Abstract
Objectives
The purpose of this study was to assess the predictors of successful catheter ablation in patients with ventricular arrhythmias arising from the papillary muscles (PAPs).
Background
Ablation of arrhythmias arising from the PAPs is challenging.
Methods
Forty consecutive patients (15 women, mean age: 51±14 years, left ventricular ejection fraction: 46±13%) with refractory PAP arrhythmias underwent mapping and ablation. Catheter stability was assessed with intracardiac echocardiography. Activation mapping and/or pace mapping were performed to identify the site of origin. Electrophysiologic data and anatomic characteristics were assessed in patients with effective vs ineffective ablation. Catheter stability was assessed with intracardiac echocardiography.
Results
Radiofrequency ablation was acutely effective in eliminating the targeted arrhythmia in 31 patients (78%). The presence of Purkinje potentials at the site of origin of the targeted arrhythmia was associated with an effective outcome (48% vs 0%; P=0.01). The mass of the arrhythmogenic PAPs in the LV was significantly larger in patients with failed vs effective ablation (4.7±2.2 g vs 2.3±0.6 g; P<0.0001). Also, the presence of a matching pace-map at the earliest endocardial activation time was associated with an effective procedure (71% vs 22%; p=0.02)
Conclusions
The presence of Purkinje potentials at the site of origin and a smaller size of the PAP are associated with successful ablation of PAP arrhythmias.
Introduction
Arrhythmias arising from the papillary muscles (PAPs) recently have been described in animal models (1) and also in patients with and without structural heart disease (2-4). Due to the constant motion of the PAPs, adequate catheter contact is difficult to achieve (2). Catheter contact, anatomic and electrophysiologic characteristics of PAPs may determine outcome of PAP ablation procedures. The purpose of this study was to identify the predictors of successful catheter ablation in patients with ventricular arrhythmias arising from the PAPs.
Methods
Study Population
This retrospective study included 40 consecutive patients referred for ablation of symptomatic premature ventricular complexes (PVCs; n=19) or ventricular tachycardia (n=21) originating from a PAP (age: 51±14 years, 25 women, ejection fraction 0.46±0.13). Antiarrhythmic drugs failed to control the arrhythmias in 24 patients. Twenty of 40 patients (50%) had structural heart disease: prior myocardial infarction in 10 patients, dilated cardiomyopathy in 9, and valvular heart disease in 1 patient. Twenty-three of the 40 patients were included in prior publications(2,3,5).
Electrophysiological Study
After written informed consent was obtained, the procedures were performed in the fasting state. A 6-F electrode catheter was introduced into a femoral vein and was positioned in the right ventricular apex. Programmed ventricular stimulation was performed using 1 to 4 extrastimuli to assess for inducible ventricular tachycardias (VTs). For left-sided procedures, systemic anticoagulation was achieved with intravenous heparin with an initial bolus of 5000 units of heparin and subsequent administration of heparin to maintain an activated clotting time of ≥250 sec. For right-sided procedures, a bolus of 3000 units of heparin was followed by 1000 units per hour.
Bipolar electrograms were recorded at filter settings of 50 to 500 Hz during the procedure (EP Med Systems, West Berlin, NJ). The intracardiac electrograms and leads V1, I, II, and III were displayed on an oscilloscope at a speed of 100 mm/sec.
Mapping and Ablation
An electroanatomical mapping system (CARTO, Biosense Webster, Diamond Bar, CA) was used for endocardial mapping. Activation mapping was performed for PVCs or VTs, and a voltage map was created during sinus rhythm. The presence of Purkinje potentials was assessed at the site of the earliest ventricular activation both during sinus rhythm and during ventricular arrhythmias (Figure 1A). In the setting of infrequent ventricular ectopy or if a targeted VT was not hemodynamically tolerated, pace mapping was used to identify the exit site of VT or the site of origin of a PVC. A matching pace-map was defined as a pace-map that matched the QRS complex of a targeted arrhythmia in at least 11/12 leads (Figure 1B).
Figure 1.
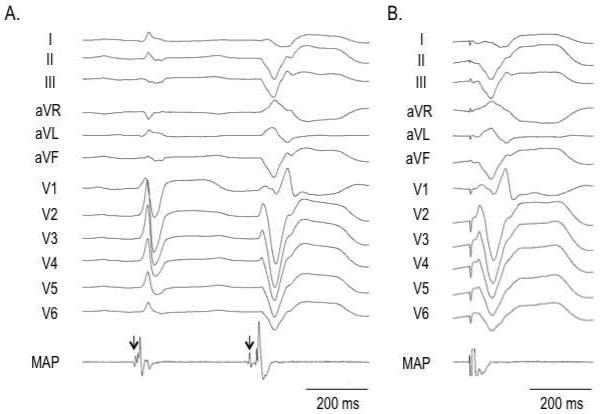
A: The site of origin of a premature ventricular complex (PVC) from the posteromedial papillary muscle (PAP). During sinus rhythm, a Purkinje potential (arrow) is present 8 ms before the onset of QRS complex. A Purkinje potential (arrow) precedes the QRS complex of the PVC by 26 ms.
B: Matching pace-map at the site of origin of the PVC in the posteromedial papillary muscle.
Catheter stability was assessed with intracardiac echocardiography (Cypres, Acuson Inc., Mountain View, CA) before and during radiofrequency (RF) ablation. RF energy was delivered via a 3.5-mm Thermocool Navistar™ (Biosense Webster) open-irrigation, deflectable tip catheter at sites with the earliest endocardial activation during the ventricular arrhythmias and/or at matching pace mapping sites. RF energy was applied at a power ranging between 30 and 50 W, with a maximum temperature of 45°C. Programmed ventricular stimulation was repeated after ablation with and without isoproterenol infusion at the end of the procedure. If catheter stability was compromised due to ventricular arrhythmias during the ablation procedure, we first attempted to pace the right ventricle at a rate slightly faster than the arrhythmia cycle length caused by RF energy delivery. If this failed to suppress the arrhythmias, cryoablation with a 6-mm Cryocatheter (Medtronic Inc, Minneapolis, MN) was used. For cryoablation, the target temperature was −80°C with a duration of 4 minutes.
For PVCs originating from a PAP, a ≥80% acute and permanent reduction of the initial PVC burden was considered an effective ablation procedure. For VT originating from a PAP, acute and permanent elimination of the VT was considered an effective procedure.
At effective ablation sites, the presence of Purkinje potentials and matching pace-maps was assessed. In patients with ineffective procedures, the presence of Purkinje potentials and matching pace-maps was assessed at the site with the earliest endocardial activation.
Cardiovascular Magnetic Resonance Imaging
In all patients without contraindications, cardiovascular magnetic resonance (CMR) imaging was performed using a 1.5-T magnetic resonance imaging scanner (Signa Excite CV/i, General Electric Medical Systems, Milwaukee, WI) with a 4- or 8-element phased-array coil placed over the chest of patients in the supine position. Images were acquired with ECG gating during breath-holds. Dynamic short- and long-axis images of the heart were acquired using a segmented, k-space, steady-state, free-precession pulse sequence (repetition time 4.2 ms, echo time 1.8 ms, 1.4×1.4-mm in plane-spatial resolution, 8-mm slice thickness). End-diastolic volume and end-systolic volume were automatically computed from contours by summation of data from multiple slices, and ejection fraction was calculated from the ventricular volumes. Epicardial contours were drawn at end-diastole at the outside border of the left ventricle to calculate the left ventricular (LV) mass.
The mass of the left sided PAPs were evaluated by CMR. Individual PAPs in the left ventricle were defined on the short-axis cine images as structures attached to the LV free wall and contiguous with the mitral valve via chordae tendineae (6,7). Ventricular trabeculations were defined as myocardium protruding more than 1.5 mm from the circumferential contour of the LV cavity with the same signal intensity as the adjacent LV wall (8), and were not included in the mass of PAPs. After identification of the primary anterolateral and posteromedial PAPs, the papillary muscles were traced manually on each end-diastolic frame with the Osirix (version 2.7.5, Geneva, Switzerland) Dicom Viewer software and the mass of each PAP was calculated after the exclusion of the LV wall and its trabeculations and the intertrabecular blood pool (Figure 2). Other smaller PAPs with chordal attachments to the valvular apparatus inserting into the ventricular wall were considered as accessory PAPs. The mass of the PAPs was calculated by 2 independent observers from the volumes based on a myocardial density of 1.05 g/cm (3,7). The observers were blinded to the outcome of the procedure. The morphological variations of the PAPs were also evaluated. A bifid PAP was defined as a PAP with >1 head on multiple cine-images (9).
Figure 2.
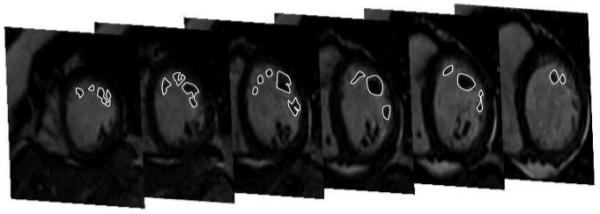
Stack of short-axis images in end-diastole show contouring of the left anterolateral papillary muscle (PAP) from base to apex. All intra-cavity structures that were unambiguously identifiable as muscle were defined as PAP and contributed to the assessment of the PAP mass. This patient had a failed ablation procedure. The PAP mass of the anterolateral PAP was 7.85 g.
Fifteen minutes after administration of 0.20 mmol/kg of intravenous gadolinium diethylenetriamine penta-acetic acid (Magnevist, Schering AG, Berlin, Germany), 2-dimensional delayed enhancement (DE) imaging was performed using an inversion-recovery sequence (repetition time 6.7 ms, echo time 3.2 ms, in-plane spatial resolution 1.4×2.2 mm, slice thickness 8 mm) in the short axis and long axis of the left ventricle at matching cine-image slice locations. The inversion time (250 to 350 ms) was optimized to null the normal myocardium. The presence of DE in the LV wall and PAPs was assessed using a threshold of ≤6 SD greater than the mean signal intensity of the nulled myocardium (10,11). In 14 patients, the CMR was repeated 10±6 months post ablation to assess for new areas of DE. The volume of DE was quantified and related to the ablation lesions (Figure 3) (12).
Figure 3.
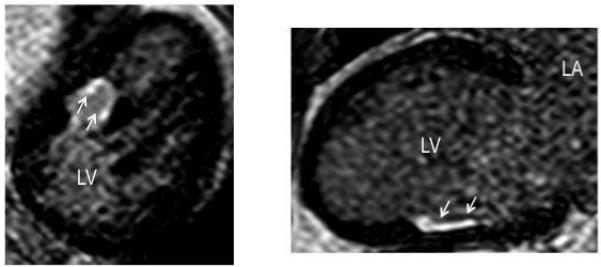
An oblique reformatted image of the mid-left ventricle (Left panel) and a long-axis view (Right panel) on delayed enhancement (DE) cardiovascular magnetic resonance imaging 41 months after ablation in a patient with failed ablation of an arrhythmia originating from the posteromedial papillary muscle (PAP). The majority of the lesion is confined to the free-wall surrounding the PAP.
Follow-up
If the procedure was acutely effective, antiarrhythmic drug therapy was discontinued. If the procedure was acutely ineffective, treatment with the prior medication was continued. Patients returned to our ambulatory clinic at 3-6 month intervals and underwent 24-h Holter monitoring and transthoracic echocardiography. Patients with ICDs were seen at 3-6 months intervals in the device clinic.
Statistical Analysis
Continuous variables, expressed as means±SD, were compared using Student’s t-test. One-way analysis of variance was followed by Scheffé’s method to test the significance of differences between the means. Categorical variables were compared with the Chi square test. If the expected sample size was smaller than 5 cells, Fisher’s exact test was used. A P value <0.05 was considered significant.
Results
Clinical characteristics (Table 1)
Table 1.
Clinical Characteristics
| Effective Ablation (n=31) |
Ineffective Ablation/Recurrence (n=9) |
P value | |
|---|---|---|---|
| Age, years | 53±14 | 42±11 | 0.02 |
| Male, n (%) | 21 (68) | 4 (44) | 0.26 |
| Left ventricular ejection fraction, % | 45±14 | 50±9 | 0.36 |
| Structural heart disease, n (%) | 17 (55) | 5 (56) | 1.0 |
| Prior-myocardial infarction, n (%) | 8 (26) | 2 (11) | |
| Dilated cardiomyopathy, n (%) | 8 (26) | 1 (11) | |
| Valvular heart disease, n (%) | 1 (3) | 2 (22) | |
| Hypertension, n (%) | 10 (32) | 1 (11) | 0.40 |
| Nonsustained VTs/PVCs, n | 14/17 | 7/2 | 0.13 |
| PVC burden, % | 14±12 | 19±16 | 0.40 |
Values are means±SD. PVC=premature ventricular complex; VT=ventricular tachycardia.
RF ablation was acutely effective in eliminating the targeted ventricular arrhythmias in 33 of 40 patients (83%). Two patients had recurrent arrhythmias during follow-up after an initial effective ablation and these patients were analyzed together with the patients in whom the procedure was acutely ineffective. Except for a significantly higher age in the effective group, the baseline characteristics of the study groups were similar.
Electrophysiological and Echocardiographic Findings
Table 2 shows the characteristics of the electrophysiological findings and ablation procedures in each study group. At the beginning of the procedure, programmed ventricular stimulation induced sustained VTs in 3 of 10 patients (30%) with prior myocardial infarction and 2 of 9 patients (22%) with dilated cardiomyopathy. The site of origin was in the left ventricle in 32 patients and in the right ventricle in 8 patients. It was located on a LV PAP in 23 patients (74%) with effective ablations and in 9 patients with failed ablations (P=0.22). Twenty-six patients (84%) with effective ablations and 5 patients (56%) with ineffective ablations had pleomorphic PVCs (≥ 1 PVC morphology), ranging from 2 to 15 different PVC morphologies (P=0.17). There was no significant difference in the number of pleomorphic PVCs in the effective versus the ineffective ablation group (4±3 vs 4±5; P=0.60). Three patients in the group with effective ablations had pleomorphic PVCs originating from 2 different PAPs (LV anterolateral and posteromedial in 2, righ ventricular posterior and septal in 1).
Table 2.
Electrophysiological Findings
| Effective Ablation (n=31) |
Ineffective Ablation/Recurrence (n=9) |
P value | |
|---|---|---|---|
| Site of origin of PAP arrhythmias | 0.16 | ||
| Left ventricle, n (%) | 23 (74) | 9 (100) | |
| Anterolateral PAP, n | 9 | 4 | |
| Posteromedial PAP, n | 16 | 5 | |
| Right ventricle, n (%) | 8 (26) | 0 (0) | |
| Anterolateral PAP, n | 4 | 0 | |
| Posteromedial PAP, n | 4 | 0 | |
| Septal muscle, n | 1 | 0 | |
| Pleomorphic PVCs, n (%) | 26 (84) | 5 (56) | 0.17 |
| Number of PVC morphology, n | 4±3 | 4±5 | 0.60 |
| Inducibility of VT, n (%) | 4 (13) | 0 (0) | 0.56 |
| PP at ablation site, n (%) | 15 (48) | 0 (0) | 0.02 |
| S-QRS interval at ablation site | 30±14 | 26±4 | 0.50 |
| Amplitude during sinus rhythm, mV | 1.3±0.9 | 1.0±0.6 | 0.50 |
| Activation time, msec | −31±18 | −28±7 | 0.66 |
| Matching pace map, n (%) | 22 (71) | 2 (22) | 0.02 |
| RF induced arrhythmias, n (%) | 27 (87) | 9 (100) | 0.56 |
| Number of RF application, n | 27±22 | 42±19 | 0.08 |
| Total RF duration, min | 22±14 | 33±9 | 0.03 |
| Procedure time, min | 305±90 | 387±110 | 0.03 |
Values are means±SD. PAP=papillary muscle; PP=Purkinje potential; RF=radiofrequency; PVC=premature ventricular complex; VT=ventricular tachycardia.
Intracardiac echocardiography was useful in identifying the PAP and confirming catheter stability on the PAP in all patients. Using intracardiac echocardiography, catheter dislocation from the targeted PAP occurred with delivery of RF energy secondary to bursts of non-sustained VT in 27 patients (87%) with effective ablation, and in all patients with an ineffective ablation procedure (p=0.6). Right ventricular pacing at a cycle length shorter than the non-sustained ventricular tachycardia was performed in 6 patients and in 2 patients in whom overdrive pacing failed to eliminate ventricular ectopy, cryoablation was performed.
Ablation
The local endocardial activation time at the site of origin was similar in effective and ineffective procedures (−31±16 ms vs −28±7 ms; P=0.7). In the effective ablation group, a matching pace-map at the site of origin of the targeted arrhythmia was more common compared to the group with ineffective procedures (71% vs 22%; P=0.02, Table 2).
Purkinje potentials at the site of origin were significantly more prevalent in patients with an effective ablation compared to patients with a failed ablations (48% vs 0%; P=0.01, Figure 1). The average voltage at ablation sites was equally low during sinus rhythm in patients with and without effective ablations (1.3±0.9 mV vs 1.0±0.6 mV; P=0.5).
In patients with effective ablation procedures, the duration of RF delivery was significantly shorter than in patients with ineffective procedures (22±14 min vs 33±9 min; P<0.03). The overall procedure duration was also significantly shorter in the effective group (305±90 min) as compared to the ineffective group (387±110 min; P=0.03).
Cardiovascular Magnetic Imaging
CMR images were obtained in 35 of 40 patients (88%) before ablation. In 27 of 35 patients, the PAP arrhythmias originated from the left ventricle and the remaining 8 from the right ventricle. The CMR findings (Table 3) were analyzed in the 27 patients with LV PAP arrhythmias. The mass of the arrhythmogenic PAP was significantly larger in the patients with failed ablations as compared to the patients with effective ablations (4.6±2.2 g vs 2.3±0.6 g; P<0.001, Figure 2). However, no significant difference was observed in the prevalence of bifid arrhythmogenic PAPs (60% vs 78%; P=0.43). At baseline, DE was identified on the arrhythmogenic PAP in 2 patients with an effective and in 1 patient with an ineffective procedure. A repeat CMR was performed in 14 patients after a mean of 10±6 months. New areas of DE were identified post ablation in 8 patients. The area of new DE was larger in patients with ineffective as compared to patients with effective procedures (Figure 3).
Table 3.
Cardiovascular Magnetic Resonance Imaging
| Effective Ablation (n=18) |
Ineffective Ablation/Recurrence (n=9) |
P value | |
|---|---|---|---|
| Body surface area, m2 | 2.1±0.4 | 2.2±0.4 | 0.68 |
| Left ventricular mass, g | 136±42 | 112±43 | 0.19 |
| Left ventricular end-diastolic volume, ml | 197±49 | 193±51 | 0.88 |
| Left ventricular ejection fraction, % | 41±18 | 52±8 | 0.13 |
| Total PAP mass, g | 5.3±1.8 | 9.1±4.8 | <0.01 |
| Arrhythmogenic PAP mass, g | 2.3±0.6 | 4.7±2.2 | 0.0001 |
| Bifid arrhythmogenic PAP, n (%) | 12/20 (60) | 7/9 (78) | 0.43 |
| Delayed enhancement prior to ablation | |||
| - Lesion on LV free wall (%) | 6 (33) | 1 (11) | 0.36 |
| - Lesion on PAP (%) | 2 (11) | 1 (11) | 1.0 |
| Assessment of ablation lesions | |||
| Lesion volume, cm3 | 0.5±0.6 | 2.1±1.2 | 0.046 |
| Endocardial lesion area, cm2 | 1.2±1.3 | 3.0±2.2 | 0.19 |
| Lesion depth, cm | 0.3±0.3 | 0.8±0.2 | 0.02 |
Abbreviations as above; Values are means±SD. DE=delayed enhancement
Follow-up
Total follow-up time was 32±20 months. At 3 months post-ablation, the PVC burden on 24-h Holter was significantly reduced in patients with effective (15±11% to 3±3%; P<0.01), but not in patients with ineffective procedures (20±16% to 15±11%; P=0.19). Two patients with initially effective procedures had recurrent arrhythmias 1 and 3 months post-ablation. These 2 patients and 4 patients with acutely ineffective ablation underwent a repeat ablation procedure. Five of the 8 patients who underwent a repeated procedure had a successful outcome during a mean follow-up of 14±11 months after the ablation.
Discussion
Main Findings
Patients with failed ablation procedures for arrhythmias originating from PAPs had a larger mass of the arrhythmogenic PAP compared to patients with effective procedures. Furthermore, Purkinje potentials and matching pace-maps at the earliest endocardial activation sites were more often present in case of effective compared to ineffective procedures.
Morphology and Mass of PAP
The larger mass of PAPs in patients with failed ablation procedures suggests that the site of origin in some patients might be within the PAP and not reachable from the endocardial surface. This is further supported by the fact that Purkinje potentials, which are identified on the endocardial surface, were not recorded at sites with the earliest endocardial activation in patients with failed procedures. Single RF lesions eliminated PAP arrhythmias in patients in whom Purkinje potentials were identified. In patients with unsuccessful procedures, Purkinje potentials were not identified at any of the target sites. Also, in patients with ineffective procedures, more ablation lesions were delivered. However, most of the patients with a failed initial ablation had a subsequently successful ablation during the repeat procedure. Pace-mapping also was less helpful in patients with an ineffective procedure compared to patients with effective ablations, suggesting that a deeper focus might have been the cause of the pace-map mismatches. The data suggest that PAP arrhythmias can originate at the surface of a PAP or from within the body of the PAP.
Presence of Structural Heart Disease
There were no differences in electrophysiological and MRI parameters between patients with and without structural heart disease. The presence of Purkinje potentials at effective ablation sites in patients with and without structural heart disease points towards a key role of the Purkinje fiber system in arrhythmias originating from PAPs. Regardless of the presence of structural heart disease, matching pace-maps identified the site of origin of PAP arrhythmias. The data suggest that the determinants of effective ablation are similar in patients with and without structural heart disease.
Catheter Stability
With respect to catheter stability, there was no difference between both groups. Catheter dislocation was seen as often in patients with as compared to patients without effective ablation procedures. Intracardiac echocardiography was essential in documenting adequate catheter contact. It is also important to realize that catheter stability is imperative and that the measures used in this study (overdrive right ventricular pacing and cryoablation) to assure catheter stability, may need to be employed to assure an effective ablation procedure.
Study limitations
This study has several limitations. For assessment of the papillary muscle mass we assessed only the left ventricular PAP mass. Right sided papillary muscle mass is difficult to accurately assess due to the presence of prominent trabeculations (13). Therefore, we limited our analysis of the PAP mass to left ventricular PAPs where criteria to differentiate PAP from trabeculations have been described (13).
A single definite reason for ablation failure is difficult to ascertain. The inability to eliminate a particular arrhythmia can have multiple reasons: failure to identify the site of origin, failure to reach the site of origin or failure to deliver enough radiofrequency to the site of origin. Based on mapping results, the earliest activation times were always identified on the PAPs and the best pace-maps were on the PAPs, indicating that the site of origin was located within the PAP. Determination whether or not the earliest recorded site is the site of origin is more difficult, especially if ablation there fails to eliminate the arrhythmia. The possibility that the site of origin on the endocardium was missed cannot be ruled out. Lastly, because only 2 patients underwent cryoablation, it is unclear whether the need for cryoablation was a predictor of outcome.
Conclusion
In a patient with PAP arrhythmias, preprocedural imaging can help to assess the PAP mass of the arrhythmogenic PAP. A larger PAP mass is often present in patients with failed ablation procedures. If Purkinje potentials or a matching pace-map at the earliest endocardial activation can be identified, a successful ablation outcome is likely.
Table 4.
Electrophysiological Findings and Cardiovascular Magnetic Resonance Imaging
| Effective ablation (n=31) | Ineffective ablation/Recurrence (n=9) | |||||
|---|---|---|---|---|---|---|
| Structural heart disease (n=17) |
No Structural heart disease (n=14) |
P Value | Structural heart disease (n=3) |
No Structural heart disease (n=6) |
P Value | |
| Electrophysiological findings | ||||||
| Inducibility of VT, n (%) | 4 (24) | 0 (0) | 0.11 | 0 (0) | 0 (0) | 1.0 |
| PP at ablation site, n (%) | 10 (59) | 5 (36) | 0.29 | 0 (0) | 0 (0) | 1.0 |
| S-QRS interval at ablation site | 34±17 | 25±6 | 0.30 | 28±1 | 26±5 | 1.0 |
| Amplitude during sinus rhythm, mV | 1.1±0.9 | 1.5±0.9 | 0.61 | 0.9±0.6 | 1.1±0.7 | 0.99 |
| Activation time, msec | −37±21 | −23±8 | 0.13 | −33±7 | −26±6 | 0.93 |
| Matching pace map, n (%) | 12 (71) | 10 (71) | 1.0 | 0 (0) | 2 (33) | 0.50 |
| RF induced arrhythmias, n (%) | 15 (88) | 12 (86) | 1.0 | 3 (100) | 6 (100) | 1.0 |
| Cardiovascular magnetic resonance imaging | ||||||
| Left ventricular ejection fraction,% | 38±17 | 56±16 | 0.23 | 51±1 | 52±9 | 1.0 |
| Total PAP mass, g | 5.5±2.0 | 5.0±1.7 | 0.99 | 7.0±2.0 | 10.1±5.6 | 0.58 |
| Arrhythmogenic PAP mass, g | 2.5±0.8 | 2.3±0.5 | 0.99 | 3.6±1.4 | 5.2±2.4 | 0.43 |
Abbreviations: as above; Vales are mean±SD.
Acknowledgments
Drs Bogun was supported by a grant from the Leducq foundation, Dr Desjardins was supported by an NIH grant (EB 006481)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim YH, Xie F, Yashima M, et al. Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation. 1999;100:1450–9. doi: 10.1161/01.cir.100.13.1450. [DOI] [PubMed] [Google Scholar]
- 2.Good E, Desjardins B, Jongnarangsin K, et al. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: A comparison with fascicular arrhythmias. Heart Rhythm. 2008;5:1530–7. doi: 10.1016/j.hrthm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Bogun F, Desjardins B, Crawford T, et al. Post-infarction ventricular arrhythmias originating in papillary muscles. J Am Coll Cardiol. 2008;51:1794–802. doi: 10.1016/j.jacc.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Doppalapudi H, Yamada T, McElderry HT, et al. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol. 2008;1:23–9. doi: 10.1161/CIRCEP.107.742940. [DOI] [PubMed] [Google Scholar]
- 5.Crawford T, Mueller G, Good E, et al. Ventricular arrhythmias originating from papillary muscles in the right ventricle. Heart Rhythm. 2010 doi: 10.1016/j.hrthm.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Harrigan CJ, Appelbaum E, Maron BJ, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:668–73. doi: 10.1016/j.amjcard.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Vogel-Claussen J, Finn JP, Gomes AS, et al. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr. 2006;30:426–32. doi: 10.1097/00004728-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Papavassiliu T, Kuhl HP, Schroder M, et al. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology. 2005;236:57–64. doi: 10.1148/radiol.2353040601. [DOI] [PubMed] [Google Scholar]
- 9.Kwon DH, Setser RM, Thamilarasan M, et al. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008;94:1295–301. doi: 10.1136/hrt.2007.118018. [DOI] [PubMed] [Google Scholar]
- 10.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 11.Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilg K, Baman T, Gupta S, et al. Assessment of Radiofrequency Ablation Lesions by Magnetic Resonance Imaging After Ablation of Idiopathic Ventricular Arrhythmias. 2009 doi: 10.1016/j.jcmg.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Axel L. Papillary muscles do not attach directly to the solid heart wall. Circulation. 2004;109:3145–8. doi: 10.1161/01.CIR.0000134276.06719.F3. [DOI] [PubMed] [Google Scholar]


