Abstract
TAK1 kinase activates multiple transcription factors and regulates the level of reactive oxygen species (ROS). We have previously reported that ablation of TAK1 in keratinocytes causes hyper-sensitivity to ROS-induced cell apoptosis. It is known that some tumor cells produce ROS at higher levels compared to normal cells. We utilized inducible epidermal specific TAK1 knockout mice, and examined whether ablation of TAK1 in pre-exiting skin tumors could cause an increase in ROS and result in tumor cell death. Deletion of tak1 gene in skin tumors caused the accumulation of ROS and increased apoptosis, and skin tumors totally regressed within 5-10 days. Normal skin did not exhibit any significant abnormality upon tak1 gene deletion. Thus, TAK1 kinase could be a new and effective molecular target for ROS-based tumor killing.
Keywords: TAK1, apoptosis, reactive oxygen species
Introduction
TAK1 is a member of mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK), and is an indispensable signaling intermediate in several MAPK and NF-κB pathways (1-3). In mouse models, TAK1 is found to be essential for immune cell differentiation and activation by mediating activation of MAPK and NF-κB (4-7). In epithelial cells, we have demonstrated that TAK1 is critically involved in cell survival, and that ablation of TAK1 upregulates TNF-induced epithelial cell death (8-10). Ablation of TAK1 signaling diminishes activation of MAPK and NF-κB pathways, and decreases antioxidant capacity (9). ROS accumulation and apoptosis are observed upon TNF stimulation in cultured TAK1-deficient epidermal cells (keratinocytes) (9).
Cells constantly generate reactive oxygen species (ROS) as byproducts of energy metabolism through the electron transport chain reactions in mitochondria. Environmental factors such as commensal bacteria and stressors can additionally upregulate ROS generation in the cells. Thus, cellular antioxidant systems including TAK1 pathway are thought to be required for protecting cells from ROS-induced cell damage. Indeed, ablation of TAK1 spontaneously causes severe tissue damage in the intestinal epithelium (8). Mice having epidermal-specific constitutive deletion of TAK1 also exhibit severe skin damage in neonatal mice (10). Thus, the TAK1 pathway is important for cell survival for some epithelial tissues. In contrast, adult mice having inducible epidermal-specific deletion of TAK1 exhibit mild tissue damage and moderately elevated apoptosis upon tak1 gene deletion (9). Cultured TAK1-deficient fibroblasts and keratinocytes grow normally and do not exhibit significantly increased ROS (9). Thus, under the standard tissue culture conditions as well as in the adult skin, the TAK1 pathway is not always required for cell survival.
It is well known that tumor cells produce ROS at higher levels compared to normal cells, because tumors have increased energy metabolism and other abnormalities such as oncogenic stress and mitochondrial dysfunction which are associated with increased ROS production (11). Because of the increased intracellular levels of ROS, some tumor cells are hyper-sensitive to oxidative stress (12-14). However, tumor cells often upregulate cellular levels of antioxidant to counteract increased ROS levels (15). By decreasing the cellular antioxidant capacity in tumor cells, the increased levels of endogenous ROS production could result in tumor cell death. We hypothesized that ablation of TAK1 would decrease cellular antioxidant capacity in tumors, cause increased levels of ROS, and kill tumor cells. In this study, we tested the hypothesis that TAK1 ablation selectively kills tumor cells via ROS in a skin tumor model.
Materials and Methods
Mice
TAK1-floxed (TAK1flox/flox) mice were from Dr. Akira, Osaka University (6). K14-CreERT mice were obtained from the Jackson Labs (16). Mice harboring an epidermal-specific inducible TAK1 deletion (K14-CreERT TAK1flox/flox) were generated and tak1 gene deletion was examined by PCR described previously (9). Littermate controls were used in all experiments. All animal experiments were done with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Cell culture and reagents
T24 cells were cultured in DMEM supplemented with 10% bovine growth serum (Hyclone) and penicillin-streptomycin at 37°C in 5% CO2. Reagents used were tamoxifen (MP) 7,12-dimethylbenz(a)anthracene (DMBA; Acros), 12-O-tetradecanoylphorbol-13-acetate (TPA; LC Laboratories) 5Z-7-oxozeaenol (17), Bouin fixation fluid (EMD), anti-poly(ADP-ribose) polymerase (Cell Signaling), and anti-BrdU (Abcam).
Tumor experiments
Control (TAK1flox/flox) and inducible epidermal-specific TAK1 deletion (K14-CreERT TAK1flox/flox) mouse littermates (6–9 weeks old) were treated with a single application of 200 nmol 7,12-dimethylbenz(a)anthracene (DMBA; Acros) followed 1 week later with thrice weekly treatment of 5 nmol 12-O-tetradecanoylphorbol-13-acetate (TPA; LC Laboratories) for 19-27 weeks (18). All agents were applied in 200 ⎧l acetone. For a tumor regression assay, TPA treatment was terminated and 0.5-1 mg/mouse tamoxifen was intraperitoneally injected for 5 consecutive days. The 5th day of tamoxifen injection is designated as day 0. To test the effect of TAK1 inhibition in skin papilloma development, CD1 mice were given with a single application of 200 nmol DMBA followed one week later with twice weekly treatment of acetone + 5 nmol TPA or 100 nmol 5Z-7-oxozeaenol + 5 nmol TPA for 25 weeks. TPA was treated 30 min after acetone or 5Z-7-oxozeaenol treatment.
Immunohistochemistry
dUTP nick-end labeling (TUNEL) assay was performed on Bouin-fixed paraffin sections using an apoptotic cell death detection kit (Promega) according to the manufacturer's instructions. Sections were counterstained with DAPI.
Quantitative real-time PCR
Total RNA from keratinocytes was isolated using RNeasy Mini (Qiagen). cDNA was synthesized using TaqMan reverse transcription reagents (Applied Biosystems). mRNA levels of NAD(P)H:quinone oxidoreductase 1 (NQO1) and GAPDH were analyzed by real-time PCR with SYBR Green (Applied Biosystems). NQO1 primer, CATTCTGAAAGGCTGGTTTGA and CTAGCTTTGATCTGGTTGTCAG; and GAPDH primers, GAAGGTCGCTGTGAACGGA and GTTAGTGGGGTCTCGCTCCT were used. Expression levels of GSTP1, GSTM1, glutamylcysteine ligase catalytic subunit (GCLC), MIP2, and GAPDH were also analyzed by TaqMan gene expression assay (Applied Biosystems). Results were analyzed using the comparative Ct Method. Values were normalized to the level of GAPDH mRNA.
ROS measurement
For ROS measurement in skin tumors and normal skin, harvested skin were embedded and frozen in OCT compound and sections were prepared. Sections were stained with 5 ⎧M CM-H2DCFDA (Invitrogen) for 1.5 h at 37°C. Images were taken using a fluorescent microscope (BX41, Olympus) controlled by IPlab (Scanalytics). Randomly selected areas were photographed with the same exposure time. The images were processed using the same fixed threshold in all samples by Photoshop software, and representative images are shown.
Transient siRNA system
Non-targeting control siRNAs were purchased from Dharmacon (Non-targeting siRNA#1) and siRNAs targeted against TAK1 were generated (siTAK1#2, 5′-GAGAUCGACUACAAGGAGA- 3′; siTAK1#3, 5″-GGCAAAGCAACAGAGUGAAUCUGGA-3′). T24 cells were transfected with the siRNAs by using Lipofectamine 2000 reagent (Invitrogen).
Immunoblotting
T24 cells were washed once with ice-cold phosphate-buffered saline and whole cell extracts were prepared using a lysis buffer (20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 ⎧M aprotinin, 0.5% Triton X-100). Cell extracts were resolved on SDS-PAGE and transferred to Hybond-P membranes (GE Healthcare). The membranes were immunoblotted with various antibodies, and the bound antibodies were visualised with horseradish peroxidase-conjugated antibodies against rabbit or mouse IgG using the ECL or ECL advance Western blotting detection kit (GE Healthcare).
Annexin V-binding assay
To determine apoptosis, Annexin V-Alexa Fluor 488 binding was performed according to the manufacturer's protocol (Invitrogen). Fluorescence was detected by flow cytometry and analyzed by FlowJo software (Tree Star Inc).
Statistical analyses
Statistical comparisons were made using Student's t test or log-rank test.
Results and Discussion
Ablation of TAK1 causes regression of skin tumors in vivo
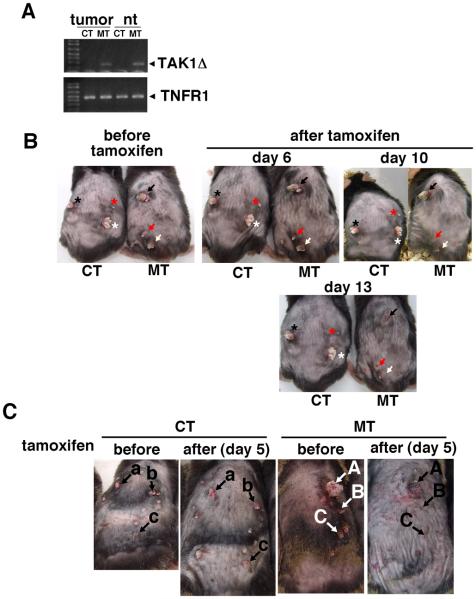
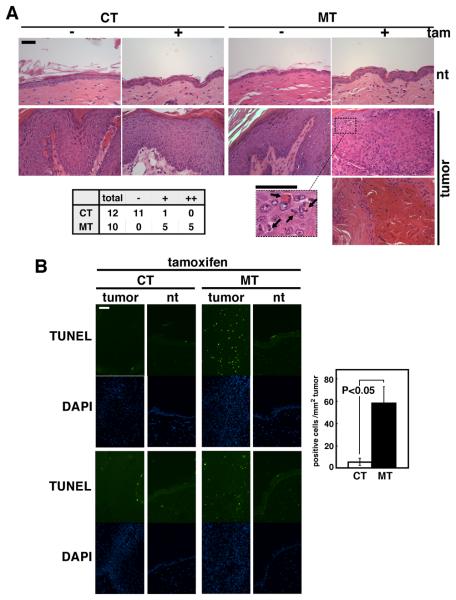
Mice having constitutive epidermal-specific deletion of TAK1 (K5-Cre TAK1flox/flox) develop severe skin damage at neonatal day 5-7 and is lethal around postnatal day 7-8 (10). We have found that this damage is blocked by deletion of TNF receptor 1 gene (TNFR1−/−). TNF is constantly expressed at some levels in the normal skin, and, when TAK1 is ablated, TNF upregulates ROS resulting in apoptosis (9,10). By using an inducible epidermal-specific gene deletion system (K14-CreERT TAK1flox/flox), we have examined the role of TAK1-ROS pathway in adult mouse skin. In adult mice, TAK1 deficiency modestly increases apoptosis; however it does not cause severe skin damage as observed in the neonatal TAK1 mutant mice. Therefore, TAK1 is important but not critically involved in cell survival in the normal adult epidermis. We then asked whether epidermal-derived tumor cells in vivo are more sensitive to TAK1 ablation. We utilized inducible epidermal-specific TAK1 knockout (K14-CreERT TAK1flox/flox) mice and examined the effect of TAK1 deletion in pre-existing epidermal-derived tumors. Skin tumors were induced by initiation with 7,12-dimethyl-benz[a]anthracene (DMBA) and promotion with 12-O-tetradecanoylphorbol-13 acetate (TPA) (18). tak1 gene deletion was induced by 5 consecutive day treatment of 0.5-1 mg/mouse/day tamoxifen. This treatment regimen induced tak1 gene deletion in both tumors and normal regions of the skin (Fig. 1A) and did not cause extensive apoptosis in the normal epidermis (Fig. 2B). We performed 4 independent experiments, and all skin tumors on K14-CreERT TAK1flox/flox mice disappeared within 5-13 days post tamoxifen treatment (n=20) (Fig. 1B, 1C and Table 1). In comparison, few tumors regressed in tamoxifen treated control TAK1flox/flox mice (n=14) (Fig. 1B, 1C and Table 1). TAK1 deleted tumors started regressing at 3 days after the completion of tamoxifen treatment (day 3). Histological analysis revealed that TAK1-deficient tumors contained large numbers of cells having fragmented nuclei (Fig. 2A) and exhibited extensive apoptosis (Fig. 2B) at day 3-6. In comparison, no pronounced increased apoptosis or tissue damages was observed in normal skin (Fig. 2A and 2B). TAK1-deficient tumors lost cellularity starting from day 6 (Fig. 2A, bottom panel). These results demonstrate that ablation of TAK1 is selectively cytotoxic to epidermal-derived tumors.
Fig. 1. Skin tumors regress by tak1 gene deletion.
(A) tak1 gene deletion was examined in tumors (tumor) or normal region of the skin (nt) at 7 days after tamoxifen treatment (0.5 mg/mouse/day for 5 consecutive days). Primers used were capable of amplifying only the shorter tak1 gene after Cre-mediated recombination. TNFR1 gene was used as a loading control (Omori et al., 2008). CT, TAK1 flox/flox; MT, K14-CreERT TAK flox/flox. (B) Tumors in the same mice before and after tamoxifen treatment are shown. Skin papillomas were induced by DMBA and TPA method. Four TAK1 flox/flox (CT) and five K14-CreERT TAK flox/flox (MT) mice were treated with tamoxifen (0.5 mg/mouse/day) for 5 consecutive days. The tumors were monitored right before the initiation of the tamoxifen injection and 6, 10 and 13 days after the tamoxifen treatment. All tumors in MT mice completely regressed by 13-14 days after the tamoxifen injection. Representative pictures are shown. The asterisk or arrow indicates the same tumor. Sizes of tumors shown before tamoxifen were 2-8 mm-diameter. (C) Similar experiments as (A) were performed using a slightly different treatment regimen. Six TAK1 flox/flox (CT) and Six K14-CreERT TAK flox/flox (MT) mice having skin papillomas were treated with tamoxifen (1 mg/mouse/day) for 5 consecutive days. The tumors were monitored right before the initiation of the tamoxifen injection (before) and 4 days after the tamoxifen treatment (after). All tumors in MT mice regressed by 4 days after the tamoxifen injection. Representative pictures of a CT and MT mouse are shown. Each labeled arrow indicates the same tumor. Sizes of tumors shown before tamoxifen were 1-8 mm-diameter.
Fig. 2. Ablation of TAK1 upregulates apoptosis in tumors.
Tumors and normal skin (normal tissue, nt) regions of the skin of TAK1 flox/flox (CT) and K14-CreERT TAK flox/flox (MT) mice were harvested when tumors started regressing at day 3-6 after the tamoxifen treatment. H&E (A) and TUNEL (B) staining was performed with Bouin-fixed sections. (A) The bottom panel shows tumors that lost cellularity. Arrows indicate fragmented nuclei. Tumors are categorized according to the level of apoptotic cells (bottom left table); −, no apoptotic cells; +, ≥10% tumor cells are apoptotic; ++, loss of cellularity similar to the bottom panel image. Scale bar, 40 μm. (B) DAPI staining (blue) images (2nd and 4th panels) are shown in the same fields of TUNEL staining (green) images (1st and 3rd panels). Data are representative images of tumors and normal regions of two different mice. TUNEL-positive cells in tumors were counted using CT and MT, and the mean±S.E. are shown (n=3). Scale bar, 40 μm.
Table 1.
Papillomas before and after tak1 gene deletion
| Exp.1a | 1-3 mm | > 3mm | ||
|---|---|---|---|---|
| Genotype | before | after | before | after |
| TAK1flox/flox | 10 | 5 | 8 | 9* |
| K14-CreERTTAK1flox/flox | 35 | 0 | 15 | 0 |
| Exp.2b | 1-3 mm | > 3mm | ||
|
| ||||
| Genotype | before | after | before | after |
|
| ||||
| TAK1flox/flox | 69 | 57 | 15 | 10 |
| K14-CreERTTAK1flox/flox | 39 | 5 | 28 | 2 |
Single-time DMBA treated mice were treated with TPA for 27 weeks and subsequently injected 1 mg/mouse tamoxifen for 5 consecutive days. The total number of papillomas in three TAK1flox/flox and seven K14-CreERTTAK1flox/flox mice were counted before tamoxifen injection (before) and 14 days after the completion of tamoxifen injection (after).
One tumour
Single-time DMBA treated mice were treated with TPA for 22 weeks and subsequently injected 1 mg/mouse tamoxifen for 5 consecutive days. The total number of papillomas in six TAK1flox/flox and six K14-CreERTTAK1flox/flox were counted before tamoxifen injection (before) and 4 days after the completion of tamoxifen injection (after).
Cell proliferation is not affected by ablation of TAK1
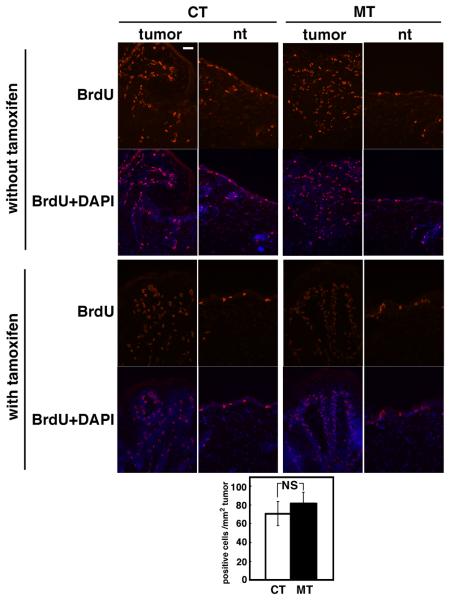
To determine the mechanism by which ablation of TAK1 causes tumor regression, we examined whether ablation of TAK1 impairs tumor cell proliferation. BrdU incorporation was examined in control and TAK1-deficient epidermis having skin tumors. Cell proliferation was not altered by TAK1 deletion in tumors or normal epidermis (Fig. 3). This indicates that the TAK1 pathway is not important for tumor cell proliferation, and that the cause of tumor regression is not due to impaired cell proliferation.
Fig. 3. Ablation of TAK1 does not affect cell proliferation.
TAK1 flox/flox (CT) and K14-CreERT TAK flox/flox (MT) mice having skin papillomas were treated with tamoxifen (0.5 mg/mouse/day) for 5 consecutive days. Mice with or without tamoxifen treatment at day 6 were injected with 0.1 mg/g of BrdU 1.5 h prior to harvest the samples, and frozen sections were subjected to immunohistochemical analysis using anti-BrdU antibody in tumors (tumor) or normal region of the skin (nt). Scale bar, 40 μm. The analysis was done in two independent experiments and photomicrographs of representative images of tumors and normal regions are shown. BrdU-positive cells in tumors were counted and means±S.E are shown (CT, n=5; MT, n=6).
ROS is upregulated in TAK1-deficient skin tumors
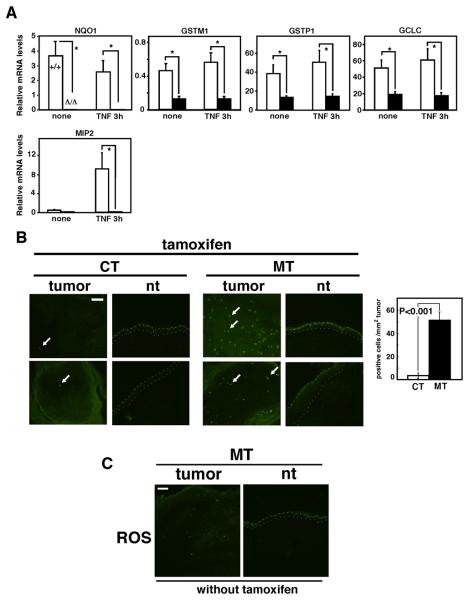
We have previously demonstrated that TAK1-c-Jun pathway regulates the levels of ROS in keratinocytes (9). To further investigate the mechanism by which TAK1 regulates antioxidant capacity in keratinocytes, we examined the expression levels of several cellular antioxidant genes with and without TNF stimulation (Fig. 4A). Among the antioxidant genes we examined, the mRNA levels of NAD(P)H:quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GSTM1 and GSTP1) and catalytic subunit of glutamylcysteine ligase (GCLC) were reduced by TAK1 ablation in both unstimulated and TNF-stimulated keratinocytes. However, other antioxidant genes such as manganese superoxide dismutase (MnSOD) were found to be unchanged by TAK1 ablation (9). Thus, ablation of TAK1 downregulates the levels of a subset of cellular antioxidant genes and lowers cellular antioxidant capacity in keratinocytes. We attempted to analyze the levels of the above cellular antioxidant genes in tumors; however we were unable to obtain enough RNA from TAK1-deficient tumors because the tumors were highly apoptotic. We anticipate that the ablation of TAK1 reduces antioxidant capacity in tumors as observed in normal keratinocytes, and that the ROS levels might be higher in tumors due to increased production of ROS in tumor cells. We tested the levels of ROS in skin tumors by staining with the ROS-reactive dye CM-H2DCFDA at days 3-6 (Fig. 4B). TAK1-deficient tumors accumulated high levels of ROS, whereas normal skin with tak1 gene deletion or tumors in control mice exhibited only marginal or no accumulation of ROS compared with the control normal skin in TAK1flox/flox mice (Fig. 4B). Tumors harvested before tak1 gene deletion did not exhibit ROS accumulation (Fig. 4C), indicating that increased ROS was caused by tak1 gene deletion. These results are consistent with the idea that the ablation of TAK1 in tumor cells reduces their antioxidant capacity resulting in increased ROS, which causes tumor cell apoptosis.
Fig. 4. Ablation of TAK1 upregulates ROS in tumors.
(A) Real-time PCR analysis to quantify mRNA levels in TAK1 wild-type (+/+) and Δ/Δ keratinocytes. Keratinocytes were left untreated or treated with 20 ng/ml TNF for 3 h. MIP2 was used as a control for TNF responsive genes. Relative mRNA levels were calculated using GAPDH mRNA. The data are means±S.E.; NQO1, n=4; GSTM1, n=4; MIP2, GSTP1, GCLC, n=10. *, P<0.05. (B) ROS were detected in unfixed fresh frozen sections from TAK1 flox/flox (CT) and K14-CreERT TAK flox/flox (MT) mice by CM-H2DCFDA. Arrows indicate examples of CM-H2DCFDA positive cells. Photomicrographs are representative images of tumors and normal regions of two different mice. Scale bars, 40 μm. ROS-positive cells in tumors were counted and the mean±S.E. are shown (CT, n=5; MT, n=9). (C) Sections from tumors and normal regions of the skin of K14-CreERT TAK flox/flox (MT) mice without tamoxifen treatment. ROS staining of unfixed frozen sections using CM-H2DCFDA are shown. Scale bar, 40 μm.
Ablation of TAK1 causes hypersensitivity to apoptosis in T24 cells
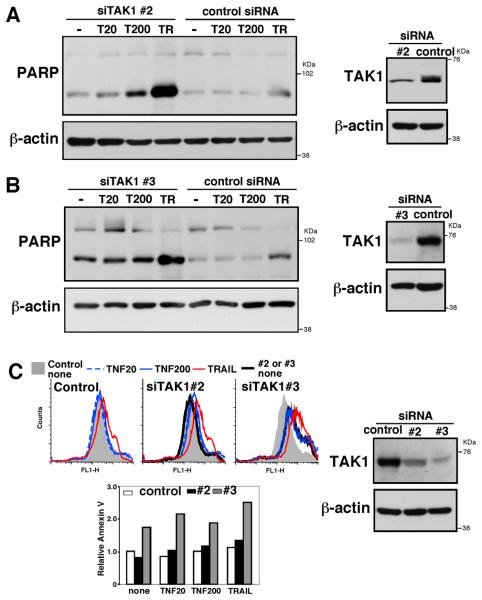
Our results thus far demonstrate that skin tumor regression is correlated with TAK1 deficiency-dependent apoptosis. To further verify whether ablation of TAK1 can directly kill tumor cells, we utilized cultured cells. DMBA-TPA skin tumors are highly associated with oncogenic mutations in Ras specifically in H-Ras (18-20). We therefore examined whether TAK1 deficiency can cause apoptosis in a tumor cell line having the H-Ras oncogenic mutation. We utilized T24 tumor cell line, which possesses the oncogenic H-Ras G12V mutation, and tested the effect of TAK1 knockdown. Non-target siRNA and 2 different siRNAs against human TAK1 (siTAK1#2 and siTAK1#3) were transfected into T24 cells, and cells were treated with apoptosis inducers, TNF and TRAIL. Caspase activation was analyzed by cleavage of poly(ADP-ribose) polymerase (PARP) and Annexin V-binding was also analyzed. In control siRNA treated T24 cells, TRAIL slightly increased cleaved PARP, and Annexin V-binding while TNF had no effect (Fig. 5A-C). TAK1 siRNA (siTAK1#2) reduced the levels of TAK1 (Fig. 5A, right panels) and increased cleaved PARP upon TNF and TRAIL treatment (Fig. 5A). siTAK1#2 also slightly increased Annexin V-binding (Fig. 5C). The other TAK1 siRNA (siTAK1#3) effectively reduced the level of TAK1 and highly increased cleaved PARP and Annexin V-binding (Fig. 5B and C). We note that we observed morphological changes consistent with apoptotic cells (shrinking cells) in T24 cells expressing siTAK1#3 even in the absence of TNF or TRAIL, and we could detect cleaved PARP in unstimulated cells (Fig. 5B, most left lane). These results indicate that the level of TAK1 is correlated with resistance to apoptosis in tumor cells having H-Ras mutation.
Fig. 5. Ablation of TAK1 in H-Ras transformed cells causes cell death.
(A, B) T24 cells were transfected with non-target siRNA (control) or TAK1 targeted siRNAs (siTAK1#2 (A) and siTAK1#3 (B)), and stimulated with 20 ng/ml TNF (T20), 200 ng/ml TNF (T200) for 4.5 h (A) or 10 h (B) or 100 ng/ml TRAIL (TR) for 4.5 h (A) or 2 h (B). Cell lysates were analyzed by immunoblotting with anti-TAK1 and anti-PAPR. (C) T24 cells were transfected with non-target, siTAK#2 or siTAK1#3, and stimulated with 20 ng/ml TNF (T20), 200 ng/ml TNF (T200) for 10 h or 100 ng/ml TRAIL (TR) for 2 h. Apoptotic cells were analyzed by Annexin V-binding assay. The staining of unstimulated non-target siRNA cells are shown in gray shadow all three panels (upper left panels). The fluorescence units relative to that of unstimulated non-target siRNA are shown (lower left panel). TAK1 expression was analyzed by immunoblotting with anti-TAK1 (right panels). Data are representative of two independent experiments with similar results.
A selective TAK1 inhibitor downregulates skin tumor development
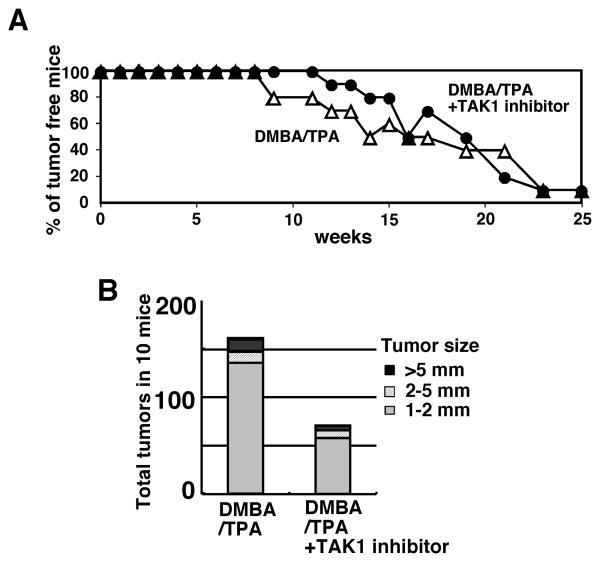
We also wanted to examine whether ablation of TAK1 could block tumor development. However, we found that induction of tak1 gene deletion together with the tumor promoter TPA treatment caused a severe inflammatory condition in normal epidermis which precluded the use of these mice. We speculated that complete deletion of TAK1 strongly enhances TPA-induced inflammation. We therefore chose to use a selective TAK1 inhibitor, 5Z-7-oxozeaenol (17), which partially blocks TAK1 activity. We examined whether inhibition of TAK1 activity could reduce tumor development in DMBA-TPA mouse skin tumor models (Fig. 6). After the initiation of tumors by DMBA treatment, 5Z-7-oxozeaenol or vehicle was applied to the skin 30 min prior to the each TPA treatment. The tumor incidence was not significantly altered by 5Z-7-oxozeaenol treatment (Fig. 6A). However, 10 mice with vehicle treatment developed total 160 tumors at 25 weeks, whereas only 71 was observed in 10 mice with 5Z-7-oxozeaenol treatment (Fig. 6B). These results suggest that inhibition of TAK1 kinase may inhibit tumor development at least in the mouse skin tumor model. Although these results raise the possibility that the TAK1 inhibitor may be useful to treat tumors, 5Z-7-oxozeaenol is a selective but not specific inhibitor of TAK1, which may cause side effects. We note here that treatment pre-existing tumors with 5Z-7-oxozeaenol did not alter the size of tumors, suggesting that a stronger inhibition of TAK1 may be required for regression of pre-existing tumors.
Fig. 6. TAK1 inhibitor inhibits skin tumorigenesis.
CD1 mice (n=10 for each treatment) were treated with a single application of 200 nmol DMBA followed 1 week later with twice weekly treatment of 5 nmol TPA for 25 weeks. Control vehicle (acetone) or 100 nmol 5Z-7-oxozeaenol was topically treated 30 min before every TPA treatment. (A) The number of mice having tumors (>1 mm) was counted every week. P=0.88 (not significant difference) by log-rank test. (B) The total numbers of tumors at 25 weeks of TPA treatment were analyzed. 5Z-7-oxozeaenol treatment alone without DMBA or TPA did not induce skin papilloma or any skin abnormality.
Highly proliferating cells such as tumor cells produce more ROS compared to normal tissues due to increased activity in respiratory chain, and balance the ROS levels by upregulating cellular antioxidant capacity. TAK1 ablation-mediated reduction of cellular antioxidant capacity highly impacts tumors which results in the selective killing of tumor cells. TAK1 is an essential intermediate activating transcription factor NF-κB and AP1, and both are involved in the transcriptional regulation of a number of genes associated with cell survival (21-23). NF-κB and AP1 regulate anti-apoptotic genes and cellular antioxidant genes. Therefore, it is likely that TAK1 regulation of both NF-κB and AP1 plays a central role in tumor cell survival not only by reducing ROS but also by inducing anti-apoptotic genes. Approaches to inhibit TAK1 signaling might provide a new avenue of cancer therapy.
Acknowledgements
We thank S. Akira for TAK1-floxed mice, B.J. Welker and M. Mattmuler for support. This work was supported by National Institutes of Health Grant GM068812 and GM084406 (to J. N-T.).
References
- 1.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark GR, Matsumoto K. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol. Cell. Biol. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 4.Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc. Natl. Acad. Sci. U S A. 2006;103:11677–11682. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat. Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 7.Pasparakis M. Regulation of tissue homeostasis by NF-κB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 8.Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K, Jobin C, Ninomiya-Tsuji J. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J. Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 12.Dolado I, Nebreda AR. AKT and oxidative stress team up to kill cancer cells. Cancer Cell. 2008;14:427–429. doi: 10.1016/j.ccr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Nogueira V, Park Y, Chen C-C, Xu P-Z, Chen M-L, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature Senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by ®-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxo-zeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPKKK. J. Biol. Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 18.Balmain A, Pragnell IB. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983;303:72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- 19.Loomis KD, Zhu S, Yoon K, Johnson PF, Smart R. C. Genetic ablation of CCAAT/enhancer binding protein 〈 in epidermis reveals its role in suppression of epithelial tumorigenesis. Cancer Res. 2007;67:6768–6776. doi: 10.1158/0008-5472.CAN-07-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown K, Buchmann A, Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci U S A. 1990;87:538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karin M, Lin A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 22.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-κB Signals Induce the Expression of c-FLIP. Mol. Cell. Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]