, but may also confer favourable modifications (Broberg et al. 2003). This study was therefore designed to determine whether chronic fetal anaemia affects myocardial infarct in adulthood and elicits functional modifications in adult coronary vasoreactivity in a sheep model.
Material and methods
Fetal preparation
All animal procedures were reviewed and approved by the Oregon Health and Science University Animal Care Committee. General anaesthesia was induced in pregnant ewes at 112–116 days gestation with intravenous ketamine–diazepam and maintained with 1.5% halothane and a 50% nitrous oxide–50% oxygen mixture as previously described (Davis et al. 2003). Following a midline peritoneal and uterine incision in the ewe, a V8 polyvinyl catheter was placed in the carotid artery in each twin and advanced into the ascending aorta. The ear of the first twin was notched and the catheter marked for identification. The uterine incision was closed and the catheters tunnelled to a pouch on the ewe’s flank. One-million units of penicillin were given in the amniotic space of each twin.
Two days following catheterization, blood gas, oxygen content and haematocrit were measured (calibrated at 39 °C). Foetuses were assigned to two groups. One twin was randomly selected for isovolaemic haemorrhage. The other served as control. In utero anaemia was induced by withdrawing 25–100 mL of blood daily and replacing with an equal volume of normal saline as previously described (Davis et al. 2003). Blood gases, oxygen content and haematocrit were measured prior to each haemorrhage and volume replacement. The withdrawn blood was stored in sterile citrate phosphate dextrose adenine solution to which penicillin was added. Phlebotomy goal was to reach a target haematocrit <50% of the initial value and to maintain this for at least 10 days. The oxygen content was desired to reduce to a nadir of 2.5 mL dL−1 (Davis et al. 2003). Control animals underwent similar measurements of haematocrit and blood gas approx. every 4 days. At approx. 138 days of gestation, anaemic foetuses were autotransfused with 120–150 mL stored packed red blood cells through a high-efficiency leucocyte filter (Y-type blood set; McGaw Inc., Irvine, CA, USA). After transfusion, the catheters were tied off at the ewes’ flank. All animals were allowed for spontaneous term delivery, during which the catheters were pulled out spontaneously. The day after delivery, the lambs were weighed with haematocrit measured. Iron dextran 1 mL containing 100 mg elemental iron was given intramuscularly. One week later, the lambs and ewes were returned to standard habitation at a local farm. At 7 months of age, the adult offspring were returned for study.
The average gestation length for sheep is about 147 days (term: 150 ± 3 days). Lambs wean at the age of 60–80 days and are considered full-grown and matured at about 1 year old with the life span of 7–13 years.
Adult studies: study of ischaemia–reperfusion injury
Experimental preparation
General anaesthesia was induced with intravenous ketamine–diazepam to allow tracheal intubation and was maintained with pentobarbital and supplemental oxygen. Temperature, end-tidal CO2 and oxygen saturation were monitored continuously throughout the study. Arterial blood pH, pO2 and pCO2 were measured intermittently to ensure adequate ventilation. Vinyl catheters were placed in the right atrium via the internal jugular vein and in the aorta via the left common carotid artery. Right atrial, aortic and left ventricular hydrostatic pressures were measured and zeroed to atmosphere at the level of the right atrium. Pressures were recorded continuously with a Gould 2800S polygraph (Gould, Valley View, OH, USA), and displayed on-line. A midline thoracotomy was performed and the pericardial sac was opened. ECG was continuously monitored during ischaemia–reperfusion (I–R).
Induction of I–R
A silicon thread (Scanlon, St Paul, MN, USA) was placed around the mid-left anterior descending artery (LAD) just distal to the second diagonal branch and through a piece of vinyl tubing to form a snare. The snare was tightened to occlude the mid-LAD for 1-h followed by 2-h reperfusion. Occlusion was verified by regional epicardial cyanosis, regional akinesis and ECG changes. Successful reperfusion was verified by regional epicardial blushing, return of local contractile function and resolution of ECG changes.
Infarct size measurement
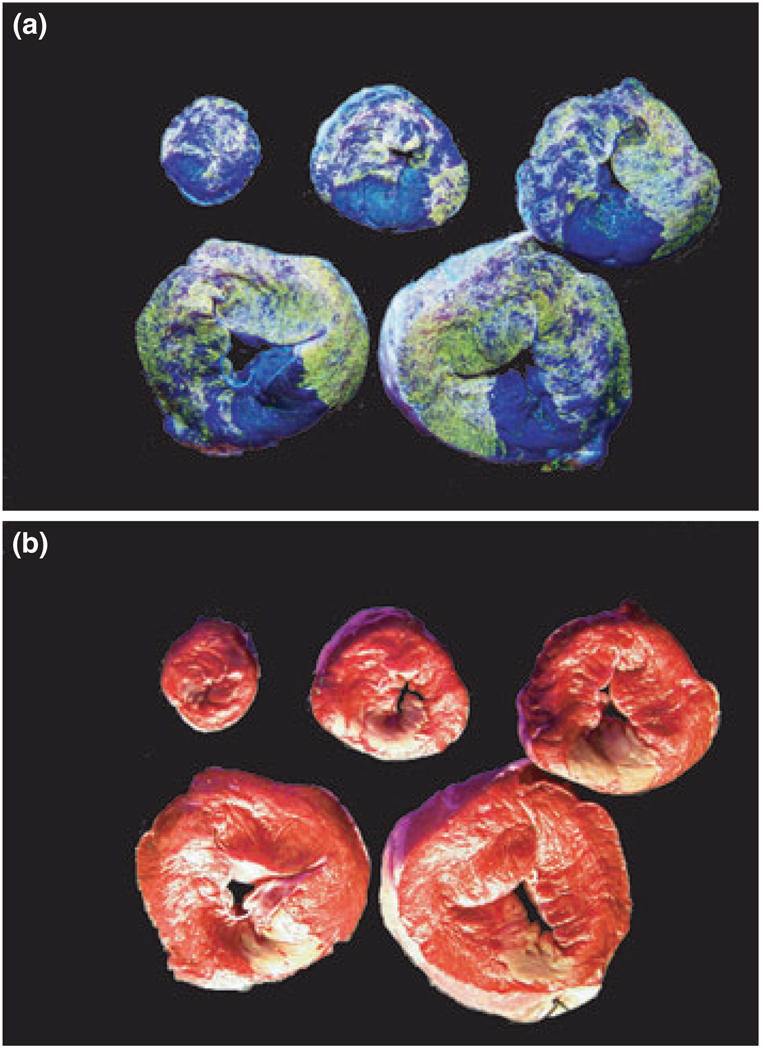
Upon completion of the reperfusion period, heparin (10 000 IU) was injected intravenously and the heart excised. A blunt-end steel tube was placed in the left main coronary orifice and tied in place. The heart was perfused with normal saline for 1 min at a pressure of 80 mmHg to flush out intravascular blood. The mid-LAD was then re-occluded and a 2 mg mL−1 suspension of fluorescent microspheres (no. 34–1, 2–8 µm diameter, Duke Scientific, Fremont, CA, USA) were infused into the left main coronary artery. These microspheres fluoresce bright yellow under ultraviolet light and thus delineate the area-at-risk as a negative image. The heart was trimmed of atria and great vessels, and weighed [right and left ventricles (RV and LV) en bloc]. The LV was cut into transverse slices of 4-mm thick and incubated in triphenyl tetrazolium chloride (TTC, 1% w/v in sodium phosphate buffer at 37 °C, pH 7.4) for 20 min. Myocardium that did not stain red was presumed to be infarcted (Fishbein et al. 1981). The slices were then placed in 4% paraformaldehyde for approx. 10–15 min to increase the contrast between stained and unstained tissue. Digital photographs of the heart slices were taken under full-spectrum and ultraviolet light (Fig. 1). Risk and infarct areas for each slice were traced and digitized using computer assisted planimetry.
Figure 1.
Left ventricle slices infused with fluorescent microspheres and stained with triphenyl tetrazolium chloride (TTC) showing the area-at-risk and infarct area. Under ultraviolet light, area-at-risk were delineated by fluorescence-negativity (a) and infarct area were red-negative under full spectrum light (b).
This method normalizes the infarct size by the area that is usually perfused by the LAD below the level of occlusion. The percentage of the absolute infarct size to this perfusion area (area-at-risk) reflects the severity of the infarct more accurately than either the absolute size or the percentage of infarct to the whole LV. This method has been well accepted previously (Miller & Van Winkle 1999).
Adult studies: vasoreactivity study
Sheep were anaesthetized with the heart quickly excised. Intramyocardial small resistance coronary arteries (diameter 200–450 µm) were dissected in ice-cold Krebs solution and cut into 2-mm-long segments. The arterial rings were mounted in a two-channel myograph (model 500A; J.P. Trading, Aarhus, Denmark). A previously described method (Yang et al. 2002, 2004) was used to normalize vascular rings under a condition simulating the transmural pressure encountered in vivo in the small coronary artery.
Vasoconstriction
Following 40-min equilibration, agonist-(thromboxin A2 mimetic U46619, −10 to −5.5 log m) and depolarizing agent (KCl, 5–120 mm)-induced vasoconstriction was determined with cumulative concentration–response curves in arteries from both in utero anaemic and control animals. The concentration of U46619 required to produce 50% (EC50) and 80% (EC80) of the maximal vasoconstriction was calculated for each artery.
Vasorelaxation
Endothelial function was assessed by the concentration-dependent response to bradykinin (−10 to −6.5 log m) in U46619-preconstricted arteries. The concentration of U46619 varied between EC50 and EC80 to ensure a similar extent of pre-constriction. To further investigate the role of endothelium-derived vasodilators including prostacyclin (PGI2), nitric oxide (NO) and non-NO–non-PGI2-component, concentration–response curves to bradykinin were repeated following a 30-min incubation with indomethacin (Indo, cyclooxygenase inhibitor, 7 µm) and/or NG-nitro-l-arginine (l-NNA, NO synthase inhibitor, 300 µm) and oxyhaemoglobin (HbO, NO scavenger, 20 µm) (Ge et al. 2000, Yang et al. 2002, 2004). Vascular relaxation in response to exogenous NO donor sodium nitroprusside (SNP, −10 to −4.5 log m) was also determined to assess vascular smooth muscle sensitivity to NO.
Data analysis
Global LV function (pressure, dP/dt) was measured prior to the occlusion, the end of occlusion and at 1- and 2-h reperfusion. Relaxation was expressed as the percentage decrease in isometric force. The EC20, EC50 or EC80 was determined from each concentration–constriction/ relaxation curve by a logistic, curve-fitting equation: E = MAP/(AP + KP), where E is the response, M is the maximal vasoconstriction/vasorelaxation, A is the concentration, K is EC20, EC50 or EC80 concentration, and P is the slope parameter. All data were expressed as mean ± SEM and were analysed by Student’s t-test, one-way, two-way anova or repeated measures, where appropriate. P < 0.05 was considered significant.
Results
General characteristics of the animals at different stages
The foetuses in both control and anaemic groups were similar in gestational age, carotid arterial blood gases, oxygen content, total haemoglobin and haematocrit. The haematocrit and haemoglobin of the daily haemorrhaged foetuses decreased gradually and maintained at <50% of the initial level for 14 ± 1 days. In control foetuses, haematocrit and haemoglobin were maintained throughout late gestation. The haematocrit level at birth was not significantly different between the controls and the in utero anaemic animals that were transfused prior to delivery (P = 0.25).
The newborn who had in utero anaemia had slightly lower average body weight (3.17 ± 0.52 kg) compared with the control (3.93 ± 0.41 kg) (P > 0.05). However, no differences in body weight were shown in the 7-month-old adults, consistent with our previous study (Broberg et al. 2003). Both groups had similar cardiac ventricle mass and ventricle-to-body weight ratio (Table 1).
Table 1.
General study characteristics of the two experimental groups (n = 5 in control and in in utero anaemia group)
| Control | In utero anaemia | |
|---|---|---|
| Prenatal | ||
| Surgery gestation age (days) | 113 ± 1 | 115 ± 1 |
| Start haemorrhage gestational age (days) | 117 ± 1 | 117 ± 1 |
| Initial carotid arterial pH | 7.398 ± 0.005 | 7.378 ± 0.017 |
| Initial pO2 (Torr) | 21.4 ± 0.5 | 21.0 ± 1.3 |
| Initial pCO2 (Torr) | 49.3 ± 1.2 | 48.9 ± 1.1 |
| Initial haematocrit (%) | 30 ± 2 | 32 ± 2 |
| Initial haemoglobin (g dL−1) | 9.84 ± 0.34 | 10.44 ± 0.64 |
| Initial oxygen content (mL dL−1) | 8.32 ± 0.40 | 7.98 ± 0.69 |
| Days anaemic (<50% initial haematocrit) | 0 | 14 ± 1* |
| Lowest haematocrit (%) | 29 ± 2 | 14 ± 1* |
| Lowest haemoglobin (g dL−1) | 9.58 ± 0.47 | 3.96 ± 0.17* |
| Lowest oxygen content (mL dL−1) | 8.06 ± 0.53 | 2.24 ± 0.10* |
| Volume blood transfused (mL) | 0 | 137.0 ± 5.4* |
| Birth | ||
| Gestation delivered (days) | 141 ± 1 | 142 ± 1 |
| Birth weight (kg) | 3.93 ± 0.41 | 3.17 ± 0.52 |
| Birth haematocrit (%) | 38 ± 7 | 29 ± 2 |
| Gender (f/m) | 3/2 | 3/2 |
| Adult | ||
| Age at final study (days) | 227 ± 3 | 228 ± 6 |
| Weight at study (kg) | 39.31 ± 2.07 | 41.86 ± 3.19 |
| Haematocrit at study (%) | 23 ± 1 | 27 ± 2 |
| Ventricle (LV + RV) weight (g) | 195.7 ± 8.7 | 229.4 ± 18.6 |
| LV weight (g) | 149.9 ± 8.5 | 176.1 ± 13.2 |
| RV weight (g) | 38.2 ± 4.6 | 53.3 ± 5.8 |
| Ventricle to body weight ratio (g kg−1) | 5.07 ± 0.32 | 5.54 ± 0.40 |
Values are mean ± SEM. pO2, arterial pO2; pCO2, arterial pCO2; f, female; m, male.
P < 0.001 compared with control.
Ischaemia–reperfusion study
At the time of final surgery, the haematocrit, oxygen content and arterial blood gases were similar in the two groups. The mean arterial, LV systolic and diastolic pressures under anaesthesia were also comparable between groups. The pH and pCO2 were maintained throughout the surgery. The pO2 remained at baseline levels in the controls while dropped in the previously anaemic group at 1-h occlusion and 1-h reperfusion (P = 0.007; Table 2). However, the oxygen content in the in utero anaemic animals was stable throughout the surgery, indicating no ventilation-related hypoxia problems.
Table 2.
Blood gases at baseline and during ischaemia–reperfusion (n = 5 in control and in in utero anaemia group)
| Baseline |
End of occlusion |
1-h reperfusion |
2-h reperfusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Anaemia | Control | Anaemia | Control | Anaemia | Control | Anaemia | |
| pH | 7.485 ± 0.017 | 7.434 ± 0.015 | 7.488 ± 0.034 | 7.430 ± 0.036 | 7.485 ± 0.032 | 7.436 ± 0.048 | 7.494 ± 0.022 | 7.464 ± 0.046 |
| pCO2 | 36.6 ± 1.7 | 41.8 ± 2.1 | 35.0 ± 2.3 | 40.6 ± 3.8 | 33.9 ± 3.4 | 42.6 ± 4.5 | 33.8 ± 3.0 | 42.2 ± 6.3 |
| pO2 | 407.0 ± 40.4 | 485.6 ± 42.5 | 407.8 ± 61.0 | 299.8 ±43.1* | 388.0 ± 65.7 | 315.8 ±22.1* | 398.3 ± 68.6 | 400.8 ± 30.3 |
| Oxygen content | 12.2 ± 0.5 | 13.1 ± 0.7 | 12.6 ± 0.4 | 12.2 ± 0.5 | 13.0 ± 0.6 | 11.9 ± 0.3 | 12.8 ± 0.9 | 12.7 ± 0.2 |
| Haemoglobin | 9.2 ± 0.4 | 10.0 ± 0.6 | 9.6 ± 0.3 | 9.3 ± 0.3 | 9.9 ± 0.5 | 9.1 ± 0.2 | 9.7 ± 0.7 | 9.7 ± 0.1 |
| O2Hb (%) | 95.0 ± 0.3 | 94.5 ± 0.6 | 94.1 ± 0.7 | 93.9 ± 0.8 | 94.6 ± 0.3 | 93.4 ± 0.9 | 94.7 ± 0.4 | 93.7 ± 0.7 |
Values are mean ± SEM. Anaemia represents in utero anaemia. pO2, arterial pO2; pCO2, arterial pCO2.
P < 0.01 compared with baseline.
The dP/dtmax was similar in the two groups under basal conditions. No differences were found at the end of ischaemia or during the 2-h reperfusion in either group, nor between groups. Likewise, the diastolic function, indicated by dP/dtmin, in these two groups did not vary, either at the baseline or during I–R (Table 3).
Table 3.
Haemodynamic parameters at baseline and during ischaemia–reperfusion (n = 5 in control and in in utero anaemia group)
| Baseline |
End of occlusion |
1-h reperfusion |
2-h reperfusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Anaemia | Control | Anaemia | Control | Anaemia | Control | Anaemia | |
| Heart rate (beats min−1) | 75 ± 8 | 115 ± 11* | 78 ± 14 | 97 ± 5 | 106 ± 11 | 104 ± 5 | 108 ± 12 | 106 ± 6 |
| LVSP (mmHg) | 112 ± 10 | 131 ±9 | 102 ± 12 | 109 ± 7 | 99 ± 9 | 115 ± 7 | 103 ± 18 | 124 ± 9 |
| dP/dtmax (mmHg s−1) | 1783 ± 491 | 2397 ± 568 | 1519 ± 455 | 1630 ± 298 | 1985 ± 717 | 1802 ± 335 | 2043 ± 709 | 2407 ± 473 |
| dP/dtmin (mmHg s−1) | −2312 ± 434 | −2830 ± 484 | −1859 ± 395 | −2014 ± 364 | −2050 ± 473 | −2520 ± 544 | −2212 ± 782 | −3130 ± 634 |
Values are means ± SEM. Anaemia represents in utero anaemia. LVSP, left ventricle systolic pressure, dP/dtmax and dP/dtmin, maximum and minimum derivative of pressure with respect to time respectively. No differences were observed at baseline, the end of ischaemia and during the 2-h reperfusion in each group (one-way anova), or between groups (repeated measures).
Not significant by anova but P < 0.05, compared to the control by unpaired t-test.
Infarct size
After 1-h occlusion of the mid of LAD distal to the second diagonal branch and 2-h reperfusion, there were no significant differences between the animals previously subjected to in utero anaemia and the control animals regarding the percentage infarct size and the area-at-risk to the LV (P > 0.05). The percentage infarct size of the area-at-risk (70.7 ± 3.5%) in the previously anaemic animals was larger than that in the controls (49.8 ± 4.5%) (P = 0.006; Table 4).
Table 4.
Infarct size and area-at-risk after ischaemia–reperfusion
| Infarct size % LV |
Area-at-risk % LV |
Infarct size % area-at-risk |
|
|---|---|---|---|
| Anaemia (n = 5) | 8.5 ± 1.5 | 12.2 ± 2.2 | 70.7 ± 3.5 |
| Control (n = 5) | 8.7 ± 1.6 | 18.4 ± 4.5 | 49.8 ± 4.5 |
| P-value | 0.903 | 0.286 | 0.006 |
Values are means ± SEM.
In this study, the ventricle size and the ventricle-to-body weight ratio from in utero anaemic animals were not significantly larger than controls. However, there is a tendency for a difference in the LV weight, RV weight and the ventricle-to-body weight ratio, although it did not reach the statistical significance (Table 1).
Vasoreactivity study
Vasoconstriction
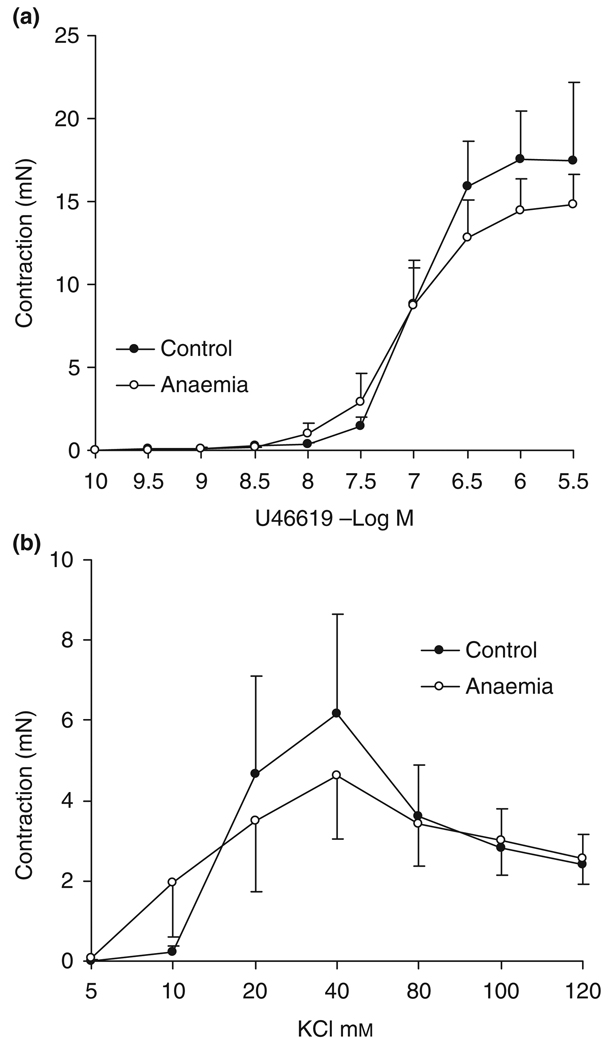
The cumulative dose-contractile responses to U46619 and KCl in small resistance coronary arteries are shown in Figure 2. Both agonist- and depolarizing agent-induced contractions were similar in the 7-month-old adults with/without in utero anaemia.
Figure 2.
Contractile responses of intramyocardial small resistance coronary arteries to (a) U46619 and (b) KCl in 7-month-old adults. No significant differences were observed between the in utero anaemic group and the control group, n = 8. Data are shown as mean ± SEM.
Vasorelaxation
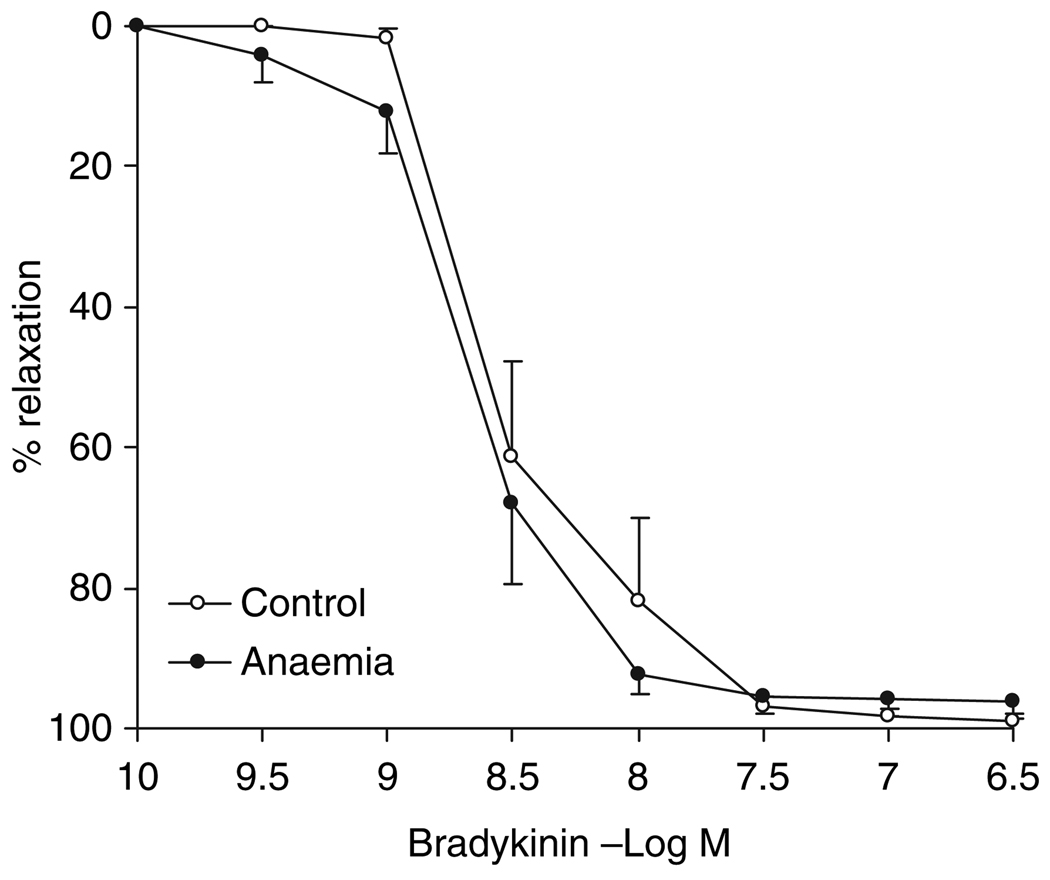
Endothelium-dependent vasorelaxation: Bradykinin-induced, endothelium-dependent vasorelaxation did not differ between the adults with/ without in utero anaemia (96.0 ± 2.6% vs. 98.8 ± 1.0%), nor was there a difference in sensitivity to bradykinin between these two groups (EC50: −8.70 ± 0.11 vs. −8.49 ± 0.12 log m in control, P > 0.05) (Fig. 3).
Figure 3.
Endothelium-dependent vasorelaxation to bradykinin in intramyocardial small resistance coronary arteries from 7-month-old adults with/without in utero anaemia, n = 8. Data are shown as mean ± SEM.
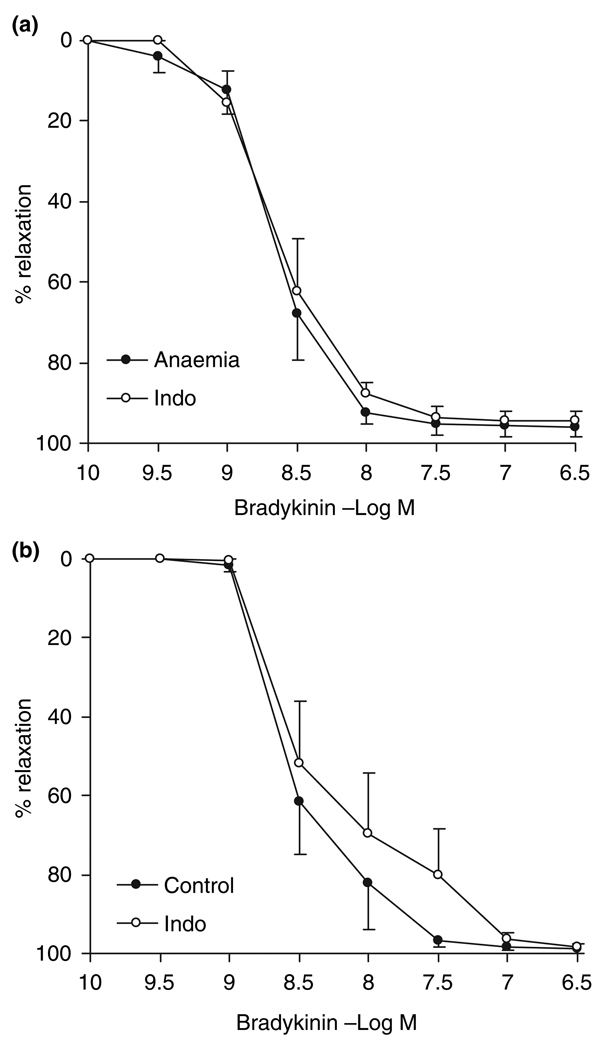
Indomethacin reduced neither the maximal vasorelaxation nor the sensitivity to bradykinin in both the in utero anaemic (relaxation: 94.6 ± 2.6%; EC50: −8.40 ± 0.19 log m) and the control animals (98.5 ± 1.0%; −8.59 ± 0.08 log m) (Fig. 4), suggesting that PGI2 plays little role in endothelium-dependent vasorelaxation in sheep small resistance coronary arteries.
Figure 4.
Endothelium-dependent vasorelaxation to bradykinin with/without the presence of indomethacin (Indo, cyclooxygenase inhibitor, 7 µm) in intramyocardial small resistance coronary arteries from 7-month-old adults (a) with or (b) without in utero anaemia, n = 8. Data are shown as mean SEM. Indomethacin had little effect on the relaxation in either group and the prostacyclin mediation of endothelium-dependent relaxation did not differ between the in utero anaemic adults and the control animals.
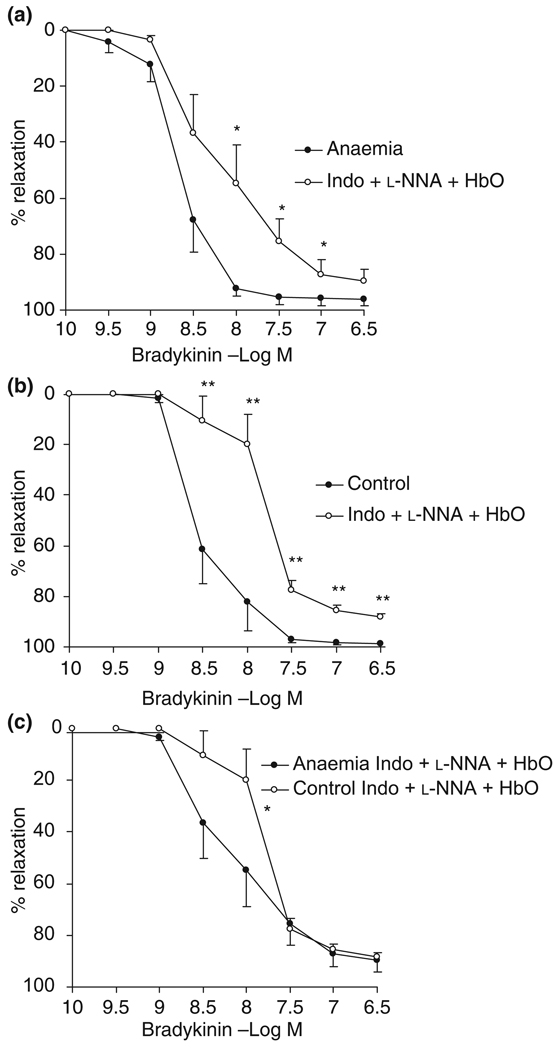
Inhibition of NO significantly reduced the relaxation in both anaemic (P < 0.05) and control groups (P < 0.001) with EC50 right-shifted significantly (anaemia: −8.70 ± 0.11 vs. −8.14 ± 0.18 log m; control: −8.49 ± 0.12 vs. −7.86 ± 0.13 log m; P < 0.01) (Fig. 5a,b), suggesting the significant role of NO in mediating endothelium-dependent vasorelaxation of small resistance coronary arteries in both groups.
Figure 5.
Endothelium-dependent vasorelaxation to bradykinin with/without the presence of indomethacin (Indo, cyclooxygenase inhibitor, 7 µm), NG-nitro-l-arginine (l-NNA, NO synthase inhibitor, 300 µm) and oxyhaemoglobin (HbO, NO scavenger, 20 µm) in intramyocardial small resistance coronary arteries. Inhibition of NO significantly reduced the relaxation in both (a) in utero anaemic adults (P < 0.05) and (b) control adults (P < 0.001) (two-way anova). (c) *In utero anaemia enhanced the non-NO–non-PGI2-mediated relaxation (P < 0.05) in response to concentration of bradykinin ranging from −9 to −7.5 log m, n = 8. Data are shown as mean SEM. *P < 0.05, **P < 0.01.
In addition to NO, the non-NO–non-PGI2 mechanism plays an important role in the sheep coronary vasodilatation. The non-NO–non-PGI2-mediated relaxation in response to −6.5 log m bradykinin reached 89.8 ± 4.4% in anaemic animals and 88.2 ± 1.4% in control siblings. This mechanism was potentiated by in utero anaemia at bradykinin concentrations ranging from −9 to −7.5 log m and the difference reached statistical significance by using two-way anova (P = 0.02) although separate unpaired t-test at these four concentrations did not have statistical difference (P = 0.07 at −8 log m). However, there were no differences regarding EC50 (P > 0.05), although there was a trend that the EC20 was slightly shifted to the left (−8.38 ± 0.18 log m in the anaemic group vs. −7.99 ± 0.13 in the control, P = 0.098) (Fig. 5c).
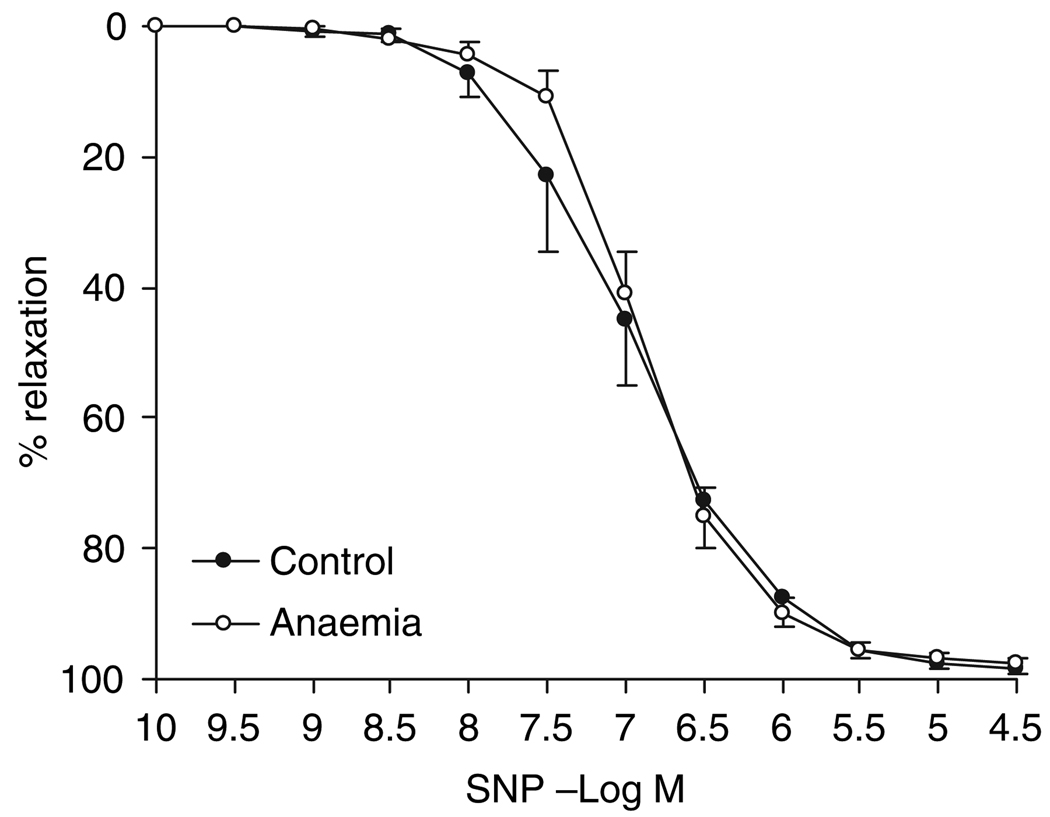
Endothelium-independent vasorelaxation: No differences were observed between the adults with/without in utero anaemia with regard to endothelium-independent vasorelaxation. The maximal vasorelaxation to SNP (97.5 ± 0.9% vs. 98.3 ± 0.7% in control) and the vascular smooth muscle sensitivity (−6.89 ± 0.07 vs. −6.96 ± 0.02 log m) to this NO donor were not significantly different in these two groups (Fig. 6).
Figure 6.
Endothelium-independent vasorelaxation to sodium nitroprusside (SNP) in intramyocardial small resistance coronary arteries from 7-month adults with/without in utero anaemia. n = 8. Data are shown as mean ± SEM.
Discussion
This study demonstrated that exposing fetal sheep to in utero anaemia in late gestation for 3 weeks does not prevent myocardial infarct under severe local I–R, although the LV function is preserved. In addition, the reactivity of coronary vasculature in these animals at the adult age of 7 month old is hardly altered.
In this study, a number of concerns were carefully taken into account. Sheep have a minimal native collateral circulation in the heart, making it an ideal model for infarct size studies (Bowen et al. 2001). In this study, the placement of the coronary occluder was carefully chosen to achieve a sizable area-at-risk while limiting the incidence of lethal arrhythmias and the method was proven to be successful.
Previous reports showed cardioprotective effect of oestrogen by the greater resistance of female hearts to I–R injury (Zhai et al. 2000, Wang et al. 2005). Therefore, ideally only one gender shall be used in this study. However, for reason of the uncontrollable gender for the sheep twins and the precious resource of the twins, either gender had to be used. However, to maximally eliminate the influence of the gender, animals involved in each group had the same ratio of male and female. In addition, to avoid the impact of temperature on the myocardial injury (Chien et al. 1994, Duncker et al. 1996, Hale et al. 2003), we strictly controlled the core body temperature of the sheep at 38.5 ± 0.5 °C with the use of heating pads and radiant lamp during the surgery. Further, no isoflurane anaesthesia was used because of its effect in limiting myocardial infarction (Kersten et al. 1997).
The present studies have demonstrated that hearts from animals with in utero anaemia had similar basal LV function compared with control hearts. The well-preserved cardiac function under resting conditions is in agreement with our previous study (Broberg et al. 2003) as well as the work from others in adult rats with prenatal hypoxia (Li et al. 2003). Likewise, mechanical function (systolic and diastolic) of the heart following the period of I–R did not differ between the control and in utero anaemic animals.
We previously showed that in utero anaemia resulted in increased adult coronary blood flow and coronary conductance (Davis et al. 2003), and augmented contractile response to hypoxic stress in adulthood (Broberg et al. 2003). This protective effect of in utero anaemia against hypoxic stress was shown in the sheep model in which the oxygen saturation was generally lowered down to 30%.
As to the correlation between the prenatal hypoxia and infarct size, Li et al. (2003) found that adult rats subjected to prenatal hypoxia showed greater infarct size after global ischaemia and reperfusion. The present study was designed to investigate whether in utero anaemia may prevent myocardial infarct because of severe ischaemia. Therefore, we have applied a new protocol in our sheep model in which we for the first time occluded the mid of LAD to produce direct and severe ischaemia in the myocardium. The results showed that after 1-h occlusion of the mid of LAD and 2-h reperfusion, there were no significant differences between the two groups with regard to the infarct size, the percentage infarct size to the LV as the whole or to the LV below the occlusion, and the area-at-risk. However, when the infarct size is divided by the area-at-risk, the value in the in utero anaemia group was higher than that in the control. These results demonstrate that the 3-week in utero anaemia may decrease myocardial ischaemic tolerance in adulthood, which is in agreement with the conclusion of Li et al. (2003). However, further studies are warranted to confirm this point.
Further, in agreement with our previous studies, in the present study the LV function in the in utero anaemia group is not significantly different from the control. However, future studies are required to further clarify whether in utero anaemia for a different period or at a different stage of gestation would limit or increase the infarct size in later life.
Notably, there is a clinically relevant difference in the heart rate of the anaemic animals being higher than controls at baseline. This difference decreased by the end of ischaemia period and disappeared after experimental procedure. Although the reason is unknown, this may be at least partially related to previous anaemia and to the tolerance of occlusion of coronary artery when the heart rate at higher baseline in the previously anaemic sheep.
In this study, the ventricle size and the ventricle-to-body weight ratio from anaemic animals were not significantly larger than controls. However, there is a tendency for a difference in the LV weight, RV weight and the ventricle-to-body weight ratio, although it did not reach the statistical significance (Table 1). There may be two reasons accounting for this. First, the sample size is not enough to detect the small difference; second, may be more important, the degree of the anaemic insult (the period lasting) may not be long enough to induce the significance. Moreover, the same results have been published by us on this point (Broberg et al. 2003).
Despite our previous observation that maximal coronary conductance and coronary reserve are increased in adults that were anaemic in utero (Davis et al. 2003), our current results show that the vasoconstriction to contractile agents was not affected by in utero anaemia. Similarly, endothelium-independent vasorelaxation did not differ between the adults with/without in utero anaemia.
With regard to the endothelium-dependent vasorelaxation, PGI2 plays a minimal role in adult sheep small coronary arteries. While NO does participate in endothelium-dependent vasorelaxation, in utero anaemia did not alter NO-mediated vasorelaxation. The unchanged NO-mediation of endothelium-dependent vasorelaxation suggests that the increased coronary conductance and coronary reserve previously found (Davis et al. 2003) in the in utero anaemic adults are unlikely because of enhanced coronary NO production. Neither are these increases explained by augmented coronary responsiveness to NO, evidenced by unchanged vasorelaxation to exogenous NO donor SNP. In contrast, non-NO–non-PGI2-mediated endothelium-dependent vasorelaxation was slightly enhanced in hearts previously subjected to prenatal anaemia but the physiological role of this is questionable because the maximal value had no significant change (Fig. 4c).
Whether structural alterations, such as increased growth of resistance vessels, underlie the increase in coronary conductance and coronary reserve needs to be further studied. Furthermore, although no apparent changes in vasoreactivity were observed in coronary vessels secondary to in utero anaemia in 7-month-old adults, prenatal anaemia may affect coronary vascular function in younger adults. Williams et al. (2005) recently demonstrated that chronic maternal hypoxia reduced the endothelium-dependent relaxation in rat mesenteric arteries in 4-month-old offspring but this impairment disappeared at the age of 7 month, suggesting the time dependency of the impact of certain prenatal event on the adult life. Therefore, further investigation is warranted.
In our experiments, in utero anaemia was imposed during the period of pregnancy at ~116 days to near birth. Anaemia during this period has been demonstrated to affect coronary flow and conductance (Davis et al. 2003). The present study demonstrates that the potentiation of non-NO-non-PGI2-mediated relaxation, although slightly, may contribute to coronary flow increase in the adults with prenatal anaemia. It is possible that anaemia in earlier pregnancy may alter other mediators of coronary vasorelaxation. This again warrants further investigations.
In conclusion, the present study demonstrated that exposing fetal sheep to in utero anaemia in late gestation for 3 weeks may increase the susceptibility of adult hearts to I–R injury without major alterations in coronary vasomotor responsiveness. The impact of in utero anaemia at earlier period of pregnancy as well as the anaemia on the earlier or later life of the adult is yet to be further investigated.
Acknowledgments
This study was supported by American Heart Association Northwest Affiliate Post-doc Fellowship Grant, Department of Surgery, Oregon Health & Science University, the Providence St Vincent Medical Foundation, Portland, Oregon and by grants from the Research Grants Council of the Hong Kong Special Administrative Region (Project No. CUHK4651/07M) and the Direct Grant 2041164, 4450171, 2041305, 2041384, 2041388 of The Chinese University of Hong Kong, Hong Kong, SAR China. The authors thank Robert Webber and Loni Scocha for valuable assistance.
Footnotes
Conflict of interest
There is no conflict of interest for this study.
References
- Bowen FW, Hattori T, Narula N, Salgo IS, Plappert T, Sutton MG, Edmunds LH., Jr Reappearance of myocytes in ovine infarcts produced by six hours of complete ischemia followed by reperfusion. Ann Thorac Surg. 2001;71:1845–1855. doi: 10.1016/s0003-4975(01)02642-x. [DOI] [PubMed] [Google Scholar]
- Broberg CS, Giraud GD, Schultz JM, Thornburg KL, Hohimer AR, Davis LE. Fetal anemia leads to augmented contractile response to hypoxic stress in adulthood. Am J Physiol Regul Integr Comp Physiol. 2003;285:R649–R655. doi: 10.1152/ajpregu.00656.2002. [DOI] [PubMed] [Google Scholar]
- Cheema KK, Dent MR, Saini HK, Aroutiounova N, Tappia PS. Prenatal exposure to maternal undernutrition induces adult cardiac dysfunction. Br J Nutr. 2005;93:471–477. doi: 10.1079/bjn20041392. [DOI] [PubMed] [Google Scholar]
- Chien GL, Wolff RA, Davis RF, van Winkle DM. “Normothermic range” temperature affects myocardial infarct size. Cardiovasc Res. 1994;28:1014–1017. doi: 10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hohimer AR, Morton MJ. Myocardial blood flow and coronary reserve in chronically anemic fetal lambs. Am J Physiol. 1999;277:R306–R313. doi: 10.1152/ajpregu.1999.277.1.R306. [DOI] [PubMed] [Google Scholar]
- Davis L, Roullet JB, Thornburg KL, Shokry M, Hohimer AR, Giraud GD. Augmentation of coronary conductance in adult sheep made anaemic during fetal life. J Physiol. 2003;547:53–59. doi: 10.1113/jphysiol.2002.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol. 1996;270:H1189–H1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110:S99–S107. doi: 10.1016/s0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Ge ZD, Zhang XH, Fung PC, He GW. Endo-thelium-dependent hyperpolarization and relaxation resistance to N(G)-nitro-L-arginine and indomethacin in coronary circulation. Cardiovasc Res. 2000;46:547–556. doi: 10.1016/s0008-6363(00)00040-7. [DOI] [PubMed] [Google Scholar]
- Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res. 2003;59:715–722. doi: 10.1016/s0008-6363(03)00456-5. [DOI] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Miller DL, Van Winkle DM. Ischemic preconditioning limits infarct size following regional ischemia-reperfusion in in situ mouse hearts. Cardiovasc Res. 1999;42:680–684. doi: 10.1016/s0008-6363(99)00005-x. [DOI] [PubMed] [Google Scholar]
- Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol. 2005;288:E321–E326. doi: 10.1152/ajpendo.00278.2004. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol. 2005;565:125–135. doi: 10.1113/jphysiol.2005.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Liu YC, Zou W, Yim AP, He GW. Protective effect of magnesium on the endothelial function mediated by endothelium-derived hyperpolarizing factor in coronary arteries during cardioplegic arrest in a porcine model. J Thorac Cardiovasc Surg. 2002;124:361–370. doi: 10.1067/mtc.2002.122548. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zhang RZ, Yim AP, He GW. Histidine-tryptophan-ketoglutarate solution maximally preserves endothelium-derived hyperpolarizing factor-mediated function during heart preservation: comparison with University of Wisconsin solution. J Heart Lung Transplant. 2004;23:352–359. doi: 10.1016/S1053-2498(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol. 2000;279:H2766–H2775. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]
- Zhang L. Prenatal hypoxia and cardiac programming. J Sol Gynecol Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]