Summary
Pathogenic substitutions in the Lrrk2 protein have been shown to be an important cause of both familial and sporadic parkinsonism. The molecular pathway involved in Lrrk2 dopaminergic neuron degeneration remains elusive. Employing a combination of Lrrk2-mediated protein precipitation and tandem mass spectrometry we identified 14 potential Lrrk2 binding partners. The majority of these interactions may be subgrouped into three functional cellular pathways: (i) chaperone-mediated response, (ii) proteins associated with the cytoskeleton and trafficking and (iii) phosphorylation and kinase activity. Future investigation of these candidates is now warranted and may help resolve the pathomechanism behind Lrrk2 neurodegeneration.
Keywords: Lrrk2, interactors, tandem mass spectrometry
Introduction
Parkinson's disease (PD) characterized by resting tremor, rigidity and bradykinesia is a common neurodegenerative disorder affecting approximately 1-2% of the population over 60 years of age. Although the vast majority of PD cases are thought to be sporadic, the identification of mutations in several genes causative for rare familial cases has helped to elucidate the mechanisms involved in the development of disease [1].
Pathogenic mutations in the recently discovered gene LRRK2 have been shown to be one of the most important genetic causes of both familial and sporadic late-onset parkinsonism [1]. LRRK2 encodes a protein of approximately 286KDa encompassing numerous functional domains [2,3]. Separate studies suggest that enhanced Lrrk2 kinase activity due to mutations in the kinase domain might be involved in the mechanism leading to disease [4-6]. However, little is known about the physiological function of the other Lrrk2 domains, their pathways and how they are involved in the development of parkinsonism [7-8].
In-silico models of the Lrrk2 protein suggest the presence of several functional domains associated with protein-protein interactions. These include an armadillo, ankyrin and leucine-rich-repeat domain at the N-terminal end and the WD40 domain following the Roc-COR-kinase domains at the C-terminal [3]. The identification of direct-interacting partners of Lrrk2 represents a key step in elucidating the normal function of Lrrk2, and the pathomechanism by which mutant Lrrk2 causes parkinsonism. We have coupled immunoprecipitation with tandem mass spectrometry to identify 14 potential Lrrk2 interactors that may help to reveal further insights into Lrrk2 biology.
Materials and Methods
Immunoprecipitation studies
HEK293T cells, maintained in Opti-mem 1 supplemented with fetal calf serum and penicillin/streptomycin (Gibco), were transfected with MOCK or a pcDNA3 vector encoding full length LRRK2-V5 using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Cells were harvested and lysed in 50mM Tris/HCL, 150mM NaCl and 0.1% Triton-X-100. For immunoprecipitation, processed lysates with equal protein concentrations were incubated with the respective antibody (anti-V5/Invitrogen, anti-MYC/Roche) preconjugated to Dynabeads Protein G for four hours at 4°C. Washing and elution procedures were performed according to the manufacturer's instructions (Invitrogen).
Mass spectrometry analysis
Three combined immunoprecipitates were subjected to SDS-PAGE on 16cm gels of varying percentages to achieve coverage and high resolution separation for proteins of a wide range of molecular weight. The excised gel bands were subsequently destained, reduced and alkylated with dithiothreitol and iodoacetamide. Proteins were digested for four hours with 0.6μg trypsin (Promega) in digestion buffer (20 mM Tris pH 8.1 / 0.0002% Zwittergent 3-16, at 37°C) followed by peptide extraction with 60μl of 2% trifluoroacetic acid, then 60μl of acetonitrile. Pooled extracts were concentrated and then brought up in 0.1% formic acid for protein identification by nano-flow liquid chromatography tandem mass spectrometry (nanoLC-MS/MS) analysis using a ThermoFinnigan LTQ Orbitrap Hybrid Mass Spectrometer (ThermoElectron Bremen) coupled to an Eksigent nanoLC-2D HPLC system (Eksigent). The MS/MS raw data was converted to DTA files using ThermoElectron Bioworks 3.2 and correlated to theoretical fragmentation patterns of tryptic peptide sequences from the NCBI nr database (downloaded March 21, 2006) using both SEQUEST™ 2 (ThermoElectron, San Jose, CA) and the Mascot™ 3 (Matrix Sciences London, UK) search algorithms running on a10 node cluster. All searches were conducted with fixed cysteine modifications of +57 for carboxamidomethyl-cysteines and variable modifications allowing +16 with methionines for methione sulphoxide, and +42 for protein N-terminal acetylation. The search was restricted to trypsin generated peptides allowing for 2 missed cleavages and was restricted to human. Peptide mass search tolerances were set to 20 ppm and fragment mass tolerances set to ± 0.8 Daltons. Protein identifications were considered when Mascot search results gave at least two unique consensus peptides, each with peptide probability scores at or exceeding the 95% probability cut off which was 29 for these searches and ranking as the number one match for the respective MS/MS spectra. The MS/MS spectra from all the Mascot identified peptides were inspected manually for spectra quality and fragment matching. Matches to poor quality spectra or spectra with numerous unmatched ions were not included.
Results
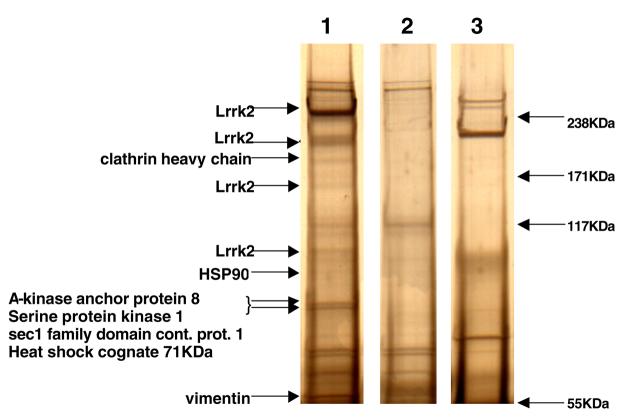
To identify potential protein interactors of Lrrk2 we adopted an immunoprecipitation approach followed by in-gel digestion and tandem mass spectrometry (MS/MS). False positive results are a common caveat of single step immunoprecipitation procedures, due to the co-isolation of non-specific proteins. Thus gels were screened for silver-stained protein bands that were only present when overexpressed Lrrk2-V5 was immunoprecipitated with V5- antibody. Negative controls included a “pull down” of Lrrk2-V5 lysate with a monoclonal mouse antibody directed against the MYC-epitope, and a MOCK transfected HEK293T lysate subjected to immunoprecipitation with V5-antibody (Figure 1). Corresponding regions of equal molecular size were cut out from adjacent control lanes and analyzed by MS/MS in parallel. To be considered a “hit” at least two unique peptide sequences with a probability score at or exceeding the 95% had to be identified for the respective protein (probability score > 29).
Figure 1.
Representative silver stained gel showing examples of the identified proteins. Lysates of Lrrk2-V5 (1, 3) or MOCK (2) transfected HEK293T cells were subjected to immunoprecipitation using V5- (1,2) or myc-antibodies (3) and separated by SDS-PAGE electrophoresis.
Table 1 gives an overview of 14 proteins identified with this approach. The potential Lrrk2 interactors were separated into four subgroups according to their physiological function. Group 1 lists interactors associated with the chaperone pathway and cellular stress, group 2 focuses on proteins linked to the cytoskeleton and protein/membrane trafficking. Group 3 includes candidates ascribed a role in phosphorylation and kinase activity; and group 4 includes the remaining proteins with various cellular functions.
| Identified proteins | Molecular weight (KDa) |
No. of peptides |
NCBI protein accession |
|---|---|---|---|
| Lrrk2 | 289 | 86 | 55740398 |
| 1. Proteins linked to the cytoskeleton and trafficking | |||
| Clathrin heavy chain | 191 | 23 | 29983 |
| Vimentin | 54 | 6 | 37852 |
| Sec1 family domain containing protein 1 | 72 | 2 | 51316882 |
| 2. Proteins associated with phosphorylation | |||
| PRKDC-DNA dependent protein kinase catalytic subunit |
469 | 62 | 38258929 |
| A-kinase anchor protein 8 | 77 | 9 | 5031579 |
| Serine protein kinase 1 | 75 | 5 | 630737 |
| 3. Chaperone proteins | |||
| Heat shock protein HSP90-alpha4 | 85 | 21 | 61656605 |
| Heat shock cognate 71KDa | 70 | 19 | 123648 |
| Heat shock protein HSP90 1 beta | 84 | 18 | 34304590 |
| Stress-70 protein, mitochondrial precursor | 74 | 11 | 21264428 |
| Midasin | 632 | 3 | 24212017 |
| 4. Other | |||
| Eukaryotic translation initiation factor 2C 1 | 98 | 23 | 6912352 |
| Eukaryotic translation initiation factor 2C 2 | 98 | 26 | 29171734 |
| Bifunctional amino-acyl tRNA synthetase | 162 | 13 | 135104 |
Four additional protein bands of various molecular sizes were identified to be either full-length or truncated species of Lrrk2-V5 protein. Apart from the highly abundant full length band at approximately 280KDa (86 peptide matches throughout the protein from amino acid residue 74-2468), three amino-terminally truncated species of Lrrk2 with the approximate molecular sizes of 200KDa, 170KDa and 100KDa were identified. According to the identified peptide matches these truncated species correspond to Lrrk2 lacking the entire N-terminus including half of the ankyrin domain (Nterm-AA757), Lrrk2 lacking the entire ankyrin domain (Nterm-AA970), and a Lrrk2 variant including only part of the kinase domain and the entire C-terminus (AA1896-Cterm).
Discussion
Lrrk2 pathogenic substitutions have been demonstrated in Roc (R1441C/G/H), COR (Y1699C) and kinase (G2019S, I2020T) domains, and a common risk factor (G2385R) has been identified in the WD40 domain [9-11]. Additional coding variants have been found throughout the protein and may also be associated with disease [12]. Protein interactions are likely to have a central role in the normal function of Lrrk2 and may play a role in the development of disease. A combination of immunoprecipitation with tandem mass spectrometry identified 14 potential protein–Lrrk2 interactors, which according to their cellular function may be separated into four groups (Table 1). Of note, our experiments confirmed the interaction of Lrrk2 with HSP90, recently described by Gloeckner and colleagues [4]. Since transfection and over-expression of proteins in general may activate a cellular stress response to unfolded protein, the importance of this interaction remains to be determined. Three other proteins of the chaperone family were also identified (Group 1).
Three interacting proteins are involved in trafficking and the cytoskeleton (Group 2). A functional link between Lrrk2 and one of these, vimentin, was reported in HEK293T cells over-expressing Lrrk2; inclusions observed were shown to be surrounded by a ‘cage-like’ structure of vimentin, reminiscent of aggresomes [5]. It is suggested that these inclusions are dependent on Lrrk2 kinase activity which may be enhanced for specific pathogenic substitutions [4-6]. However, Lrrk2 inclusions are not observed in overexpression models in vivo and may be non-physiological artifacts [13]. An interaction between Lrrk2 and phosphorylation substrate(s) may be temporary and escape detection with our experimental approach. However, co-regulators or scaffold proteins involved should still be detectable and we identified three phosphorylation associated-proteins (Group 3). The final category contains other proteins, including two eukaryotic translation initiation factors (Group 4).
Data published on Lrrk2 function, including this study, are based almost exclusively on non-neuronal HEK293T cellular model systems over expressing Lrrk2 protein, and the physiological relevance of these observations remains to be proven. A limitation of our approach is that potential brain-specific interactors of Lrrk2 protein may escape identification. However, lrrk2 expression is not restricted to the central nervous system, endogenous lrrk2 is present in HEK293T cells, and protein interactors identified may highlight more general functional pathways in which lrrk2 is involved. HSP90 and vimentin are consistently observed as Lrrk2 interactors in HEK293T cells. These proteins, indeed all potential Lrrk2 interactors identified, should be confirmed by immunoprecipitation and immunohistochemical methods of endogenous protein in human brain samples.
Previous studies report the presence of C-terminal fragments of Lrrk2 in human brain [14, 15]. Our identification of truncated species suggests the Lrrk2 protein may also be cleaved at the N-terminal. Whether these truncated species are the result of specific cleavage under physiological conditions or of protein degradation remains to be determined. In vivo models to investigate normal Lrrk2 function are at an early stage, and will provide physiological insight into the mechanism whereby LRRK2 mutations lead to the development of PD.
Supplementary Material
Acknowledgements
Mayo Clinic Jacksonville is a Morris K. Udall Parkinson's Disease Research Center of Excellence (NINDS P50 #NS40256). We thank Minnie Schreiber for technical assistance. We would like to thank all those who have contributed to our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 2.Marin I. The Parkinson Disease Gene LRRK2: Evolutionary and Structural Insights. Mol Biol Evol. 2006;23:2423–2433. doi: 10.1093/molbev/msl114. [DOI] [PubMed] [Google Scholar]
- 3.Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 5.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 8.Macleod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The Familial Parkinsonism Gene LRRK2 Regulates Neurite Process Morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Di Fonzo A, Wu-Chou YH, Lu CS, van Doeselaar M, Simons EJ, Rohe CF, et al. A common missense variant in the LRRK2 gene, Gly2385Arg, associated with Parkinson's disease risk in Taiwan. Neurogenetics. 2006;7:133–138. doi: 10.1007/s10048-006-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.Mata IF, Kachergus JM, Taylor JP, Lincoln S, Aasly J, Lynch T, et al. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 11.Tan EK, Zhao Y, Skipper L, Tan MG, Di Fonzo A, Sun L, et al. The LRRK2 Gly2385Arg variant is associated with Parkinson's disease: genetic and functional evidence. Hum Genet. 2006 doi: 10.1007/s00439-006-0268-0. E-pub Sept 30. [DOI] [PubMed] [Google Scholar]
- 12.Farrer MJ, Ross OA, Stone JT. GeneTests: Medical Genetics Information Resource. University of Washington; Seattle: 1997-2006. LRRK2-Related Parkinson Disease: GeneReviews. Copyright. http://www.genetests.org. [Google Scholar]
- 13.Melrose HL, Taylor JP, Lincoln SJ, Tyndall GM, Dachsel JC, Kent CB, et al. Characterization of mice expressing human wild-type Lrrk2. Mov Disord. 2006;21:S602. [Google Scholar]
- 14.Giasson BI, Covy JP, Bonini NM, Hurtig HI, Farrer MJ, Trojanowski JQ, Van Deerlin VM. Biochemical and pathological characterization of Lrrk2. Ann Neurol. 2006;59:315–322. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 15.Miklossy J, Arai T, Guo JP, Klegeris A, Yu S, McGeer EG, McGeer PL. LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol. 2006;65:953–963. doi: 10.1097/01.jnen.0000235121.98052.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.