Abstract
Repeated administration of cocaine induces heightened behavioral hyperactivity termed sensitization. Although NK-3 receptors have been shown to modulate acute cocaine-induced behaviors, their role in behavioral sensitization is unknown. The present study investigated whether NK-3 receptor blockade altered behavioral sensitization to cocaine. Additionally, glycogen synthase kinase-3 (GSK3) has been shown to be involved in dopamine receptor signaling and in development of sensitization, therefore regulation of GSK3 activity in the nucleus accumbens was also investigated. Administration of the NK-3 receptor antagonist SB 222200 (5 mg/kg, s.c.) prior to repeated cocaine (20 mg/kg, i.p.) prevented the development of sensitized responses after a cocaine challenge. Pretreatment with SB 222200 before a cocaine challenge also blocked expression of sensitization. Decrease in GSK3 activity demonstrated by increased phosphorylation of GSK3α and GSK3β was detected 20 mins after an acute cocaine injection. In contrast, a cocaine challenge failed to alter phosphorylation of GSK3α and GSK3β in sensitized mice. SB 222200 prior to repeated cocaine resulted in increased phosphorylation of GSK3α and GSK3β akin to changes following acute cocaine. Collectively, these findings demonstrate the involvement of NK-3 receptors in development and expression of behavioral sensitization and in regulation of GSK3 activity in the nucleus accumbens after repeated cocaine.
Keywords: NK-3R, cocaine, dopamine, sensitization, GSK3, nucleus accumbens
INTRODUCTION
Drugs of abuse that are reinforcing share a common mechanism in causing elevated synaptic dopamine levels in the nucleus accumbens (Di Chiara & Imperato 1988). In particular, cocaine produces its locomotor and its reinforcing effects by inhibiting re-uptake of the monoamines dopamine, norepinephrine and serotonin into pre-synaptic terminals, causing elevated levels of neurotransmitters in the synapse (Ritz et al. 1987, Heikkila et al. 1979). Another feature of cocaine and other similar psychostimulants is repeated intermittent exposure induces behavioral sensitization, characterized as a progressively heightened behavioral response after abstinence and re-exposure (Post & Rose 1976, Heidbreder et al. 1996). Behavioral sensitization has been suggested to be a useful model in revealing neuroadaptations that contribute to the compulsive craving characteristic of cocaine-seeking behaviors and drug addiction (Berke & Hyman 2000, Nestler 2001, Robinson & Berridge 2001, Vanderschuren & Kalivas 2000).
Alterations in dopaminergic neurotransmission from the ventral tegmental area (VTA) to the nucleus accumbens are part of the underlying mechanism which brings about behavioral sensitization. Two distinct components of behavioral sensitization are understood to be differentially mediated by these brain regions. Development of behavioral sensitization results from changes in the cell bodies of dopaminergic neurons in the VTA and its afferent inputs from other brain regions, whereas the expression of sensitization involves changes in the dopaminergic synaptic terminals in the nucleus accumbens (Kalivas & Stewart 1991, Pierce & Kalivas 1997). Therefore, it is postulated that modulation of dopaminergic neurotransmission from the VTA to the nucleus accumbens can impact the development and expression of behavioral sensitization produced by repeated cocaine exposure.
NK-3 receptors are G-protein coupled receptors activated by mammalian tachykinins, which can modulate dopaminergic neurotransmission, and thus may play a role in development and expression of behavioral sensitization. Activation of NK-3 receptors stimulates dopaminergic neuronal activity (Keegan et al. 1992, Overton et al. 1992) and increases dopamine release and metabolism in the striatum and prefrontal cortex (Bannon et al. 1995, Humpel et al. 1991, Marco et al. 1998). The influence of NK-3 receptors on dopaminergic neurotransmission also results in altered dopamine-mediated behaviors. Behaviors produced by cocaine and dopamine receptor agonists can be attenuated by NK-3 receptor antagonist administration (Bishop & Walker 2004, Jocham et al. 2006, de Souza Silva et al. 2006b, Nwaneshiudu & Unterwald 2009). In addition, NK-3 receptor agonists can potentiate cocaine-induced behaviors (Jocham et al. 2007, de Souza Silva et al. 2006a). NK-3 receptors therefore modulate dopaminergic neurotransmission and behaviors, however a role in behavioral sensitization to cocaine remains to be studied.
Glycogen synthase kinase-3 (GSK3) is recognized as an important downstream substrate of dopamine receptor signaling (Grimes & Jope 2001, Beaulieu et al. 2004, Prickaerts et al. 2006). In addition, GSK3 plays a role in cocaine-induced behavioral sensitization (Miller et al. 2009, Xu et al. 2009). GSK3 is expressed in the brain in two isoforms, namely GSK3α and GSK3β, which are regulated by phosphorylation at serine 21 and 9 residues respectively. Phosphorylation of GSK3α and GSK3β leads to inhibition of GSK3 activity (Grimes & Jope 2001), and the phosphorylation state of GSK3 can be regulated by PKA, protein kinase B (Akt), PKC, DARPP-32 and protein phosphatase 1, among others (Alessi et al. 1996, Li et al. 2000, Svenningsson et al. 2003). Genetic or pharmacological inhibition of GSK3 activity can attenuate dopamine-mediated behaviors (Beaulieu et al. 2004, Miller et al., Miller et al. 2009). Studies have reported that inhibition of GSK3 activity blocks the development of behavioral sensitization to cocaine (Miller et al. 2009, Xu et al. 2009). Overall, there is evidence in support of GSK3 as an important molecular substrate involved in dopamine neurotransmission and in behavioral sensitization to cocaine, however interconnection of GSK3 with NK-3 receptors remains largely unknown.
The present study examined the role of NK-3 receptors in the development and expression of sensitization to locomotor behaviors and also in neuroplastic changes in GSK3 activity in the nucleus accumbens resulting from repeated cocaine administration. Since NK-3 receptors modulate dopaminergic neurotransmission, and NK-3 receptor blockade attenuates acute dopamine and cocaine-induced behaviors (Bishop & Walker 2004, de Souza Silva et al. 2006b, Jocham et al. 2006, Nwaneshiudu & Unterwald 2009), we hypothesized that activation of NK-3 receptors is necessary for the development and expression of locomotor sensitization to cocaine. In addition, given the role of GSK3 as a molecular substrate of dopamine receptor signaling and in behavioral sensitization to cocaine (Beaulieu et al. 2004, Miller et al. 2009, Xu et al. 2009), we sought to determine if NK-3 receptors are involved in changes in GSK3 phosphorylation in the nucleus accumbens produced by cocaine administration.
MATERIALS AND METHODS
Animals
Adult male CD-1 mice (Charles River Laboratories, Raleigh, NC, USA) were group-housed (4–6 per cage) in a temperature and humidity controlled environment on a 12-h light–dark cycle (lights on at 7AM) with ad libitum access to food and water. Animals were handled daily prior to the beginning of the study. All experiments were conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and with approval from Temple University School of Medicine Institutional Animal Care and Use Committee.
Drugs
Cocaine hydrochloride was generously provided by the National Institute on Drug Abuse and dissolved in a sterile 0.9% saline solution. (S)-3-methyl-2-phenyl-N-(1-phenylpropyl)-4-quinolinecarboxamide (SB 222200) was obtained from Sigma Aldrich and dissolved in a vehicle composed of 60% polyethylene glycol (PEG-200) and 40% distilled water. Saline control or cocaine was injected intraperitoneally in a volume of 3 ml/kg body weight, and vehicle control or SB 222200 was injected subcutaneously in a volume of 2 ml/kg. The doses and schedule of SB 222200 administration were chosen based on its in vivo pharmacological properties previously reported (Sarau et al. 2000), and also its effects on cocaine-induced hyperactivity (Nwaneshiudu & Unterwald 2009).
Drug treatment and behavioral assessment
In order to examine effects of the NK-3 receptor antagonist SB 222200 on the development of cocaine behavioral sensitization, adult male CD-1 mice were administered either vehicle or SB 222200 (2.5 or 5 mg/kg, s.c.) followed 30 mins later by an injection of either saline or cocaine (20 mg/kg, i.p.) once daily for 5 days (days 1–5) in their home cages. After a 7-day drug-free period (day 13), all mice were challenged with cocaine (20 mg/kg, i.p.) in the absence of SB 222200, and ambulatory activity was measured for 30 mins in a novel environment. To examine effects of SB 222200 administration on the expression of behavioral sensitization to cocaine, mice were injected once daily with either saline or cocaine (20 mg/kg, i.p.) for 5 days (days 1–5), followed by a 7-day drug-free period. On day 13, mice were pretreated with either vehicle or SB 222200 (5 mg/kg, s.c.) 30 mins prior to a challenge injection of cocaine (20 mg/kg, i.p.), and ambulatory activity was measured for 30 mins.
Behavioral activity was measured using a Digiscan DMicro System (Accusan, Columbus, OH, USA) that consists of clear 20 × 20 × 42 cm plastic cages lined with horizontal photo-beams and detectors that are interfaced with an output computer. Ambulatory activity was recorded as counts of consecutive photo-beam breaks.
Tissue preparation and immunoblotting
In a separate study, mice were injected once daily with either vehicle or SB 222200 (5 mg/kg, s.c.) followed 30 mins later by either saline or cocaine (20 mg/kg, i.p.) for 5 days (Days 1–5), and left drug-free for 7 days. On day 13, mice were challenged with either saline or cocaine (20 mg/kg, i.p.). All injections were given in the animals’ home cages. Twenty mins later, mice were exposed to CO2 for 15-sec, and the nucleus accumbens were rapidly dissected on ice. Tissues were homogenized using a sonicator in 100°C 1% SDS with 1 mM NaF and 1 mM Na3VO4 as phosphatase inhibitors. Samples were boiled for 5 mins and stored at −80°C.
Protein concentrations of tissue samples were determined using the Lowry protocol (Lowry et al. 1951). 30 µg of protein from each sample were loaded onto 7.5% Tris-HCl Bio-Rad Ready-gels, separated by SDS-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. Membranes were stained with 2.5% Ponceau S dye in 1% acetic acid/dH2O to insure integrity of transferred protein. Membranes were washed in Tris buffered saline with Tween-20 (TTBS), and blocked with 5% non-fat dry milk in TTBS for 1hr at room temperature. Membranes were incubated with the following primary antibodies diluted in 5% non-fat dry milk in TTBS, 1:2000 for anti-phospho-GSK3α/β (Cell Signaling Technology, Beverly, MA, USA), and 1:5000 for anti-GSK3α/β (Santa Cruz Biotechnology, Santa Cruz, CA). The phospho-GSK3α/β antibody recognizes the phosphorylated form of GSK3α at serine 21(52 kDa) and GSK3β phosphorylated at serine 9 (47 kDa). Membranes were washed in TTBS and incubated with anti-rabbit or anti-mouse secondary antibodies conjugated to two different infra-red dyes (LI-COR Biosciences, Lincoln NE) at room temperature for 1h in a dark room. Secondary antibodies were diluted 1:10,000 in Odyssey blocking buffer with 0.2% Tween-20 (LI-COR). Membranes were visualized and proteins were quantified using the Odyssey infrared imaging system and software (LI-COR). Phosphorylated and total forms of GSK3 were detected simultaneously as the colors green and red respectively. Membranes were stripped of antibodies using the New Blot nitro stripping buffer (LI-COR) and re-probed with anti-α-tubulin [1:80,000 (Sigma-Aldrich)] to control for differences in protein loading and during protein transfer. Ratios of densities of phosphorylated GSK3α/β to total GSK3α/β, and total GSK3α/β to α-tubulin were calculated.
Data analysis
Data were analyzed by one-way ANOVA with Bonferroni post hoc tests (GraphPad Prism V.4, La Jolla, CA). Statistical significance was determined at the alpha level of 0.05.
RESULTS
Pretreatment with the NK-3 receptor antagonist SB 222200 blocked the development of behavioral sensitization to cocaine
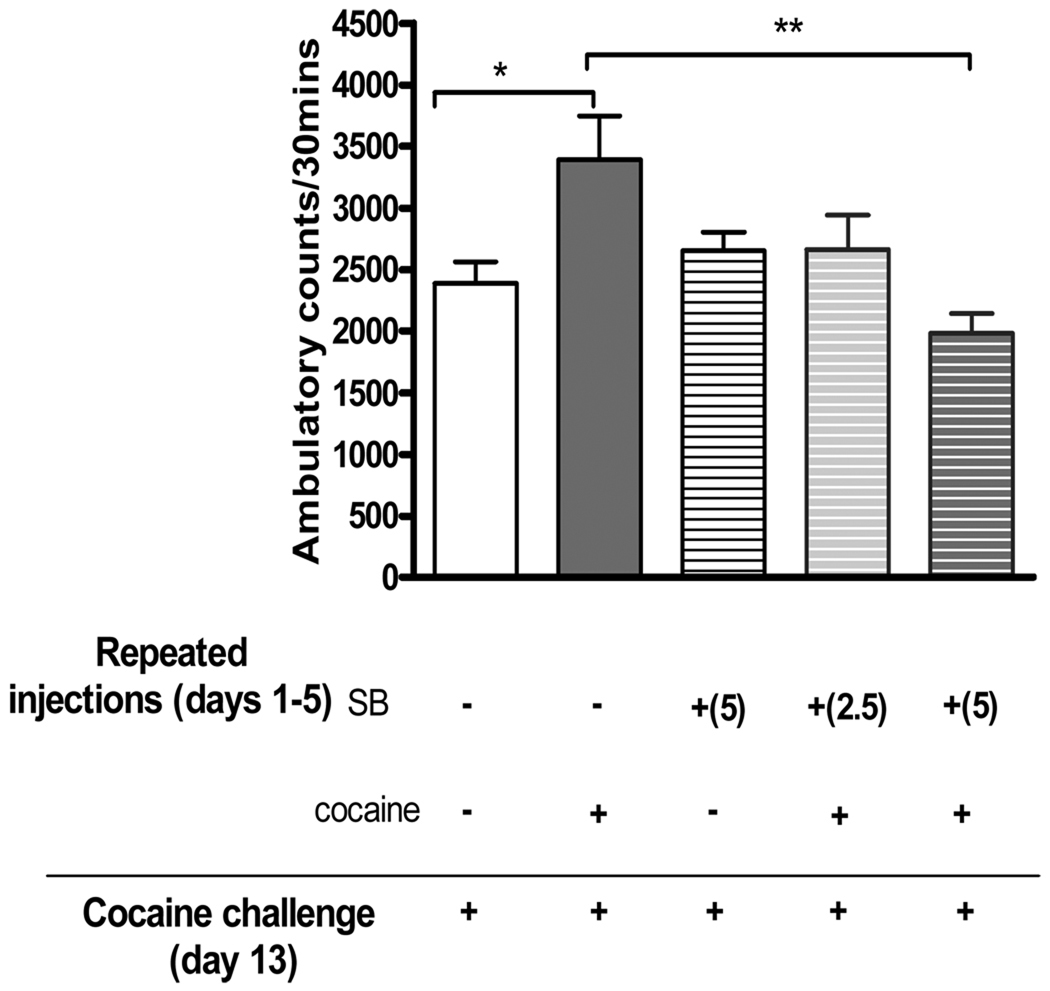
Ambulatory responses to a cocaine challenge after 7 days drug-free (day 13) were measured in mice pretreated for 5 days with SB 222200 and/or cocaine. Statistical analyses of total ambulatory activity revealed a significant difference between treatment groups (F(4,39)=3.93, p< 0.01, Figure 1). Bonferroni post hoc comparisons showed that a cocaine challenge induced significantly higher ambulatory activity in mice administered repeated cocaine than in mice given repeated saline (p <0.05) demonstrating behavioral sensitization. Mice administered SB 222200 (5 mg/kg, s.c.) prior to repeated cocaine for 5 days did not show a sensitized response to a subsequent cocaine challenge and showed significantly less activity in response to a cocaine challenge as compared to mice administered vehicle prior to repeated cocaine (p<0.01). Therefore, daily pretreatment with SB 222200 blocked the development of cocaine-induced behavioral sensitization. Baseline ambulatory activity prior to cocaine challenge was not significantly different among the treatment groups (F4,39)=0.68, p>0.05.
Figure 1. Effect of NK-3 receptor blockade on development of behavioral sensitization to cocaine.
Adult male CD-1 mice were injected with either vehicle or the NK-3 receptor antagonist SB 222200 (2.5, 5 mg/kg s.c.) 30 mins prior to a cocaine (20 mg/kg, i.p.) injection for 5 days. After a seven day drug-free period, all mice were challenged with cocaine (20 mg/kg, i.p.) and behavioral responses were measured. Within vehicle treatment groups, mice injected repeatedly with cocaine had significantly increased ambulatory activity to a subsequent cocaine challenge compared to saline animals indicating a sensitized behavioral response. Administration of SB 222200 blocked this sensitized behavioral response in mice injected with repeated cocaine. Data are presented as mean ± SEM; N=7–12 mice/group (* p<0.05, **p< 0.01).
Effect of SB 222200 on the expression of behavioral sensitization to cocaine
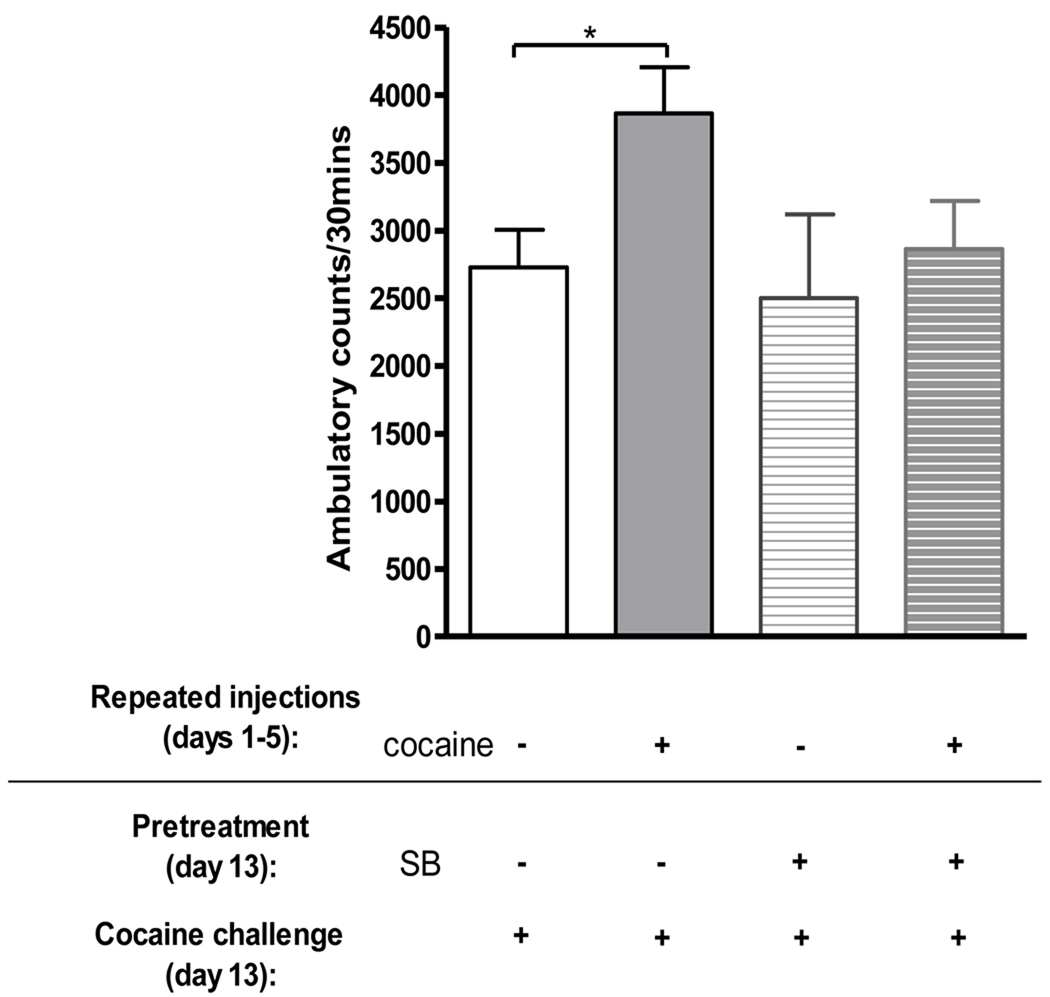
Ambulatory responses were measured in mice injected with SB 222200 30 mins prior to a cocaine challenge on day 13 after 5 days of repeated cocaine. Statistical analyses of ambulatory activity revealed a significant difference between treatment groups (F(3,38)=2.87, p< 0.05, Figure 2). Bonferroni post hoc comparisons showed that mice administered repeated cocaine had significantly higher ambulatory responses to a subsequent cocaine challenge than mice given repeated saline (p <0.05), which demonstrates behavioral sensitization. The sensitized response was not detected after administration of SB 222200 30 mins prior to the cocaine challenge on day 13. Activity of mice pretreated with SB 222200 prior to a cocaine challenge was not significantly different from non-sensitized controls (p>0.05), in agreement with our previous study (Nwaneshiudu & Unterwald 2009). Baseline ambulatory activity prior to cocaine challenge did not significantly differ among treatment groups (F3,38)=1.27, p>0.05).
Figure 2. Effect of NK-3 receptor blockade on expression of behavioral sensitization to cocaine.
Adult male CD-1 mice were injected once daily with either saline or cocaine (20 mg/kg, i.p) for five days. After a seven day drug-free period, they were administered either vehicle or the NK-3 receptor antagonist SB 222200 (5 mg/kg, s.c.) 30 mins prior to a cocaine challenge (20 mg/kg, i.p.). SB 222200 administration 30 mins prior to a cocaine challenge did not significantly alter ambulatory activity in saline treated mice, but blocked the enhanced behavioral response to cocaine in mice given repeated cocaine. Data are presented as mean ± SEM; N=7–14 mice/group (* p<0.05).
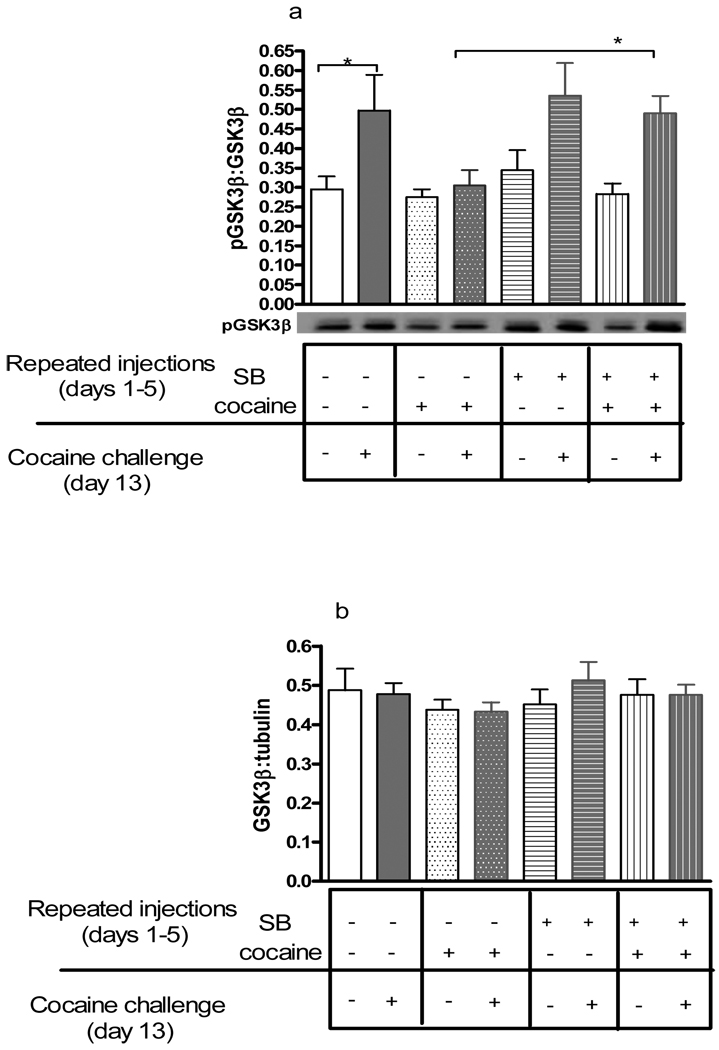
Pretreatment with SB 222200 prior to repeated cocaine altered GSK3α phosphorylation in the nucleus accumbens
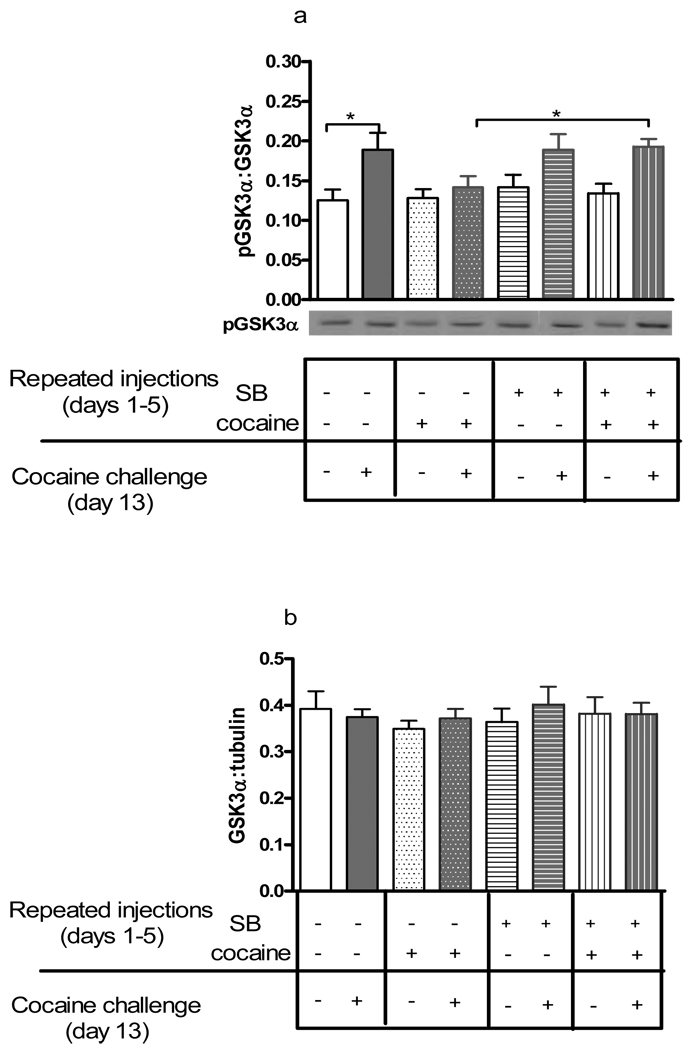
Phosphorylated GSK3α (Ser-21) and total GSK3α proteins were measured in the nucleus accumbens from mice given SB 222200 and cocaine for 5 days, and challenged with cocaine after a 7-day drug-free period. Representative immunoblots of phosphorylated GSK3α (pGSKα) (green) and total GSK3α (red) are shown in Figures 3 and 4a.GSK3α was visualized as the upper protein band (approximately 52 kDa) (Figure 3). Statistical analysis of ratios of pGSK3α to total GSK3α demonstrated significant differences between treatment groups (F(7,61)= 3.67, p< 0.01, Figure 4a). Bonferroni post hoc comparisons showed higher levels of phosphorylated GSK3α in the nucleus accumbens 20 mins after the cocaine challenge in control mice pretreated with vehicle and saline (p<0.05). In contrast, pGSK3α was unaltered following the cocaine challenge in mice that received repeated cocaine on days 1–5 (p>0.05), demonstrating tolerance to repeated cocaine administration. This tolerance to GSK3α regulation was abolished by pretreatment with SB 222200 prior to daily cocaine. Phosphorylated GSK3α was elevated 20 mins following a cocaine challenge in mice pretreated SB 222200 and repeated cocaine (p<0.05). A saline challenge after repeated cocaine and/or SB 222200 administration did not significantly alter levels of pGSK3α (p>0.05). Ratios of total GSK3α to α-tubulin proteins were unaltered by any drug treatment (Figure 4b). These data suggest that administration of acute cocaine increased phosphorylation of GSK3α at serine 21 in the nucleus accumbens measured 20 mins later, and tolerance to the increase in GSK3α phosphorylation occurred with repeated cocaine administration and challenge. Furthermore, pretreatment with SB 222200 prior to repeated cocaine reversed tolerance to the increase in GSK3α phosphorylation induced by repeated cocaine.
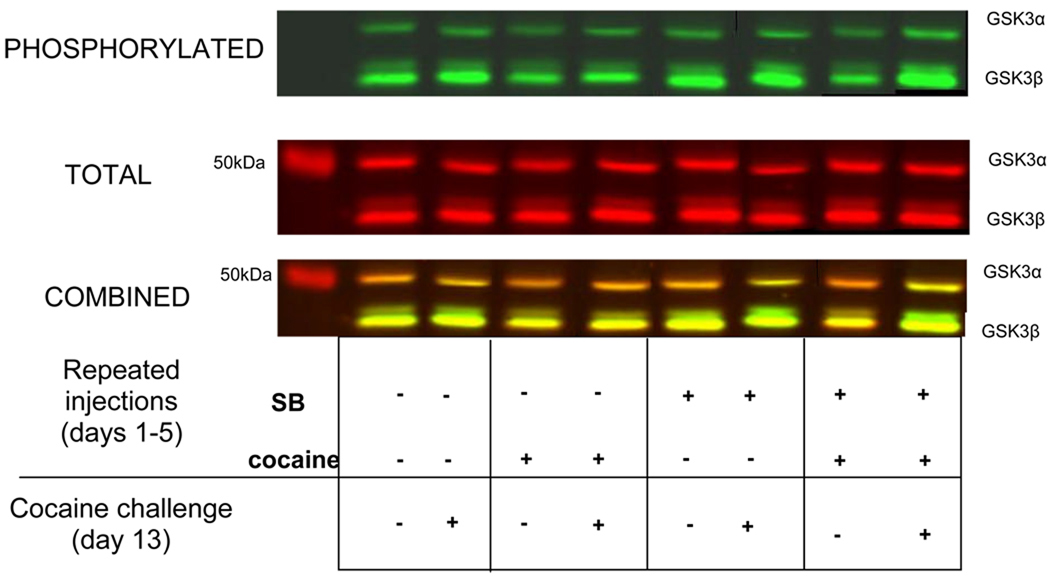
Figure 3. Representative immunoblots of phosphorylated and total GSK3α and GSK3β from the nucleus accumbens.
Phosphorylated and total GSK3 proteins were detected simultaneously using the Odyssey infrared imaging system. GSK3α was visualized at 52 kDa and GSK3β at 47 kDa.
Figure 4. GSK3α phosphorylation in the nucleus accumbens after repeated cocaine administration and cocaine challenge.
Adult male CD-1 mice were injected once daily with vehicle or SB 222200 (5 mg/kg, s.c) and saline or cocaine (20 mg/kg), and 7 days later were challenged with either saline or cocaine. The nucleus accumbens was examined for changes in GSK3α phosphorylation 20 mins after the challenge. Representative immunoblots of phosphorylated GSK3α (Ser-21) protein of tissues from the nucleus accumbens of each treatment groups are also shown. Data are presented as mean ± SEM; N=6–10/group (* p<0.05).
SB 222200 administration prior to repeated cocaine altered GSK3β phosphorylation in the nucleus accumbens
Phosphorylated GSK3β (pGSK3β) (Ser-9) and total GSK3β protein were also measured similarly to GSK3α in the nucleus accumbens from mice pretreated with SB 222200 and cocaine for 5 days and challenged with cocaine after a 7-day drug-free period. Representative immunoblots of pGSK3β and total GSK3β are shown in Figures 3 and 5a. GSK3β is seen as the denser protein band at approximately 47 kDa. Statistical analyses of ratios of pGSK3β to total GSK3β showed significant differences between treatment groups (F(7,58)= 3.7, p< 0.01, Figure 5a). Post hoc comparisons revealed increased levels of pGSK3β in the nucleus accumbens 20 mins after the cocaine challenge as compared to saline-injected controls (p<0.05). No significant changes in pGSK3β levels were detected after a subsequent cocaine challenge in mice that received repeated cocaine as compared to mice with repeated saline (p>0.05), demonstrating tolerance following repeated cocaine administration. However, there were increases in pGSK3β following a cocaine challenge in mice after prior administration of SB 222200 with repeated cocaine (p<0.05). Repeated cocaine and/or SB 222200 administration did not significantly alter pGSK3β after a saline challenge (p>0.05). Additionally, ratios of total GSK3β to α-tubulin were not altered by any drug treatment (Figure 5b). These data demonstrate that, similar to GSK3α, there is also increased phosphorylation of GSK3β at serine-9 20 mins after an acute injection of cocaine in the nucleus accumbens, and tolerance to a subsequent cocaine challenge after repeated cocaine administration. Furthermore, tolerance was prevented by pretreatment with SB 222200 prior to repeated cocaine.
Figure 5. GSK3β phosphorylation in the nucleus accumbens after repeated cocaine administration and subsequent challenge.
Adult male CD-1 mice were injected once daily with vehicle or SB 222200 (5 mg/kg s.c.) and saline or cocaine (20 mg/kg). Seven days later, they were challenged with either saline or cocaine and examined for changes in GSK3β phosphorylation 20 mins later. Representative immunoblots of phosphorylated GSK3β (Ser-9) protein of tissues from the nucleus accumbens of each treatment groups are also shown. Data are presented as mean ± SEM; N=6–10/group (* p<0.05).
DISCUSSION
Sensitization to cocaine-induced locomotor activity has been shown to result from neuroplastic changes in the mesolimbic dopaminergic neurotransmission (Anderson & Pierce 2005, Pierce & Kalivas 1997, Vanderschuren & Kalivas 2000). Structural changes in dopaminergic neurons produced by repeated cocaine administration (Robinson & Kolb 1999) and their increased responsivity to glutamate have been reported (Zhang et al. 1997). Other studies report enhanced inhibition of nucleus accumbens neurons to local application of dopamine (Beurrier & Malenka 2002, Henry & White 1991). In addition, repeated cocaine results in increased activation and expression of dopamine receptors (Alburges et al. 1993, McCreary & Marsden 1993, Unterwald et al. 1994), increased dopamine receptor activation of cAMP signaling (Unterwald et al. 1996) and induction of dopamine receptor-mediated gene expression in the nucleus accumbens (Zhang et al. 2005). Therefore, we postulate that agents can alter behavioral sensitized responses to cocaine by preventing changes in dopaminergic neurotransmission to the nucleus accumbens induced by repeated cocaine administration.
NK-3 receptors have been shown to modulate dopamine neurotransmission to the nucleus accumbens (Bannon et al. 1995, Humpel et al. 1991, Keegan et al. 1992, Marco et al. 1998, Overton et al. 1992). In the present study, the role of NK-3 receptors in the regulation of locomotor sensitization produced by repeated cocaine administration was examined. Our findings show that administration of the NK-3 receptor antagonist SB 222200 before daily injections of cocaine blocked the development of a sensitized locomotor response after a subsequent cocaine challenge. These findings suggest that NK-3 receptors play a role in the development of behavioral sensitization to cocaine, and may do so through altering dopaminergic transmission in the nucleus accumbens.
The ability of the NK-3 receptor antagonist SB 222200 to inhibit the development of behavioral sensitization to cocaine suggests a mechanism involving cocaine-induced release of endogenous ligands that activate NK-3 receptors. Brain regions involved in motor behaviors, such as the substantia nigra, VTA, caudate-putamen, nucleus accumbens and cerebral cortex, express neurokinin-B and substance P (Burgunder & Young 1989, Marksteiner et al. 1992, Warden & Young 1988), endogenous ligands that can activate NK-3 receptors (Krause et al. 1990). Acute and repeated cocaine administration increases preprotachykinin mRNA levels in the striatum (Adams et al. 2001, Mathieu-Kia & Besson 1998), which encodes the precursors for the endogenous ligands substance P and neurokinin-B. In the present study, antagonism of NK-3 receptors prevented the development of locomotor behavioral sensitization to cocaine. For this reason, we hypothesize that activation of NK-3 receptors, through the release of its endogenous ligands, alters dopaminergic neurotransmission to the nucleus accumbens (Bannon et al. 1995, Humpel et al. 1991, Keegan et al. 1992, Marco et al. 1998, Overton et al. 1992), which can contribute to behavioral sensitization.
GSK3 plays an important role as a molecular substrate of dopamine-mediated behaviors and in behavioral sensitization to cocaine. Modulation of GSK3 activity has been shown to alter psychostimulant-induced behaviors (Beaulieu et al. 2004, Miller et al. 2009, Prickaerts et al. 2006) and behavioral sensitization to cocaine (Miller et al. 2009, Xu et al. 2009). GSK3β heterozygous null mice have attenuated behavioral responses to amphetamine (Beaulieu et al. 2004). In addition, changes in GSK3 phosphorylation occur after administration of the psychostimulants amphetamine and cocaine, which are time-dependent. Increases in phosphorylation of GSK3β and Akt in the striatum have been reported at early time points after acute amphetamine and cocaine (Brami-Cherrier et al. 2002, McGinty et al. 2008, Svenningsson et al. 2003), and decreases in GSK3α and β and Akt phosphorylation have been reported at later times (Beaulieu et al. 2004, McGinty et al. 2008, Shi & McGinty 2007, Miller et al. 2009). Our present study demonstrates GSK3 phosphorylation is increased in the nucleus accumbens 20 mins after an acute injection of cocaine, and tolerance to this increase in GSK3 phosphorylation develops with repeated cocaine administration. Our study shows changes in GSK3 phosphorylation in the nucleus accumbens occurring after acute administration of cocaine, and novel changes that coincide temporally with manifestation of locomotor behavioral sensitization after repeated cocaine administration.
The present findings indicate that NK-3 receptors are involved in neuroplastic changes in GSK3 activity in the nucleus accumbens produced by repeated cocaine administration that contribute to bring about locomotor behavioral sensitization. Currently there are no studies demonstrating direct modulation of GSK3 activity through NK-3 receptors. There is evidence to support modulation of GSK3 activity by dopamine receptors via its downstream substrates (Grimes & Jope 2001). The activity of GSK3α and GSK3β is inhibited by phosphorylation at the serine-21 and -9 residues respectively and Akt, DARPP-32, P70 S6 kinase, MAP kinase-activated protein kinase-1 (MAPKAP-1), and protein kinase A can regulate GSK3 phosphorylation (Alessi et al. 1996, Li et al. 2000, Svenningsson et al. 2003). Since NK-3 receptors modulate dopamine neurotransmission to the nucleus accumbens (Bannon et al. 1995, Humpel et al. 1991, Keegan et al. 1992, Marco et al. 1998, Overton et al. 1992), we postulate that NK-3 receptors indirectly alter GSK3 activity in the nucleus accumbens by modulating dopamine receptor activation. In support of this, our present findings show that administration of the NK-3 receptor antagonist SB 222200 reversed the tolerance to increased GSK3 phosphorylation produced by repeated cocaine administration, and also prevented locomotor behavioral sensitization to cocaine.
Behavioral sensitization is a model that has been suggested to be useful in revealing neuroadaptations that contribute to the compulsive craving seen in cocaine seeking behaviors (Robinson & Berridge 2001, Vanderschuren & Kalivas 2000, Kalivas et al. 1998). This is based in part on observations that brain regions such as the VTA and nucleus accumbens that are involved in drug-induced sensitization also mediate behaviors such as salient-motivation and drug reward (Robinson & Berridge 2001). These behaviors are said to be influenced by environmental factors such as stress and conditioned cues, which also affect behavioral sensitization to cocaine and cocaine-seeking behaviors (Hinson & Poulos 1981, Nestler 2001, Pierce & Kalivas 1997, Pierce et al. 1998, Vezina & Leyton 2009). It would be of interest to investigate the possible interplay between NK-3 receptors, stress and/or conditioned cues in the manifestation of locomotor behavioral sensitization to cocaine. In addition, elucidating the exact mechanism whereby NK-3 receptors can regulate GSK3 activity would significantly enrich our present findings as it pertains to locomotor behavioral sensitization.
In summary, the present study demonstrates that antagonism of NK-3 receptors blocks the development and expression of cocaine-induced locomotor behavioral sensitization. Our findings suggest that cocaine causes activation of NK-3 receptors which in part contributes either directly or indirectly through alterations in dopaminergic transmission, to locomotor behavioral sensitization. In addition, cocaine acutely increases GSK3 phosphorylation in the nucleus accumbens, and this effect is absent after repeated cocaine exposure. Tolerance to the increase in GSK3 phosphorylation following repeated cocaine administration also appears to involve NK-3 receptors. These findings suggest that neuroplastic changes in GSK3 phosphorylation in the nucleus accumbens in part play a role to bring about locomotor behavioral sensitization to cocaine, and these changes require the activation of NK-3 receptors. In conclusion, these findings point to an important interaction between NK-3 receptors and GSK3 activity in the nucleus accumbens in the manifestation of locomotor behavioral sensitization induced by cocaine.
Acknowledgements
We would like to thank Ms. M. McCafferty and Dr. M. Simmons for their expertise in contributing to the successful completion of this study. This work was supported by the National Institutes of Health grant DA09580-S1.
REFERENCES
- Adams DH, Hanson GR, Keefe KA. Differential effects of cocaine and methamphetamine on neurotensin/neuromedin N and preprotachykinin messenger RNA expression in unique regions of the striatum. Neuroscience. 2001;102:843–851. doi: 10.1016/s0306-4522(00)00530-3. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Narang N, Wamsley JK. Alterations in the dopaminergic receptor system after chronic administration of cocaine. Synapse (New York, N.Y. 1993;14:314–323. doi: 10.1002/syn.890140409. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS letters. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Brownschidle LA, Tian Y, Whitty CJ, Poosch MS, D'sa C, Moody CA. Neurokinin-3 receptors modulate dopamine cell function and alter the effects of 6-hydroxydopamine. Brain research. 1995;695:19–24. doi: 10.1016/0006-8993(95)00791-n. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodget JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci. 2002;22:5817–5822. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Walker PD. Intranigral antagonism of neurokinin 1 and 3 receptors reduces intrastriatal dopamine D1 receptor-stimulated locomotion in the rat. Brain research. 2004;1023:126–133. doi: 10.1016/j.brainres.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgunder JM, Young WS., 3rd Distribution, projection and dopaminergic regulation of the neurokinin B mRNA-containing neurons of the rat caudate-putamen. Neuroscience. 1989;32:323–335. doi: 10.1016/0306-4522(89)90081-x. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Mello EL, Jr, Muller CP, Jocham G, Maior RS, Huston JP, Tomaz C, Barros M. Interaction of the tachykinin NK3 receptor agonist senktide with behavioral effects of cocaine in marmosets (Callithrix penicillata) Peptides. 2006a;27:2214–2223. doi: 10.1016/j.peptides.2006.03.005. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Mello EL, Jr, Muller CP, Jocham G, Maior RS, Huston JP, Tomaz C, Barros M. The tachykinin NK3 receptor antagonist SR142801 blocks the behavioral effects of cocaine in marmoset monkeys. European journal of pharmacology. 2006b;536:269–278. doi: 10.1016/j.ejphar.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. The Journal of pharmacology and experimental therapeutics. 1996;278:490–502. [PubMed] [Google Scholar]
- Heikkila RE, Cabbat FS, Duvoisin RC. Motor activity and rotational behavior after analogs of cocaine: correlation with dopamine uptake blockade. Commun Psychopharmacol. 1979;3:285–290. [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. The Journal of pharmacology and experimental therapeutics. 1991;258:882–890. [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Humpel C, Saria A, Regoli D. Injection of tachykinins and selective neurokinin receptor ligands into the substantia nigra reticulata increases striatal dopamine and 5-hydroxytryptamine metabolism. European journal of pharmacology. 1991;195:107–114. doi: 10.1016/0014-2999(91)90387-6. [DOI] [PubMed] [Google Scholar]
- Jocham G, Lauber AC, Muller CP, Huston JP, de Souza Silva MA. Neurokinin 3 receptor activation potentiates the psychomotor and nucleus accumbens dopamine response to cocaine, but not its place conditioning effects. The European journal of neuroscience. 2007;25:2457–2472. doi: 10.1111/j.1460-9568.2007.05491.x. [DOI] [PubMed] [Google Scholar]
- Jocham G, Lezoch K, Muller CP, Kart-Teke E, Huston JP, de Souza Silva MA. Neurokinin receptor antagonism attenuates cocaine's behavioural activating effects yet potentiates its dopamine-enhancing action in the nucleus accumbens core. The European journal of neuroscience. 2006;24:1721–1732. doi: 10.1111/j.1460-9568.2006.05041.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Keegan KD, Woodruff GN, Pinnock RD. The selective NK3 receptor agonist senktide excites a subpopulation of dopamine-sensitive neurones in the rat substantia nigra pars compacta in vitro. British journal of pharmacology. 1992;105:3–5. doi: 10.1111/j.1476-5381.1992.tb14199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause JE, Hershey AD, Dykema PE, Takeda Y. Molecular biological studies on the diversity of chemical signalling in tachykinin peptidergic neurons. Ann N Y Acad Sci. 1990;579:254–272. doi: 10.1111/j.1749-6632.1990.tb48367.x. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marco N, Thirion A, Mons G, Bougault I, Le Fur G, Soubrie P, Steinberg R. Activation of dopaminergic and cholinergic neurotransmission by tachykinin NK3 receptor stimulation: an in vivo microdialysis approach in guinea pig. Neuropeptides. 1998;32:481–488. doi: 10.1016/s0143-4179(98)90075-0. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. The Journal of comparative neurology. 1992;317:341–356. doi: 10.1002/cne.903170403. [DOI] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res Mol Brain Res. 1998;54:141–151. doi: 10.1016/s0169-328x(97)00338-0. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Marsden CA. Cocaine-induced behaviour: dopamine D1 receptor antagonism by SCH 23390 prevents expression of conditioned sensitisation following repeated administration of cocaine. Neuropharmacology. 1993;32:387–391. doi: 10.1016/0028-3908(93)90161-u. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Shi XD, Schwendt M, Saylor A, Toda S. Regulation of psychostimulant-induced signaling and gene expression in the striatum. Journal of neurochemistry. 2008;104:1440–1449. doi: 10.1111/j.1471-4159.2008.05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, Unterwald EM. Inhibition of GSK3 attenuates dopamine D1 receptor agonist-induced hyperactivity in mice. Brain Res Bull. 2010;82:184–187. doi: 10.1016/j.brainresbull.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, Unterwald EM. Cocaine-induced hyperactivity and sensitization are dependent on GSK3. Neuropharmacology. 2009;56:1116–1123. doi: 10.1016/j.neuropharm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nwaneshiudu CA, Unterwald EM. Blockade of neurokinin-3 receptors modulates dopamine-mediated behavioral hyperactivity. Neuropharmacology. 2009;57:295–301. doi: 10.1016/j.neuropharm.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton P, Elliott PJ, Hagan RM, Clark D. Neurokinin agonists differentially affect A9 and A10 dopamine cells in the rat. European journal of pharmacology. 1992;213:165–166. doi: 10.1016/0014-2999(92)90251-x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. The Journal of pharmacology and experimental therapeutics. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction (Abingdon, England) 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. The European journal of neuroscience. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Sarau HM, Griswold DE, Bush B, et al. Nonpeptide tachykinin receptor antagonists. II. Pharmacological and pharmacokinetic profile of SB-222200, a central nervous system penetrant, potent and selective NK-3 receptor antagonist. The Journal of pharmacology and experimental therapeutics. 2000;295:373–381. [PubMed] [Google Scholar]
- Shi X, McGinty JF. Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. Journal of neurochemistry. 2007;103:706–713. doi: 10.1111/j.1471-4159.2007.04760.x. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. European journal of pharmacology. 1996;318:31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. The Journal of pharmacology and experimental therapeutics. 1994;270:1387–1396. [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56 Suppl 1:160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MK, Young WS., 3rd Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. The Journal of comparative neurology. 1988;272:90–113. doi: 10.1002/cne.902720107. [DOI] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, Shi J, Lu L. Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. Journal of neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.06414.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. The Journal of pharmacology and experimental therapeutics. 1997;281:699–706. [PubMed] [Google Scholar]