Abstract
The Hippo Pathway regulates organ size and tumorigenesis in Drosophila and mammals and is altered in a variety of human cancers, yet it remains unclear if the Hippo Pathway is of prognostic significance to cancer patients. Here we show that Yap, the human homolog of the key transcriptional target of the Hippo pathway, plays a conserved role in promoting tumorigenesis in both the fly ovary and human ovarian cancer. While studies linking Yap to cancer in other tissues have focused on overall Yap levels, we demonstrate for the first time that subcellular levels of Yap show an exceptionally strong association with poor patient survival. Specifically, high levels of nuclear Yap, or low levels of cytoplasmic phosphorylated-Yap, associated with poor survival from ovarian cancer. Patients with both high nuclear Yap and low cytoplasmic phosphorylated-Yap had approximately 50% lower 5-year survival, and this combination is an independent prognostic marker for survival, with an exceptionally high hazard ratio of 7.8. We find that Yap2 is the predominantly expressed Yap isoform in both the ovarian surface epithelium and epithelial ovarian cancers. Overexpression of Yap2 and phosphorylation-defective Yap2-5SA in immortalized ovarian surface epithelium cells resulted in increased cell proliferation, resistance to cisplatin-induced apoptosis, faster cell migration, and anchorage independent growth, while Yap knockdown resulted in increased sensitivity to cisplatin-induced apoptosis. Findings argue that the Hippo signaling pathway defines an important pathway in progression of ovarian cancer.
Keywords: Drosophila Follicular Epithelium, Human Ovarian Cancer, Yap, Hippo Pathway, Basolateral Junction Signaling
Introduction
Epithelial ovarian cancer is the most lethal gynecologic malignancy and is the fifth most prevalent cause of cancer death in women in the United States (1). Due to the internal localization of the ovaries, lack of specific symptoms, and lack of effective screening methods, ovarian cancer usually remains undetected until it has reached an advanced stage (2). Nearly 70% of patients present with late stage disease that has spread to other organs in the abdominal cavity and the 5 year survival for these patients remains at only 30%(1). The current standard of care includes surgical resection of the tumor followed by treatment with platinum- and taxane-based chemotherapies (3).
An important step in the development of more targeted and personalized treatments for ovarian cancer is identification of molecules involved in its development and progression. One method for identifying such molecules is through the study of model organisms. Several laboratories have developed mouse models of ovarian cancer through targeted disruption or expression of candidate ovarian cancer tumor suppressors and oncogenes in the proposed site of origin of ovarian cancer- the ovarian surface epithelium (OSE) (4). Although useful for the characterization of candidate molecules, the mammalian ovary does not lend itself to large-scale forward genetic screens that can identify new molecules.
We hypothesized that the Drosophila ovary might serve as a powerful genetic model to screen for molecules disrupted inhuman ovarian cancer. Mutations in basolateral junction proteins discs large, lethal giant larvae, and scribble in the fly ovary result in tumor-like phenotypes, including overproliferation, loss of cell polarity, and invasion (5–7). In order to identify new molecules involved in ovarian tumorigenesis, we performed a large-scale genetic modifier screen for genes that enhance the discs large ovarian tumor phenotype, and identified warts. Further analysis revealed that warts loss alone caused tumors in the Drosophila ovary (7).
Warts is a kinase that regulates organ size and tumorigenesis in many fly tissues. Warts acts in a network of tumor suppressors that encode receptor, scaffolding, and signaling molecules, collectively known as the Hippo pathway, whose crucial function is to repress oncogene Yki, a transcriptional coactivator (8). In mammals, Warts homologs Lats-1 and Lats-2 phosphorylate the Yki homolog Yap (Yes associated protein (9)) at S127, allowing 14-3-3 to bind Yap and retain it in the cytoplasm, thus blocking Yap’s ability to coactivate transcription in the nucleus (8). Yap is located in a genomic region, 11q22, which is amplified in a variety of cancers including ovarian cancer, and Yap levels predict patient outcome in hepatocellular carcinoma (11, 12). Further, Yap overexpression in the murine liver causes massive liver overgrowth and tumorigenesis (10).
Here we report that overexpression of human Yap induces tumorigenesis in the Drosophila ovary, suggesting that it plays a conserved role in ovarian tumorigenesis. Consistent with a role for Yap in human ovarian cancer, we found that high nuclear Yap (nYap) and low cytoplasmic phosphorylated-S127-Yap (cpYap) are associated with poor survival. Further, we found that overexpression of Yap2, or a phosphorylation resistant allele of Yap2, Yap2-5SA, in immortalized OSE cells (IOSE) resulted in increased proliferation, resistance to cisplatin-induced apoptosis, loss of contact inhibition, increased cell migration, and anchorage independent growth. Together, these findings indicate that Yap acts as an oncogene in ovarian cancer by promoting disease progression and development of chemoresistance.
Materials and Methods
Drosophila
Yap overexpressing flies were constructed using the FLP-out technique. Adult female y,w,hs-FLP; act>y+>Gal4, UAS-GFP/UAS-Yap-S127A flies were heat shocked at 37 °C for 2 min, then incubated at 25 °C for 3 days. Ovaries were dissected and processed as described (7).
Human Tissue Samples
The tissue microarray (TMA) was comprised of 2 blocks with cores in duplicate from formalin fixed, paraffin embedded archival primary ovarian carcinoma samples from 70 patients (median age 59.8, range 22–93)(Table 1). The patients had been treated at the Massachusetts General Hospital from 1989–2004 under an IRB approved protocol. H&E sections of all samples were reviewed by a certified gynecologic pathologist who selected tumor cores.
Table 1.
Distribution of clinical characteristics of ovarian cancer patients included in the study.
| N | % | |||
|---|---|---|---|---|
| Total |
70 | 100.0 | ||
| FIGO Stage |
||||
| Stage I | 23 | 32.9 | ||
| Stage II | 11 | 15.7 | ||
| Stage III | 29 | 41.4 | ||
| Stage IV | 7 | 10.0 | ||
| Tumor Type |
||||
| Serous | 26 | 37.1 | ||
| Clear Cell | 17 | 24.3 | ||
| Endometriod | 17 | 24.3 | ||
| Mucinous | 6 | 8.6 | ||
| Transitional | 4 | 5.7 | ||
| Tumor Grade |
||||
| Grade 1 | 7 | 10.0 | ||
| Grade 2 | 22 | 31.4 | ||
| Grade 3 | 39 | 55.7 | ||
| Missing | 2 | 2.9 | ||
| nYap |
||||
| Low | 42 | 60.0 | ||
| High | 25 | 35.7 | ||
| Missing | 3 | 4.3 | ||
| cpYap |
||||
| Low | 39 | 55.7 | ||
| High | 28 | 40.0 | ||
| Missing | 3 | 4.3 | ||
| nY/cpY Category |
||||
| nYap | cpYap | |||
| 0 | low | high | 22 | 31.4 |
| 1 | low | low | 19 | 27.1 |
| 2 | high | high | 6 | 8.6 |
| 3 | high | low | 18 | 25.7 |
| Missing | 5 | 7.1 | ||
nY/cpY Category 0 =low nYap, high cpYap, 1 = low nYap, low cpYap, 2 = high nYap, high cpYap, and 3=high nYap, low cpYap. FIGO = International Federation of Gynecology and Obstetrics.
Immunohistochemistry
Normal ovary sections and the ovarian cancer TMA were analyzed by immunohistochemistry as described (13). No antigen retrieval was used for anti-Yap 1:100 (H-125, Santa Cruz Biotechnology, Inc.), and 0.01M citrate buffer (pH 6.0) was used for anti-phosphorylated-S127-Yap 1:50 (4911S, Cell Signaling Technologies). Immunostained slides were evaluated for both nuclear and cytoplasmic Yap and pYap as described (14) by three independent scorers, TW (certified pathologist), CH, and SO or SG, who were blinded to the data. Scores were combined using 2/3 majority. Proportion scores did not vary considerably across samples and were not analyzed. Intensity scores 0 (none) and 1 (weak) were assigned to the low category, and 2 (intermediate) and 3 (strong) were assigned to the high category for both Yap and pYap.
Cell Lines
All cell lines were grown in a 1:1 mixture of MCDB 105 medium (Sigma-Aldrich Co., M6395) and Medium 199 (Sigma-Aldrich Co., M5017) supplemented with 10% FBS, 25ug/ml gentamycin, 10 U/ml Penicilin, and 10 ng/ml streptomycin. TheIOSE-80 cell line (15), which normally senesces around passage 20, was immortalized by infection with a hTERT expressing retrovirus (Addgene Plasmid #1774), followed by selection with 100 ug/ml G418 for 10 days. The resulting cell line, 80T, is able to grow for at least 90 passages. Yap knockdown lines were made by infecting 80T cells with pGIPZ lentiviruses from Open Biosystems per manufacturer instructions followed by selection with 1ug/ml puromycin for 10 days. shYap-7 corresponds to plasmid Cat. No. RHS4430-98818907, shYap-8 to RHS4430-98525388, and shYap-9 to RHS4430-98893379. Yap overexpressing cell lines were made by infecting 80T cells with pQCXIH-myc retroviruses containing Yap2 or Yap2-5SA (16), followed by selection with 200 μg/ml hygromycin for 10 days. The approximate percentage of cells in each culture expressing the indicated constructs after selection was: Nontargeting (90%), shYap7 (50%), shYap8 (80%), shYap9 (80%), empty vector (n/a), Yap2 (60%), and Yap2-5SA (90%).
Sequence Analysis
Whole RNA was isolated from tumors and reverse transcribed using the iScript cDNA synthesis kit (BioRad). Yap fragments were PCR amplified from cDNAs. T7 or T3 sequence was added upstream of the following Yap specific primers: Yap Left Forward: CCGGGCATCAGATCGTGCA; Yap Left Reverse: GCTGAAGCCGAGTTCATCAT; Yap Right Forward: TTCAGCCATGAACCAGAGAA; Yap Right Reverse: AGTCTGCCTGAGGGCTCTA. PCR products were sequenced (LoneStar Labs) using T7 and T3 primers and analyzed for mutation using Sequencher software.
Western Blot Analysis
Cells were harvested in RIPA buffer (Santa Cruz Biotechnology Inc., sc-24948), subjected to SDS-PAGE, and processed per antibody manufacturer instruction (BD Biosciences). Antibodies used: anti-Yap 1:500 (H-125, Santa Cruz Biotechnology, Inc.), anti-phosphorylated-S127-Yap 1:500 (4911S, Cell Signaling Technologies), anti-cleaved-Caspase-3 1:1000 (9661S, Cell Signaling Technologies), anti-Caspase-8 1:1000 (3-1-9, BD Biosciences), anti-Lats-1 1:200 (A300-478A, Bethyl Laboratories Inc.), anti-Lats-2 1:200 (ST-3D10, CycLex Corp.), anti-actin 1:40,000 (AC40, Sigma Aldrich).
Immunofluorescence and Imaging
Cell lines and Drosophila ovaries were stained as described (7) with anti-myc 1:500 (9E10, BD Biosciences), Alexa-647 conjugated phalloidin 1:100 (Invitrogen), and propidium iodide. Tissues were visualized using a Zeiss LSM confocal microscope, and images were minimally adjusted for brightness and contrast using the Zeiss LSM510 Image Browser software.
Cell Proliferation Assays
For the cell count assay, 10,000 cells were plated in triplicate in 12 well plates and counted on days 1 and 6 (prior to confluence) using a Beckman Coulter Z1 Particle Counter. To establish a mitotic index, 10,000 cells were plated on cover slips, grown for 4 days, and stained with propidium iodide. Twenty images were captured per genotype and the mitotic index (number of nuclei with condensed chromosomes divided by total number of nuclei) was scored.
Apoptosis Assay
Cells were incubated in medium containing 30 μM Cisplatin (Alexis Biochemicals, 400-040-M050) for 48 hours. Floating and adherent cells were harvested for western analysis.
Scratch Assay
2.5 ×106 cells were plated in T25 flasks with medium containing 10 ug/ml Mitomycin C to block proliferation. Twelve hours later, cells were scratched using a 200 μL pipette and media was replaced. Images of 10 scratches per cell line were captured after 30 minute recovery and at the same location 10 hours later. Scratch areas were measured using Ziess AxioVision Software 4.6.
Soft Agar Assay
5,000 cells were plated in complete media plus 0.5% agar in 6 well plates in triplicate. Media was replaced every 3 days. After 14 days, cells were stained with 2 ml of 0.5 mg/ml MTT (dimethylthiazol diphenyltetrazolium bromide) for 2 hrs. Visible colonies were counted.
Statistical Analysis
For tissue microarray data, clinical and immunohistochemistry variables were summarized descriptively (Table 1). For the 65 subjects with complete IHC and outcome data, disease specific survival, truncated at 5 years, was estimated by the Kaplan-Meier method, and compared across groups (see below) using the log-rank test. Patients that survived, dropped out of the study, or died for reasons unrelated to cancer were censored at the lesser of the last follow-up time or 5 years. The contribution of dichotomized nYap (L=0,1 vs H=2,3), dichotomized cpYap (L=0,1 vs H=2,3), or their combined dichotomized nY/cpY Category (3vs 0,1,2), independent of other clinico-pathologic variables, was analyzed by stepwise Cox proportional hazards regression modeling. The following clinicopathologic variables were considered (categorized as indicated): Stage (1,2,3,4 treated as a single ‘continuous’ variable because univariate analysis indicated that this was a reasonable representation), dichotomized Grade (H=3 vs other including ungraded (n=2)), dichotomized histology (serous vs other). Cell culture experiments were done at least in triplicate and repeated at least three times. Western and immunofluorescence experiments were repeated at least three times. Student’s Paired t test was used to compare each measured value to control. P values <.05 were considered significant.
Results
Human Yap-S127A induces tumorigenesis in the Drosophila ovary
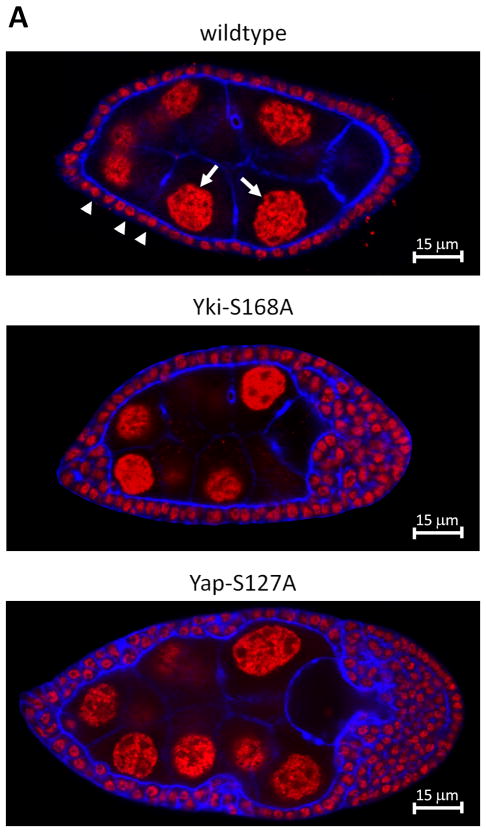
Loss of function clones of warts in the fly ovarian follicular epithelium results in tumor formation (7). We thus tested if the key Warts target, Yki, also induces tumors. Using the flp-out technique, we overexpressed wildtype Yki and phosphorylation resistantYki-S168Ain the Drosophila ovarian follicular epithelium (not shown and Fig. 1A), and found that Yki-S168A, but not wildtype Yki, induced tumorigenesis. To test if the human homolog of Yki, Yap, might play a conserved role in ovarian tumorigenesis, we overexpressed wildtype Yap and a phosphorylation resistant allele of Yap, Yap-S127A, in the Drosophila ovarian follicular epithelium (not shown and Fig. 1). Similar to Yki, expression of wildtype Yap had no effect, while expression of Yap-S127A resulted in overproliferation and loss of epithelial architecture beginning at mid-oogenesis. We conclude that the oncogenic capacity of Yki in the fly ovary is conserved in human Yap.
Figure 1.
Drosophila Yki-S168A or human Yap-S127A overexpression induces tumorigenesis in the Drosophila ovary. A. Confocal section through wildtype Drosophila egg chamber (upper) stained with propidium iodide to reveal nuclei and with phalloidin to reveal cell cortices. Follicle cells (small nuclei, arrow heads) form a monolayer epithelium around the germ cells (large nuclei, arrows). In egg chambers in which all follicle cells overexpress fly Yki-S168A (middle) or human Yap-S127A (lower) the follicular epithelium becomes multilayered and has supernumerary follicle cells.
Yap does not appear to have activating mutations in ovarian cancer
Since activating mutations in Yap are able to induce tumors in the fly ovary, we reasoned that Yap might be activated by mutation in ovarian cancer. Mutation of any of the five Lats consensus binding/phosphorylation sites in Yap (16) could result in constitutive activation of Yap. We sequenced these sites in cDNAs synthesized from whole cell RNA from 10 ovarian cancer cell lines, 2 IOSE cell lines, and 17 ovarian tumors, but did not detect any non-synonymous mutations. We conclude that Yap is not frequently mutated at any of the five Lats consensus binding/phosphorylation sites in ovarian cancer. We cannot rule out that Yap might be activated by mutation at low frequency and/or at other sites in ovarian cancer.
Yap is not overexpressed or under phosphorylated at S127 in ovarian cancer cell lines
Others have shown that Yap is overexpressed in hepatocellular carcinoma (12). To test if Yap is overexpressed in ovarian cancer, we performed western analysis on 10 ovarian cancer cell lines and two IOSE cell lines (Supplementary Fig. 1A). Using an antibody raised against a fragment of Yap1 that is contained in Yap2 and Yap2L, one prominent 65 kDa band corresponding to Yap2 (see below) was evident by western analysis. Yap2 was expressed in all cell lines and Yap2 levels varied to a similar extent in ovarian cancer cells and control cell lines. Since Yap is inhibited through phosphorylation at S127 by Lats-1 orLats-2 kinases (16), we also asked if Lats kinases are expressed and if Yap is phosphorylated in ovarian cancer cell lines (Supplementary Fig. 1A). Lats-1 and Lats-2 were expressed in all cell lines and pYap levels varied to a similar extent in ovarian cancer and control cell lines (Supplementary Fig. 1A). We conclude that Yap is not overexpressed and that Yap phosphorylation at S127 is not grossly misregulated in ovarian cancer cell lines.
Subcellular levels of Yap and pYap correlates with ovarian cancer survival
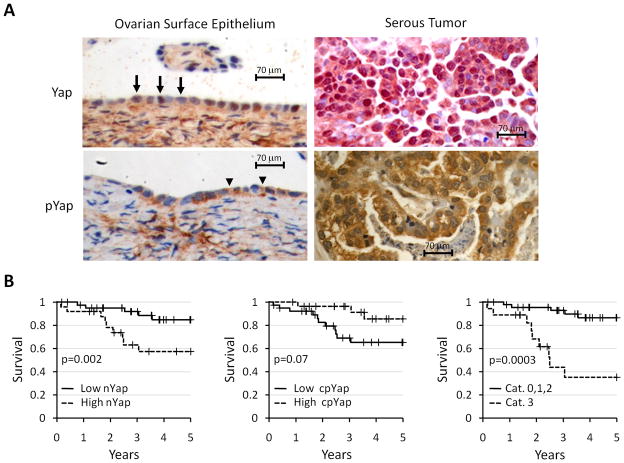
To further explore the possibility that Yap is misregulated in ovarian cancer, we reasoned that since cytoplasmic retention is the primary mechanism controlling Yap nuclear accumulation (8), Yap subcellular distibution might correlate with patient survival in ovarian cancer. After verifying the specificity of our Yap and pYap antibodies (Supplementary Fig. 1B), we established the normal expression pattern of Yap and pYap by staining normal OSE. Similar to a previous report, Yap staining was low or undetectable in most (5/7) samples (17). When Yap was expressed in the OSE, it localized predominantly to the nucleus (Fig. 2A) and pYap localized predominantly to the cytoplasm (Fig. 2A).
Figure 2.
Yap and pYap associate with disease-specific survival. A. OSE (left) and serous ovarian tumor (right) stained with Haematoxylin (blue) to reveal cell nuclei, and for Yap (top; brown in OSE, red in tumor) or pYap (bottom, brown). In OSE and tumors, Yap localizes primarily to the nucleus (arrows) and pYap localizes primarily to the cytoplasm (arrowheads). B. Kaplan-Meier estimates of disease-specific survival (truncated at 5 years) of patients with high verses low nYap (left), high versus low cpYap (middle), and nY/cpY Category 3 (high nYap and low cpYap) vs other nY/cpY Categories (0, 1, 2)(right)(see table 1). P-values from Log-rank tests are shown.
To determine if Yap distribution correlates with patient survival in ovarian cancer, Yap and pYap localization and levels were evaluated in 70 tumors on an ovarian cancer tissue microarray, which contained a mixture of serous, clear cell, endometrioid, mucinous, and transitional carcinomas (Table 1). Kaplan-Meier estimation and comparison of disease specific survival found high nuclear Yap (nYap) staining intensity (p=0.002) to be associated and low cytoplasmic pYap (cpYap) staining intensity (p=0.07) to be marginally associated with poor patient outcome (Fig. 2B). In contrast, cytoplasmic Yap staining intensity and nuclear pYap staining intensity were not associated. Although nYap staining intensity remained a significant independent marker for poor patient survival (p=.0048, HR=3.3, 95% CI=1.03–10.9) after considering other clinical factors (data not shown), we sought to determine if the combination of nYap and cpYap staining intensities showed an even stronger association with poor patient survival. We sorted each tumor into one of four categories based on the combination of nYap and cpYap staining intensities (Table 1). Cases with both high nYap and low cpYap (nY/cpY Category 3) did significantly worse than the other categories, which were combined since they were indistinguishable from each other on univariate survival analysis (Fig. 2B). Upon multivariate Cox regression analysis that considered stage, high grade, serous histology, dichotomized nYap score, dichotomized cpYap score, and the dichotomized nY/cpY Category (3 vs other), only stage (p<0.001, HR=7.0, 95% CI: 3.0–16.5) and nY/cpY Category (p=0.002, HR=7.8, 95% CI: 2.1–28.9) were significant. We conclude that the combination of high nYap and low cpYap is a strong independent predictor of disease-specific survival for ovarian cancer.
Yap overexpression promotes proliferation and resistance to apoptosis
Correlations between Yap staining and poor patient survival does not distinguish whether Yap merely correlates with ovarian cancer outcome or if Yap is an active driver of the disease. To determine if Yap is an active driver of ovarian cancer progression, we used an IOSE cell line, 80T as an in vitro model. cDNA sequencing (above) indicated that Yap2 was the predominantly expressed Yap isoform in NOSE, IOSE, and ovarian cancer cell lines. Yap1 and Yap2L transcripts were also detected but at relatively low levels.
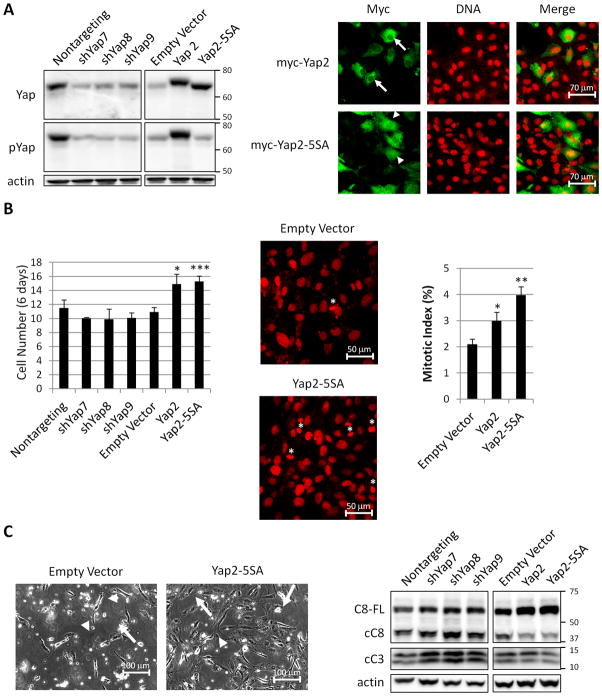
Therefore, to obtain IOSE cells with high nuclear Yap, as observed in ovarian tumors, we overexpressedYap2, which would be expected to increase nuclear Yap by mass action, and Yap2-5SA, which is resistant to cytoplasmic retention since it cannot be phosphorylated by Lats (Fig. 3A) (16). Additionally we knocked down Yap with shRNAs (Fig. 3A). Consistent with Yap2 being the predominantly expressed isoform in 80T cells, over expressed Yap2, which is myc-tagged, ran only slightly higher than the endogenously expressed isoform (Fig. 3A). We also determined the localization of Yap inYap2 and Yap2-5SAoverexpressing cell lines (Fig. 3A). Although a majority of overexpressed Yap localizes in the cytoplasm, there is more nuclear Yap in cells overexpressing Yap2 and Yap2-5SA compared to control cells (Fig. 3A and Supplementary Fig. 1B).
Figure 3.
Yap overexpression promotes proliferation and resistance to apoptosis. A. Western analysis (left) of Yap and pYap from 80T cells expressing nontargeting shRNA, shRNAs targeting Yap (shYap7,8,9), empty vector (overexpression control), Yap2-myc, and Yap2-5SA-myc. The blot on the right was exposed for shorter time. Immunofluorescence staining (right) for myc and DNA in 80T cells expressing Yap2-myc and Yap2-5SA-myc. For clarity, the fields were selected to contain fewer overexpressing cells than typically observed. A higher portion of overexpressed Yap2-5SA localizes to the nucleus (arrowheads) compared to overexpression of Yap2 (arrows). B. Quantification of cell number (left) in Yap knockdown and overexpressing cells after six days of growth. Control and Yap2-5SA cells stained for DNA (middle) to reveal condensed DNA of mitotic cells (asterics). Quantification of mitotic index (right). C. Light micrographs of cells treated with 30 μM cisplatin for 48 hours (left) to show morphology of adherent cells (arrows) and nonadherent cells (arrowheads). Western analysis (right) of full length Caspase-8 (C8-FL), cleaved Caspase-8 (cC8), and cleaved Caspase-3 (cC3) from cells treated with 30 μM cisplatin for 48 hours.*p<.05, **p<.01, and ***p<.001.
To determine if Yap regulates cell proliferation we compared cell number in Yap knockdown, overexpression, and control cells after six days in culture. 80T cells overexpressing Yap2 or Yap2-5SA contained significantly more cells than control, while cells knocked down for Yap showed no significant difference (Fig. 3B). The increase in cell number could be due to increased proliferation, decreased cell death, or both. To determine if Yap2 and Yap2-5SA overexpressing cells had increased proliferation, we established their mitotic index (Fig. 3B). Yap2 or Yap2-5SA overexpression resulted in significantly higher mitotic index compared to control, suggesting that these cultures were more proliferative. We asked if this effect might be mediated by the EGF family ligand Amphiregulin, a protein implicated in ovarian cancer, and that acts downstream of Yap in breast cells to promote proliferation (2, 18). Amphiregulin levels were unchanged in 80T cells overexpressing Yap2 or Yap2-5SA by both western and IHC analysis (not shown).
To determine if Yap expression decreases cell death, we challenged with cisplatin, one of the most commonly used ovarian cancer chemotherapeutic agents (3). After 48 hours of treatment, Yap2 or Yap2-5SA overexpressing cells remained adherent to the culture dish, while control and shYap cells rounded up and detached (Fig. 3C), suggesting that Yap2 overexpression protects from apoptosis. To test if Yap2 or Yap2-5SA cells have decreased apoptosis, we performed western analysis of initiator and effector caspases, Caspase-8 and -3, respectively (19). Yap knockdown resulted in higher levels of both cleaved Caspases, while overexpression of Yap2 and Yap2-5SA showed decreased levels of cleaved Caspase-8, increased levels of full length Caspase-8, and a small, but reproducible, decrease in cleaved caspase-3 (Fig. 3C). We conclude that overexpression of Yap2 or Yap2-5SA results in increased proliferation and decreased cisplatin-induced apoptosis, while Yap knockdown results in increased cisplatin-induced apoptosis.
Yap overexpression reduces contact inhibition and promotes movement and anchorage independent growth
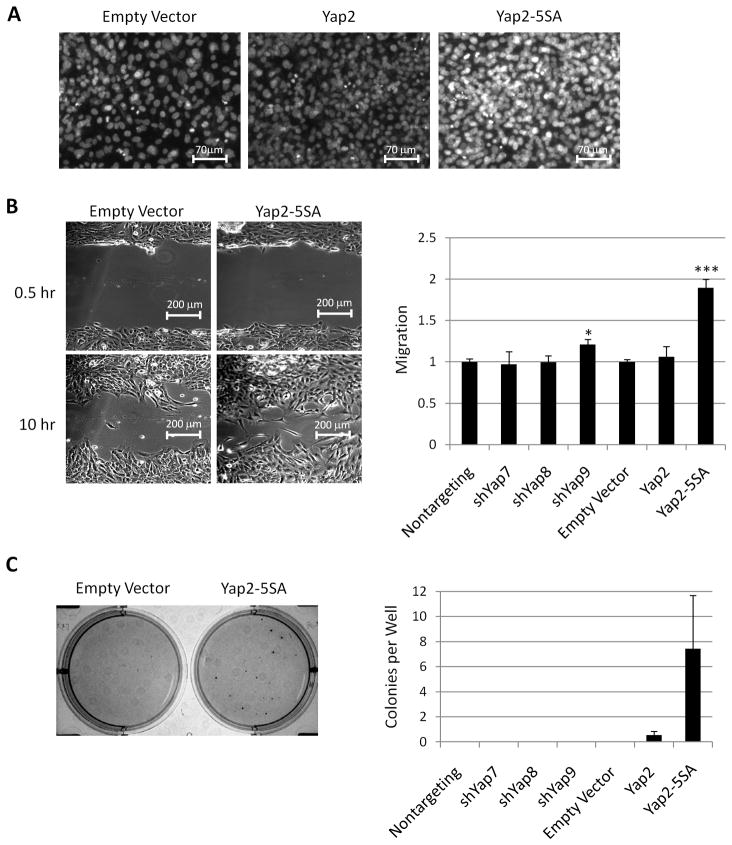
In addition to increased growth, aggressive tumor cells undergo epithelial to mesenchymal transition (EMT), lose contact inhibition, develop increased motility, and develop anchorage independent growth. We investigated these processes in Yap overexpressing and knockdown cells. We noticed that when Yap2 and Yap2-5SA overexpressing cells reached full confluence, cell density was higher (Fig. 4A), indicating loss of contact inhibition. We also found that overexpression of Yap2-5SA, but not overexpression of Yap2 or Yap knockdown, resulted in significantly faster wound closure (Fig. 4B). Further, cells overexpressing Yap2 and Yap2-5SA formed colonies in soft agar (Fig. 4C), while control cells and cells with diminished Yap were unable to grow. We conclude that Yap promotes loss of contact inhibition, cell migration, and growth in soft agar.
Figure 4.
Yap overexpression reduces contact inhibition and promotes wound closure and anchorage independent growth. A. Micrographs of confluent cultures stained for DNA to reveal cell density. Greater fluorescence intensity reveals multilayering of cells. B. Representative bright field micrographs (left) of control and Yap2-5SA cells 0.5 and 10 hours after wounding. Quantification of wound closure (right) after 10 hours. C. Representative micrograph of cell colonies visualized with MTT (left) after 14 days of growth in soft agar. Quantification of the number of colonies per well (right).*p<.05 and ***p<.001.
EMT is often associated with loss of contact inhibition, increased cell migration, and increased growth in soft agar and Yap has been shown to induce EMT in human mammary epithelial cells (20). However, 80T cells already appear mesenchymal (Fig 3A, C and Fig 4B), express mesenchymal markers (Fibronectin and Vimentin), and do not express epithelial markers (E-cadherin, Occludin, or β-Catenin)(not shown). Yap knockdown and Yap2 or Yap2-5SAoverexpression did not alter the expression of these markers (not shown). We conclude that Yap can promote loss of contact inhibition, cell migration, and growth in soft agar independent of EMT.
Discussion
We found that, similar to Yki, phosphorylation resistant human Yap is able to induce ovarian tumors when expressed in the Drosophila ovary, suggesting that the ability of Yap and Yki to induce ovarian tumorigenesis is conserved. Although we found no evidence of activating mutations in Yap in ovarian cancers, or for Yap overexpression in cell lines, we found that sub-cellular levels of Yap and pYap correlate and tend to associate, respectively, with survival of ovarian cancer patients. Consistent with a role for Yap in ovarian cancer progression, we find that overexpression of Yap2 and phosphorylation-defective Yap2-5SA in 80T cells resulted in several phenotypes associated with cancer including increased proliferation, resistance to cisplatin-induced apoptosis, faster cell migration, and anchorage independent growth, while knockdown of Yap in these cells resulted in increased sensitivity to cisplatin-induced apoptosis.
Paradoxically, Yap has been shown to act as both a tumor suppressor and as an oncogene in breast cancer (20, 21). However, neither of these studies attempted to correlate Yap expression with patient outcome, which would help resolve this controversy. Three lines of evidence refute a role for Yap as a tumor suppressor in ovarian cancer. First, low nYap expression in ovarian tumors correlated with better disease-specific survival. Second, Yap was not lost or dramatically down-regulated in 10 ovarian cancer cell lines. Third, Yap knockdown in 80T cells did not result in phenotypes associated with cancer. In fact, Yap knockdown cells were more sensitive to cisplatin-induced apoptosis than control cells (Fig. 3C).
Although the sample size is modest and should be interpreted cautiously, Yap appears to be a powerful prognostic marker of poor survival in ovarian cancer. Cox regression analysis of the nY/cpY Category (3 vs 0, 1, 2,) revealed an exceptionally high hazard ratio of 7.7(comparable to the hazard ratio of 7.0 for stage), indicating that, in our data set, patients with both high nYap and low cpYap are at nearly eight times greater risk of death from the disease than other patients. This hazard ratio is substantially higher than the hazard ratio for nYap staining alone (HR=3.3). There could be two explanations for this finding. First, immunohistochemical studies of archival tumor samples can be confounded by both systematic and random errors such as inter- and intra-sample heterogeneity and differences in the age, fixation, processing, staining, and scoring of tumor samples. Therefore, two independent measures of the same marker would be expected to reduce error. Second, the nY/cpY Category might reflect the activity of Yap in the tumor. Yap is phosphorylated at S127 by Lats kinases and retained in the cytoplasm by 14-3-3(8). When Yap is localized in the nucleus, it is likely transcriptionally active and thus intense nYap staining suggests high Yap activity. In contrast, when Yap is phosphorylated and in the cytoplasm, Yap is presumably transcriptionally inactive and thus intense cpYap staining suggests low Yap activity. Thus, tumors in nY/cpY Category 3, which have high levels of nYap and low levels of cpYap, might be expected to have the highest Yap activity. Analysis of the levels of Yap target genes in each nY/cpY Category might shed light on these two possibilities.
An important question that remains is the mechanism of Yap misregulation in ovarian cancer. We found no evidence for Yap protein amplification or for Yap activating mutations. Further, pYap staining was detectable in 63/70 ovarian tumors and all ovarian cancer cell lines (Supplementary Fig. 1A), indicating that increased nYap does not likely result from complete loss of phosphorylation. A variety of mechanisms have been described Drosophila that alter Yki levels and subcellular localization (8), providing several possible mechanisms by which nYap levels may increase in human ovarian cancer. Elucidation of these mechanisms will likely provide insight for therapeutic intervention targeting Yap.
Our results support the notion that Yap might be an excellent therapeutic target for the treatment of ovarian cancer. Though our sample size is modest and our analysis needs to be confirmed in a larger patient population, Yap distribution appears to be a very strong predictor of survival from ovarian cancer. Even if further analysis reveals that the hazard ratio for nY/cpY Category 3 is at the low end of our 95% confidence interval, (2.1–28.9), this would be on par with EGF and Her-2/neu, which are currently targeted in clinical trials for ovarian cancer treatments (22, 23). Further, Yap activation results in several cancer-associated phenotypes while reduction of Yap resulted only in increased sensitivity to chemotherapy, indicating that therapies targeting Yap could affect multiple cancer processes with minimal deleterious effects.
The Drosophila ovarian follicular epithelium and human OSE are female specific tissues that share many important properties including mesodermal origin, monolayer cuboidal epithelial organization, hormone dependency, and the ability to undergo dynamic remodeling (7, 24–26). The results presented here support the notion that the Drosophila follicular epithelium is a useful tool for the discovery of genes crucial to the pathogenesis of ovarian cancer.
Supplementary Material
Acknowledgments
We thank George Halder, Kun-Liang Guan, Marius Sudol, Craig Allred, Michael Ittmann, Wendong Yu, Jianghua Wang, Yi Cai, and Patricia Castro for reagents and advice, and anonymous reviewers for helpful suggestions. This work was supported by NIH 2RO1 CA087759-06A2 and Mary Kay Ash 038-03 grants to SG and NIH RO1 CA103924 grant to SO. CAH was supported by NIH training grant T32GM008307.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Sale S, Orsulic S. Models of ovarian cancer metastasis: Murine models. Drug Discov Today Dis Models. 2006;3:149–54. doi: 10.1016/j.ddmod.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goode S, Perrimon N. Inhibition of patterned cell shape change and cell invasion by Discs large during Drosophila oogenesis. Genes Dev. 1997;11:2532–44. doi: 10.1101/gad.11.19.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–39. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Szafranski P, Hall CA, Goode S. Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics. 2008;178:1947–71. doi: 10.1534/genetics.108.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–74. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–52. [PubMed] [Google Scholar]
- 10.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao J, Mu D, Ergel B, et al. Hepsin colocalizes with desmosomes and induces progression of ovarian cancer in a mouse model. Int J Cancer. 2008;123:2041–7. doi: 10.1002/ijc.23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 15.Auersperg N, Siemens CH, Myrdal SE. Human ovarian surface epithelium in primary culture. In Vitro. 1984;20:743–55. doi: 10.1007/BF02618290. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–9. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Ji JY, Yu M, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–50. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–13. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 20.Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–9. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 22.Mackay HJ, Oza AM. Other new targets. Int J Gynecol Cancer. 2009;19 (Suppl 2):S49–54. doi: 10.1111/IGC.0b013e3181bf830d. [DOI] [PubMed] [Google Scholar]
- 23.de Graeff P, Crijns AP, de Jong S, et al. Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer. 2009;101:149–59. doi: 10.1038/sj.bjc.6605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Tanwar PS, Raftery LA. Drosophila follicle cells: morphogenesis in an eggshell. Semin Cell Dev Biol. 2008;19:271–82. doi: 10.1016/j.semcdb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szafranski P, Goode S. A Fasciclin 2 morphogenetic switch organizes epithelial cell cluster polarity and motility. Development. 2004;131:2023–36. doi: 10.1242/dev.01097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.