Abstract
The significance of angiogenesis in cancer biology and therapy is well established. In this study, we utilized the prototypical RIP-Tag model of multistage pancreatic islet tumorigenesis to show that the nuclear receptor COUP-TFII is essential to regulate the balance between pro- and anti-angiogenic molecules that influence the angiogenic switch in cancer. Conditional ablation of COUP-TFII in the tumor microenvironment severely compromised neoangiogenesis and lymphangiogenesis during pancreatic tumor progression and metastasis. We found that COUP-TFII plays a cell autonomous role in endothelial cells to control blood vessel sprouting by regulating cell proliferation and migration. Mechanistic investigations revealed that COUP-TFII suppressed VEGF/VEGFR-2 signaling by transcriptionally repressing expression of VEGFR-1, thereby curtailing a central angiogenic driver for vascular growth. Together, our results implicate COUP-TFII as a critical factor in tumor angiogenesis via regulation of VEGF/VEGFR-2 signaling, suggesting COUP-TFII as a candidate target for anti-angiogenic therapy.
Keywords: Nuclear Receptor, Endothelial Sprouting, VEGFR-2 signaling
Introduction
Tumor angiogenesis, the development of new capillaries from preexisting blood vessels, is indispensable for tumor growth and metastasis (1–3). Without the concomitant growth of new blood vessels, tumors cannot expand beyond a minimal size, invade locally, or metastasize to distant sites (4). It is widely accepted that angiogenesis is regulated by a balance of pro- and anti-angiogenic molecules, when the balance shifts in favor of angiogenesis inducers, and an angiogenic switch activates the normally quiescent vasculature to develop new blood vessels (2, 5). Thus, the “angiogenic switch” during tumorigenesis is recognized as a rate-limiting secondary event that can be effectively targeted as a therapeutic approach to treat cancer (4). Pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), acidic and basic fibroblast growth factors (FGF1 and FGF2), members of the angiopoietin family (Ang1 and Ang2), and Platelet-derived growth factor (PDGF), have been shown to induce signaling cascades to increase endothelial cell proliferation, survival, and migration to promote angiogenesis (6). Among them, VEGF is a major mediator of angiogenesis, which transmits its signals primarily through binding to its receptor VEGFR-2, a receptor tyrosine kinase, on the surface of blood endothelial cells to promote angiogenesis (7).
Using a rat insulin promoter (RIP) to ectopically express the oncoprotein SV40 T antigen in the β-cells of pancreatic islets (RIP1-Tag2) (8), it has been realized that the angiogenic switch plays a crucial role in the development of multistage β-cell carcinogenesis (2, 8). Histopathological studies have revealed three distinct stages of tumor progression in this model, with hyperplastic lesions observed at 5 weeks of age, followed by the development of angiogenic islets at 7–10 weeks of age. A few weeks later, encapsulated adenomas emerge, of which about 25% progress into invasive carcinomas (3).
COUP-TFII, a member of the steroid/thyroid nuclear hormone receptor superfamily (9), plays critical roles in organogenesis (10–14). We showed that COUP-TFII is essential for tumor angiogenesis through regulation of a pericyte-derived paracrine signal (Ang1/Tie2 signaling) that impacts the endothelium (15). However, the fact that providing Ang1 can only partially rescue the angiogenic defect suggested that COUP-TFII might regulate other factors to control angiogenesis. To further dissect the underlying mechanism, we took advantage of the RIP1-Tag2 spontaneous pancreatic cancer model to investigate the role of COUP-TFII in pancreatic cancer progression. Here we showed that ablation of COUP-TFII in adult mice markedly compromised neoangiogenesis and lymphangiogenesis. We demonstrated that COUP-TFII plays an autonomous role in blood endothelial cells to control vessel sprouting by directly regulating VEGFR-1 expression. These findings elucidate the important roles of COUP-TFII within the blood endothelial cells to regulate tumor angiogenesis.
Materials and Methods
Animal experiments
RIP1-Tag2 mice were obtained from the MMHCC Repository and COUP-TFIIflox/flox and ROSA26CRE-ERT2 mice were as previously described (12, 16). Mice were maintained according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The RIP1-Tag2 mice were crossed with ROSA26CRE-ERT2/+; COUP-TFIIflox/flox mice to generate mice with genotypes of RIP1-Tag2+/−/ROSA26CRE-ERT2/+/COUP-TFIIflox/flox and RIP1-Tag2+/−/COUP-TFIIflox/flox, which were then treated with Tamoxifen at the age of 5 weeks (17).
Assessment of angiogenic islets, tumor burden and tumor frequency
Angiogenic islets, morphologically exhibited a reddish patch in a white nodular background, were isolated after mice were perfused with collagenase IV solution (18). In other experiments, tumors were isolated by microdissection, and tumor number and weight were measured.
Cell cultures and reagent
Primary human umbilical vein endothelial cells (HUVEC) were purchased from Lonza and cultured according to the supplier's recommendations. Human primary LEC cells isolated from neonatal human foreskin as previously described (19), were kindly provide by Dr. Young-Kwon Hong (Keck School of Medicine, University of Southern California). Small interfering RNA duplexes targeting COUP-TFII, VEGFR-1 and Control Non-Target siRNA were purchased from Dharmacon. Cells were transfected with siRNA duplexes by using Oligofectamine (Invitrogen).
HUVEC proliferation and migration analysis
For cell cycle analysis, cells were stained with propidium iodide and analyzed by a FACScan flow cytometer (Becton Dickinson). The number of viable cells was measured using MTT cell proliferation kit (Promega). Modified Boyden chambers with filter inserts (BD) were employed for the Chamber migration assay, and blood endothelial cells were stimulated by VEGF (5 ng/ml).
Western blot analysis
Primary antibodies used in this study were as follows: phospho-VEGFR-2 (Tyr1175) and VEGFR-2 (Cell Signaling), COUP-TFII (Perseus Proteomics), and VEGFR-1 (Acris Antibody). Tumor lysates were immunoprecipitated with anti-VEGFR-2 overnight and subjected to immunoblotting with anti-phospho-VEGFR-2 antibodies. Protein loading amount was normalized against total VEGFR-2. For VEGFR-1 antibody neutralization assay, anti-VEGFR-1 antibody (R&D) or control IgG was added at a concentration of 250 ng/ml.
HUVEC sprouting assay
HUVEC spheroids of a defined cell number were generated overnight and embedded into collagen gels as described (20). VEGF was used to stimulate cell sprouting at a concentration of 5 ng/ml.
Histology and immunohistochemistry
Primary antibodies used were as follows: CD31 (BD), NG2 (Millipore), cleaved Caspase 3 (Cell Signaling), phophorylated-histone H3 (Cell Signaling), LYVE-1 (AngioBio), Insulin (DAKO) and large T antigen (Calbiochem).
Quantitative real-time RT-PCR
Gene expression assay was performed using the ABI PRISM 7500 Sequence Detector System (Applied Biosystems), and all mRNA quantities were normalized against 18S RNA or CD31. The primers/probes used in this study were purchased from Applied Biosystems.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using an assay kit (Millipore) and monoclonal anti-COUP-TFII (Perseus Proteomics) following the manufacturer’s recommendation. The primers used for detection of the VEGFR-1 promoters were as follows: 5’-CTGGGAGGAAGAAGAGGGTAGGTG-3’ and 5’-CGAGGGCGGGGGCGATTTAT-3’. The primers for negative control were: 5’-ACATTGGCAAGCTGAGAACC-3’ and 5’-GACATTGCTTCCCGGATATG-3’.
Image quantification and statistics analysis
Images of fluorescent staining were analyzed and quantified using National Institutes of Health (NIH) Image J. Data represent mean ± SEM. Statistical significance was calculated by Student’s t test or the Fisher Exact test.
Results
Efficient deletion of COUP-TFII in RIP-Tag2 mice
To explore the role of COUP-TFII on pathological vascular processes and tumorigenesis, we inactivated the COUP-TFII gene in the adult using the tamoxifen inducible Cre-recombinase system (ROSA26CRE-ERT2/+ mice) (16). RIP1-Tag2 mice were crossed with COUP-TFIIflox/flox and ROSA26CRE-ERT2/+ mice to generate genotypes of RIP1-Tag2+/−/COUP-TFIIflox/flox (RIP/+; F/F) and RIP1-Tag2+/−/ROSA26CRE-ERT2/+/COUP-TFIIflox/flox (RIP/+; Cre/+; F/F). Both genotypes were subjected to tamoxifen treatment at the age of 5 weeks. COUP-TFII were ablated in RIP/+; Cre/+; F/F mice, while RIP/+; F/F mice served as controls. Before assessing the role of COUP-TFII in pancreatic β-cell carcinogenesis, we used double immunostaining of COUP-TFII and T antigen to evaluate the efficiency of COUP-TFII deletion (Supplementary Fig. 1A). T antigen, specifically expressed in β tumor cells, was used to mark the tumor cells. The loss of COUP-TFII positive cells in Supplementary Fig 1A clearly showed that COUP-TFII was efficiently deleted in the mutant mice, which was further confirmed by western blot analysis (Supplementary Fig. 1B). Since COUP-TFII expression was not co-localized with T antigen expression, it suggests that COUP-TFII is expressed within the tumor microenvironment, but not in the tumor cells. To determine the cell types that expressed COUP-TFII, we used CD31 (endothelial cell) and NG2 (pericyte) as markers. We observed that COUP-TFII was strongly expressed in endothelial cells (Supplementary Fig. 1C; arrow) and pericytes (arrowhead) within the tumor vasculature.
Involvement of COUP-TFII in the angiogenic switch and tumor progression
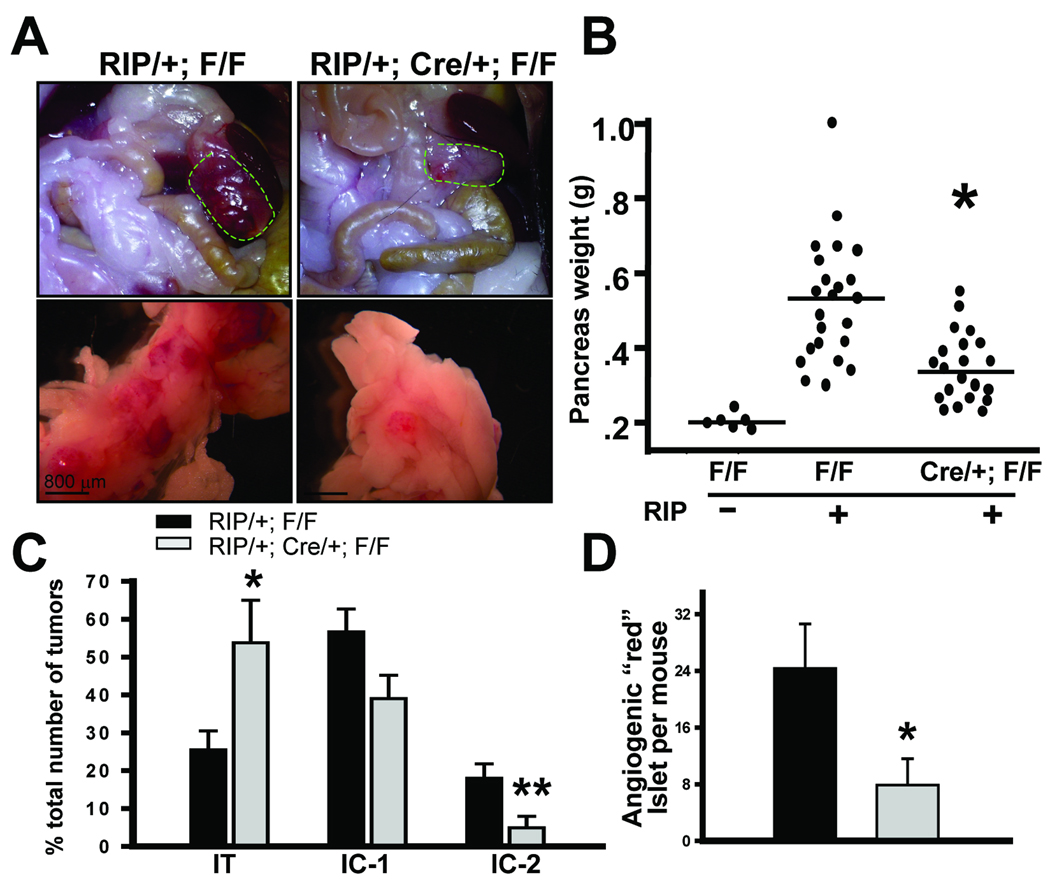
Next, we examined the effects of COUP-TFII depletion on RIP-Tag2 tumorigenesis. Control and COUP-TFII mutant mice were sacrificed at the age of 12 weeks, shortly before the mice succumbed to hypoglycemia caused by the insulin-expressing tumors. We observed that tumors from COUP-TFII deficient mice were significantly smaller and appeared much paler compared to the control mice (Fig. 1A). In accordance, COUP-TFII mutant animals displayed a significant decrease in aggregate tumor burden in comparison with control animals (Fig. 1A and B).
Fig. 1. Importance of COUP-TFII in angiogenic switch and tumorigenesis in RIP1-Tag2 mice.
(A) Representative images of tumors (circle) from control and COUP-TFII mutant mice. (B) Aggregate tumor burden per mouse is depicted for control and COUP-TFII mutant mice (N=20). The weight of the pancreas in COUP-TFIIflox/flox mice was used as an indicator for tumor-free basal weight. (C) Quantification of tumor invasiveness by H&E-stained sections of pancreatic tissue from control and mutant mice. (D) Quantification of angiogenic islets in control and COUP-TFII mutant mice (N=5). * P<0.05, ** P<0.01
Pancreatic tumors in the RIP1-Tag2 model can be classified into three types: encapsulated tumors (IT), microinvasive carcinomas (IC1), and highly invasive carcinomas (IC2) according to established criteria (21). IT have well-defined margins and frequent fibrous capsules, and IC1 have focal regions of invasion, while IC2 have wide fronts of invasion that prominently intermingled with the surrounding acinar tissue. To evaluate whether loss of COUP-TFII would affect tumor malignance, we performed histological analysis to compare the abundance of the various stages of tumors from control and mutant mice. Fig. 1C showed that COUP-TFII deficient mice displayed a higher incidence of encapsulated islet tumors (IT), concomitantly with a decrease of widely invasive carcinomas (IC2). The decreased incidence of IC2 suggested that loss of COUP-TFII inhibited the invasion of pancreatic β tumor cells.
It has been shown that angiogenic islets are formed during 5 to 10 weeks of age and play a crucial role during β cell tumorigenesis. We hypothesized that the inhibited tumor growth by loss of COUP-TFII might be due to a defect in the angiogenic switch. To this end, we isolated angiogenic islets from 10.5-week-old control and mutant mice to address a possible role for COUP-TFII in the angiogenic switch. We observed that COUP-TFII-deficient mice exhibited a 60% decrease in the number of reddish angiogenic islets compared with control animals (Fig. 1D). The angiogenic switch defects exhibited by COUP-TFII mutant mice indicated that COUP-TFII is critically involved in the regulation of the balance between pro- and anti-angiogenic factors during pancreatic tumorigenesis.
Loss of COUP-TFII impairs tumor angiogenesis
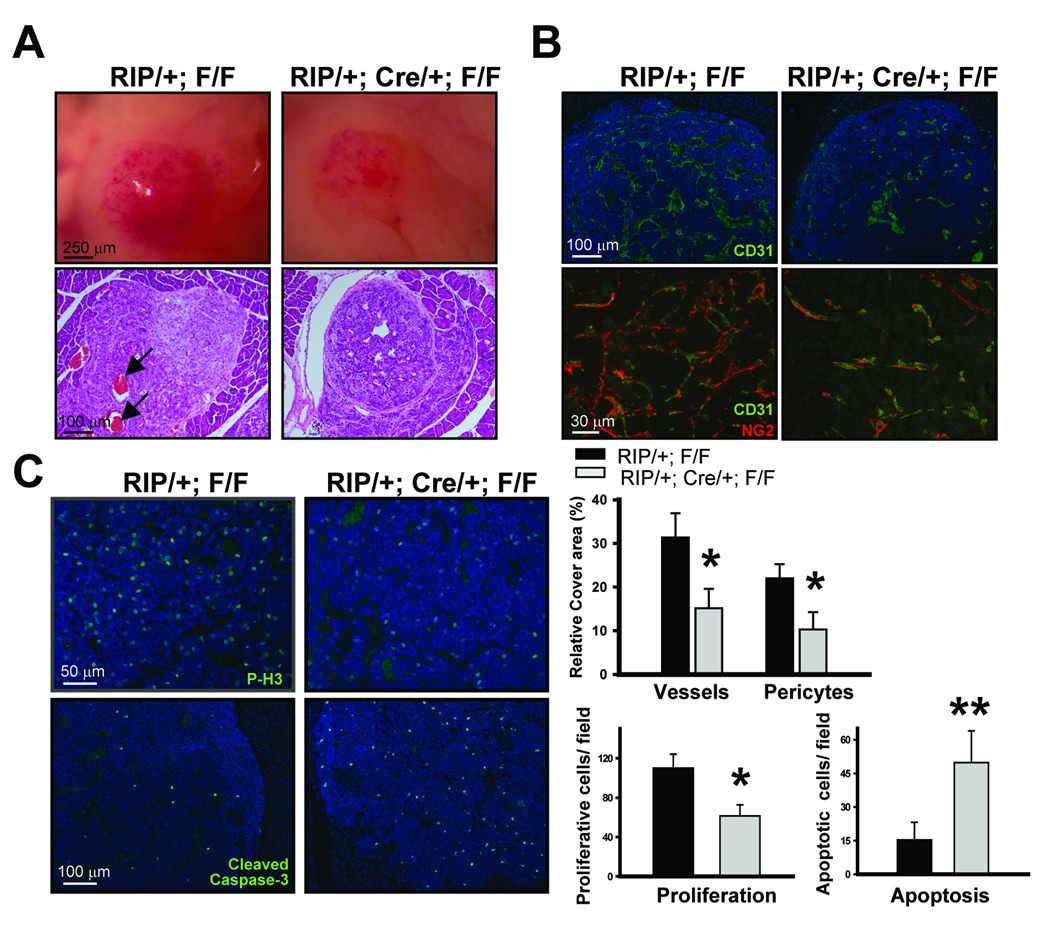
Since loss of COUP-TFII resulted in a significant reduction of angiogenic islet formation, we hypothesized that COUP-TFII might be important for tumor angiogenesis. Morphological analysis of lesions from control mice showed hypervascularized “red islets” with a microhemorrhaging vasculature, indicative of a robust angiogenic response in control mice (Fig. 2A, upper panel). In sharp contrast, the islets from COUP-TFII deficient mice displayed a decrease in the vasculature network, suggesting that angiogensis was compromised in the absence of COUP-TFII. Furthermore, histological analysis revealed that there was microhemorrhaging as observed by the accumulation of erythrocytes inside the islets in the control mice (Fig. 2A, lower panel), whereas this was largely absent in COUP-TFII mutant mice. To ensure that the difference observed was due to the angiogenic defect of mutant mice, we performed CD31 and NG2 immunostaining to examine vessel density within the angiogenic islets. It is clear that the vascular network was well formed in the control angiogenic islet, but not in COUP-TFII mutant mice (Fig. 2B). Quantification of the vascular network showed that there was about 50% reduction of vessel density and the number of pericytes in the tumors from the mutant mice. Collectively, these results support the notion that COUP-TFII is important for pathological neoangiogenesis.
Fig. 2. Roles of COUP-TFII in tumor vasculature.
(A) Image shows the appearance of angiogenic islets from control and COUP-TFII mutant mice (upper panel). H&E staining (lower panel) shows that microhemorrhaged erythrocytes have accumulated in the islet tumor of control (arrow) but not COUP-TFII mutant mice. (B) Visualization of the tumor vascularization by immunostaining with CD31 and NG2. Quantification of vessel abundance (lower panel) was shown (N=6). (C) Immunostaining of phophorylated-histone H3 and cleaved Caspase 3 to determine the index of proliferation and apoptosis, respectively. The positive number was counted and calculated from several fields of each tumor from a total of 5 animals analyzed. * P<0.05; ** P<0.01
The angiogenic defects observed in the tumor regions from the mutant mice suggested that tumor cells might be deprived of nutrients and oxygen and would exit the cell cycle and undergo apoptosis. In agreement with this notion, we found that there was a substantial reduction of proliferation and survival of tumor cells as evaluated by phosphorylated-H3 and cleaved caspase-3 immunostaining, respectively, and the results are quantified in Fig. 2C. Taken together, these data suggest that the loss of COUP-TFII results in angiogenesis defects and subsequent reduction of tumor growth.
Inactivation of COUP-TFII inhibits tumor metastasis and lymphangiogenesis
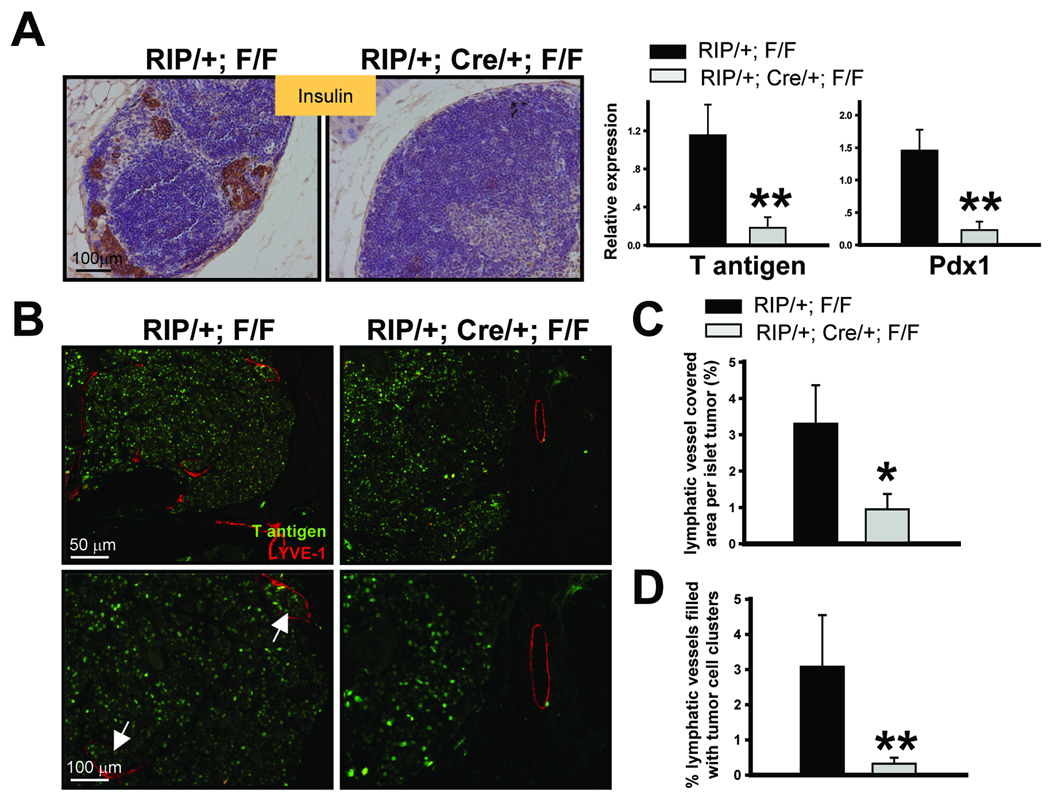
Since angiogenesis and lymphangiogenesis are necessary for the growth and metastatic spread of solid tumors, we asked whether metastasis was also inhibited by the loss of COUP-TFII. In the RIP-Tag2 model, β tumor cells would follow routes of natural drainage and reach the marginal sinus of pancreatic mesenteric lymph node, thus forming metastases at these sites (3). We therefore analyzed regional lymph nodes in control and COUP-TFII mutant mice at 14 weeks of age. Immunohistochemical staining of insulin expression in mesenteric lymph nodes showed that metastatic tumors cells were indeed observed in about 28% of control mice (6 of 21) (Fig. 3A). In contrast, metastasis was not observed in COUP-TFII mutant mice (0 of 21). In accordance, qRT-PCR analysis showed that transcripts of T antigen and Pdx1 mRNA, which are expressed specifically in the pancreatic β tumor cells, were indeed detectable in insulin positive lymph nodes from control mice, but were largely absent in COUP-TFII deficient mice (Fig. 3A, right panel). Collectively, our results showed that the loss of COUP-TFII inhibits tumor metastasis to regional lymph nodes.
Fig. 3. Inactivation of COUP-TFII inhibits tumor metastasis and lymphangiogenesis.
(A) Immunohistochemical staining of insulin to indicate metastatic β tumor cells in pancreatic lymph nodes from control and COUP-TFII mutant mice (left panel). qRT-PCR analysis of the expression of Pdx1 and large T antigen in pancreatic lymph nodes. Control samples were first screened based on whether insulin was expressed. Mutant samples were randomly chosen since insulin expression was negative (N=6). (B) Determination of peritumoral lymphatic density by LYVE-1 immunostaining. T antigen immunostaining was used to indicate β tumor cells. Arrow indicates lymphatic vessel filled with disseminated tumor cells. (C) Quantification of the percentage of lymphatic vessel covered area per islet tumor in control and mutant mice (N=6). (D) Quantification of the percentage of lymphatic vessels with disseminated tumor cell clusters inside the lymphatic vessel (N=6). * P<0.05, ** P<0.01
The inhibition of lymph node metastasis by loss of COUP-TFII may either arise directly from the impact of COUP-TFII on the lymphatic system, such as decreased lymphangiogenesis, or indirectly affected by the invasive capacity of the tumor cell itself. Since we recently showed that COUP-TFII modulates lymphatic vessel development by regulating the expression of neuropilin-2 (22), we asked whether tumor lymphangiogenesis was also compromised in the absence of COUP-TFII. To this end, the abundance and distribution of lymphatic vessels in the vicinity of control or mutant islet tumors were examined by staining for the lymphatic marker LYVE-1. We found that LYVE1-expressing lymphatic vessels were distributed largely around but also within the islet tumors in control mice (Fig. 3B). In contrast, the density of lymphatic vessels surrounding the islet tumors was markedly decreased in the absence of COUP-TFII. Furthermore, lymphatic vessels were never observed inside the islet tumors of the mutant mice. Quantification results showed a significant decrease in the percentage of the LYVE1 covering areas per islet tumor of the mutant mice (Fig. 3C). During the analysis of sections stained for LYVE-1 antibody, we found a significant decrease in the number of disseminated tumor cell emboli within mutant lymphatic vessels. There was around 3.2% lymphatic vessels filled with disseminated tumor cells in the control mice, but the incidence was greatly reduced in the mutant mice (Fig. 3D). This result is consistent with the above observation that the tumors from COUP-TFII mutant mice appeared to be less invasive and less likely to metastasize to other sites. Taken together, we conclude that the loss of COUP-TFII inhibits lymphangiogenesis and tumor metastasis.
Depletion of COUP-TFII impairs endothelial sprouting
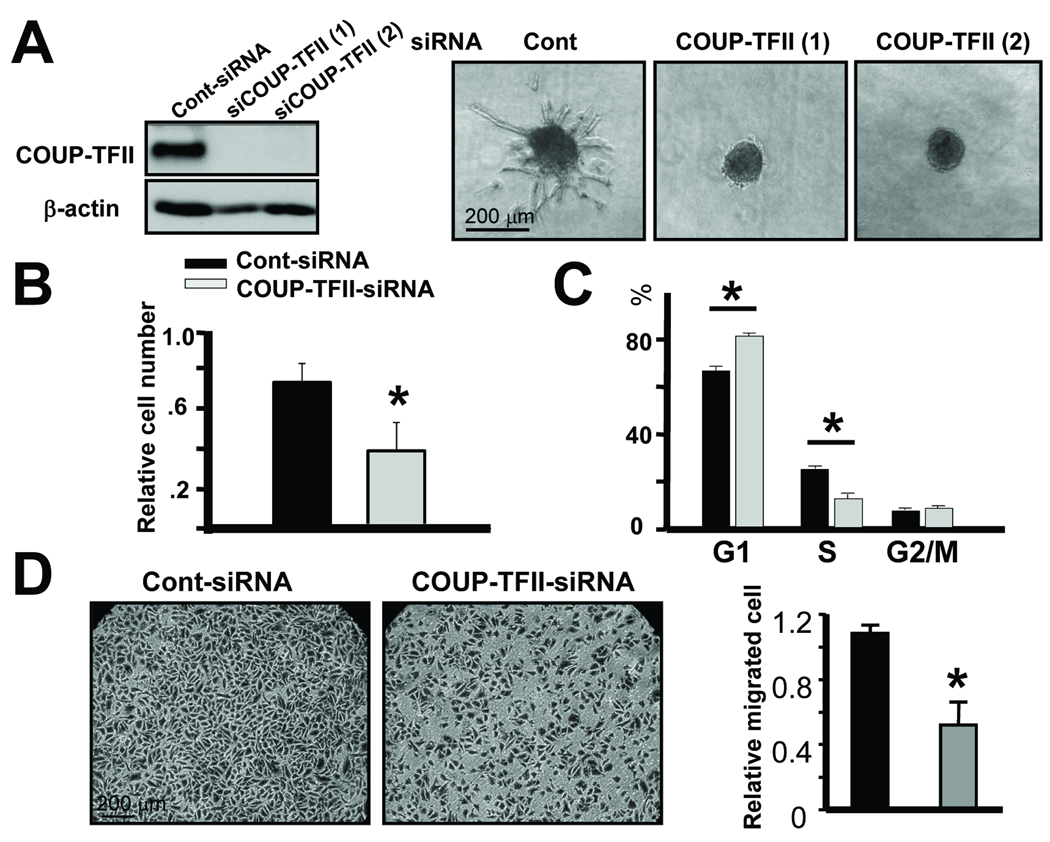
We showed previously that COUP-TFII regulated a pericyte-derived paracrine signal (Ang1/Tie2 signaling) for the endothelium (15). However, the profound angiogenic defect displayed by COUP-TFII mutant mice in the RIP-Tag2 model is unlikely only due to the regulation of Ang1 expression by COUP-TFII. Since COUP-TFII was also strongly expressed in endothelial cells, we hypothesized that COUP-TFII might have an intrinsic function in endothelial cells to regulate tumor angiogenesis. To answer this question, we employed a three-dimensional spheroidal sprouting assay to examine a possible autonomous role of COUP-TFII in endothelial sprouting. We knocked down COUP-TFII in human primary endothelial (HUVEC) cells, and COUP-TFII was efficiently depleted as shown in Fig. 4A. Outgrowth and branching of capillary-like structures, also known as endothelial sprouting, were assessed. We observed that depletion of COUP-TFII abolished endothelial sprouting (Fig. 4A). Endothelial sprouting as measured by the cumulative sprouting length was substantially decreased in the COUP-TFII knockdown cells (14.5 ± 5.7 µm) in comparison to control cells (924.5± 77.7 µm). Similarly, the number of sprouts per spheroid was also reduced in COUP-TFII knockdown cells (0.78 ± 0.22) versus controls (11.0 ± 0.58). These results indicated that COUP-TFII plays an autonomous role in blood endothelial cells to control vessel sprouting.
Fig. 4. Important role of COUP-TFII in endothelial sprouting.
(A) HUVEC were transfected with COUP-TFII-targeting siRNA or control siRNA, and COUP-TFII expression was examined by immunoblotting analysis (left panel). Three-dimensional spheroids generated from control or COUP-TFII knockdown HUVEC cells were stimulated with VEGF and spheroid sproutings were analyzed (right panel). (B) MTT assay indicated that depletion of COUP-TFII decreased the number of proliferating cells. (C) FACS analysis of the proportions of cells in the cell cycle distribution from control and COUP-TFII depleted cells. (D) Chamber migration assay was used to determine cell migration rate upon VEGF stimulation. Quantification of two chamber migration assays is shown. * P<0.05
Endothelial sprouting is a multi-step process, involving both proliferation and migration. Indeed, we observed a decrease in the number of proliferating cells subsequent to COUP-TFII depletion over a 4-day period (Fig. 4B). Furthermore, 3H-thymidine incorporation assays revealed that depletion of COUP-TFII resulted in a defect in cell proliferation (Supplementary Fig. 2A). Moreover, flow cytometry analysis indicated that cells with reduced COUP-TFII expression showed a defect in G1/S phase transition (Control: 66.3% cells in G1, 25.6% cells in S versus Mutant: 78.3% cells in G1, 12.6% cells in S) (Fig. 4C). Taken together, these results indicated that COUP-TFII is essential for proliferation of blood endothelial cells.
We next used a wound healing assays to address whether COUP-TFII affects endothelial migration. We found that migration of endothelial cells was impaired when COUP-TFII was knocked down in comparison to the control (Supplementary Fig. 2B). Furthermore, the migration rate of COUP-TFII knockdown cells in response to VEGF-A decreased about 50% in a chamber migration assay (Fig. 4D). Taken together, these results demonstrated that COUP-TFII promoted vessel sprouting through regulating blood endothelial cell proliferation and migration.
Up regulation of VEGFR-1 in islet tumor of COUP-TFII mutant Mice
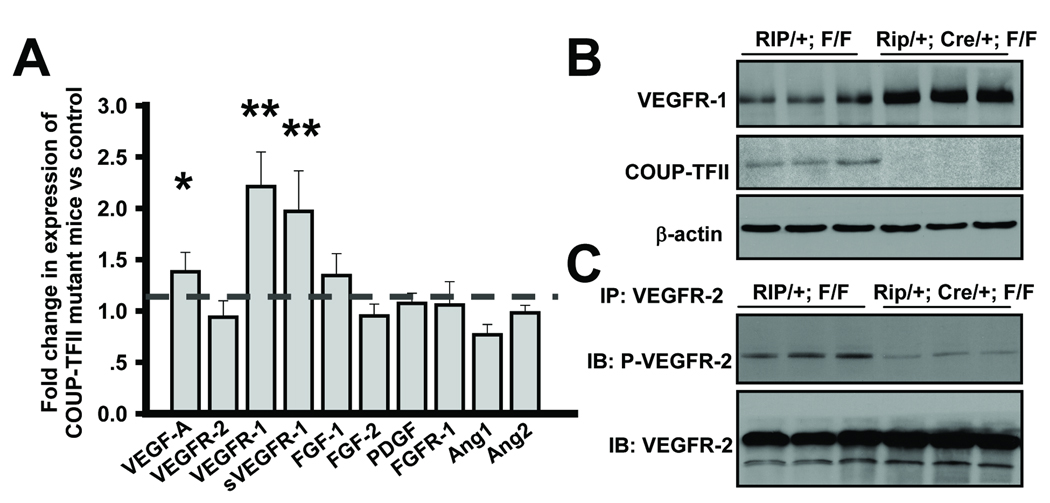
To identify COUP-TFII downstream targets that impacted on angiogenesis, we isolated the angiogenic islet tumors from control and COUP-TFII mutant mice, and performed a candidate gene profiling analysis to assess possible changes in a set of proangiogenic factors. These include VEGF-A, FGF, PDGF, VEGFR-2, VEGFR-1, sVEGFR-1, PDGFR-1, FGFR-1 and members of the Angiopoietin family (Fig. 5A). We found that the expression level of VEGFR-1 and sVEGFR-1 was significantly increased in the absence of COUP-TFII, which was further confirmed by western blot analysis (Fig. 5B). Notably, the increased expression of VEGF-A in the mutant mice was very likely due to secondary effects of the hypoxia condition and angiogenic defects in COUP-TFII deficient mice. It is well established that VEGFR-1 is expressed in blood endothelial cells and counteracts the positive mitogenic signal of VEGFR-2 by sequestering the VEGF ligand (23, 24). For this reason, we sought to examine whether VEGF/VEGFR-2 signaling, the major angiogenic signaling for vascular formation, was impaired in the tumors of COUP-TFII mutant mice. Our results revealed that the loss of COUP-TFII indeed compromised VEGFR-2 signaling as indicated by the reduced phosphorylated level of VEGFR-2 in the mutant angiogenic islet (Fig. 5C). Collectively, COUP-TFII might modulate VEGFR-2 signaling through the regulation of VEGFR-1 expression.
Fig. 5. Increased VEGFR-1 expression by loss of COUP-TFII.
(A) qRT-PCR analysis of proangiogenic factors in angiogenic islet tumors from control and COUP-TFII mutant mice. Bar graph shows fold changes of each molecule in COUP-TFII mutants relative to controls (N=6). The dotted line indicates the expression levels of those genes in control mice. * P<0.05, ** P<0.01 (B) Western blot analysis of COUP-TFII and VEGFR-1 expression in control and mutant angiogenic islet tumors. (C) Tumor cell lysates from control and mutant mice were subjected to immunoprecipitation with anti-VEGFR-2 followed by immunoblotting with phosphorylated VEGFR-2. Protein loading amount was normalized against VEGFR-2.
COUP-TFII modulates VEGF/VEGFR-2 signaling by direct regulation of VEGFR-1 expression
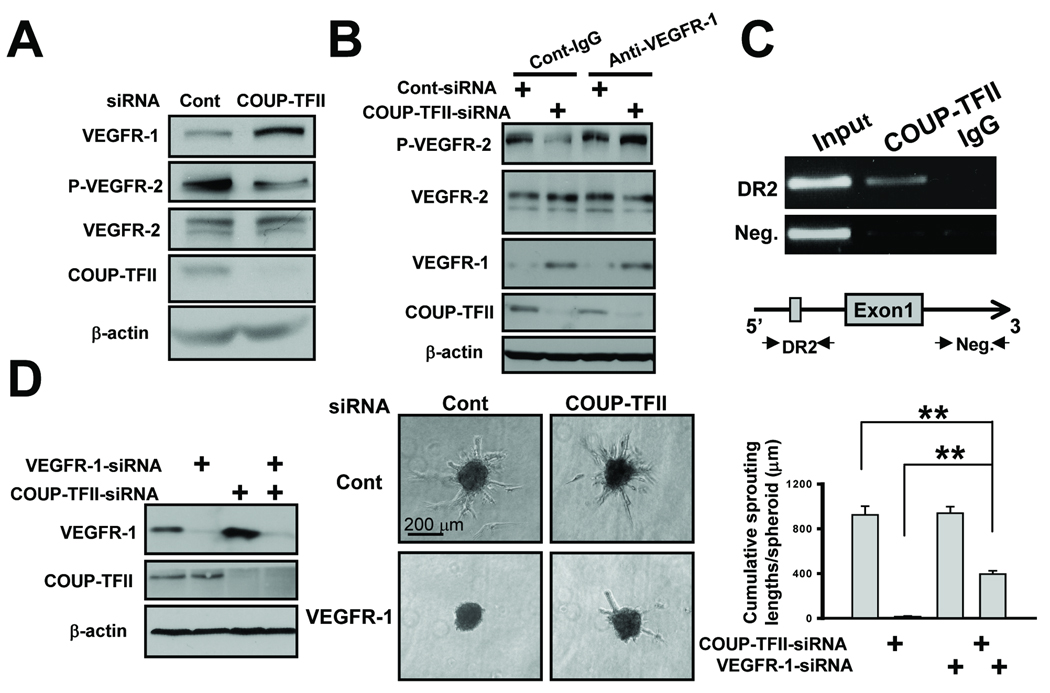
To elucidate the underlying molecular mechanism on how COUP-TFII regulated VEGFR-1 expression within endothelial cells, we knocked down COUP-TFII in HUVEC cells and examined whether the expression levels of VEGFR-1 are consistently increased in the COUP-TFII depleted cells. By qRT-PCR and immunoblotting analysis, we showed that ablation of COUP-TFII increased VEGFR-1 levels by nearly 3 fold (Supplementary Fig. 3 and Fig. 6A), a similar increase was also observed in the level of sVEGFR-1 (Supplementary Fig. 3), which primarily acts as a decoy receptor to sequester VEGF-A.
Fig. 6. COUP-TFII regulates VEGF/VEGFR-2 signaling by direct repression of VEGFR-1 expression.
(A) Immunoblotting analysis of the expression of VEGFR-1, phosphorylated VEGFR-2, VEGFR-2 and COUP-TFII. (B) Immunoblotting analysis of phosphorylated VEGFR-2, VEGFR-2, VEGFR-1 and COUP-TFII level in control and COUP-TFII knockdown cells treated with anti-VEGFR-1 antibody or control IgG. (C) ChIP analysis showed that COUP-TFII bound to the region containing a conserved DR2 binding site (ACGTCAagGGGTCC) in the VEGFR-1 promoter. Region in the first intron served as a negative control (middle). (D) siRNA-mediated knockdown of VEGFR-1 expression (left) was used to rescue endothelial cell sprouting defects in COUP-TFII knockdown cells (middle). Quantification (right) of endothelial sprout formation by counting the cumulative sprouting lengths. ** P<0.01
Since VEGFR-1 expression was increased, we asked whether VEGFR-2 signaling is perturbed. We found that the phosphorylated level of VEGFR-2 decreased concomitantly with an increase in VEGFR-1 expression, whereas the total levels of VEGFR-2 remained the same in control and COUP-TFII knockdown cells (Fig. 6A). Thus our results supported in vivo observation that loss of COUP-TFII dampened VEGF/VEGFR-2 signaling. Since we hypothesized that the up regulation of VEGFR-1 was a major reason for the decreased VEGFR-2 signaling in the absence of COUP-TFII, it is anticipated that reduced VEGFR-1 expression level in COUP-TFII-depleted cells will restore VEGFR-2 signaling. To this end, we treated COUP-TFII knockdown cells with VEGFR-1 antibody to neutralize functional VEGFR-1. As expected, we found that the phosphorylated level of VEGFR-2 was largely restored in COUP-TFII depleted cells treated with VEGFR-1 antibody, while there was no discernable effect in control cells (Fig. 6B), suggesting that the ablation of COUP-TFII impaired VEGF/VEGFR-2 signaling mainly through increasing VEGFR-1 expression.
Previous studies have shown that COUP-TFII can directly bind the conserved DR binding site to repress target gene expression (9). To address whether COUP-TFII directly regulates VEGFR-1 expression, we used ChIP assay to examine if COUP-TFII could be recruited to the VEGFR-1 promoter via conserved COUP-TFII response elements. Fig 6C shows that COUP-TFII was preferentially recruited to the region containing a conserved DR2 COUP-TFII response element in comparison to non-specific IgG, while there was no difference in COUP-TFII recruitment to an intragenic region lacking COUP-TFII binding site. Thus, COUP-TFII directly binds to the VEGFR-1 promoter through a conserved DR2 binding site to negatively regulate VEGFR-1 expression.
Finally, if the sprouting defects were largely contributed by the elevated expression of VEGFR-1, we would anticipate that an inhibition of VEGFR-1 expression might rescue the sprouting defects exhibited by COUP-TFII knockdown cells. To address this, we knocked-down VEGFR-1 expression in COUP-TFII deficient cells, and found that endothelial sprouting length and the number of sprouts were partially restored (Fig. 6D). Collectively, our results suggest that COUP-TFII regulates VEGFR-2 signaling by directly repressing VEGFR-1 expression.
Discussion
In the present study, we took advantage of a well-defined β-cell carcinogenesis model to assess the role of COUP-TFII during the angiogenic switch as well as pancreatic tumor progression. Our results highlight the notion that COUP-TFII is critical for the angiogenic switch during tumorigenesis. Notably, COUP-TFII is a member of the nuclear receptor superfamily whose activity can be regulated by small diffusible ligands. Indeed, structural analysis has shown that COUP-TFII contains a potential ligand binding pocket, and its activity could be stimulated by retinoids (25), thus raising the interesting possibility that antagonists for COUP-TFII can be found, and supporting COUP-TFII as a novel “druggable” target for the treatment of tumors.
Using a xenograft model, we showed previously that COUP-TFII directly regulates Ang1 expression in pericytes to modulate angiogenesis (15). However, angiogenic profile analysis indicated that there was little change between control and mutant animals with respect to Ang1 expression (Fig. 5). This is not unexpected since Casanovas et al. showed that Ang1 was induced by the islet tumor cells in this cancer model (26) and the increase of Ang1 in the tumor cells may mask the down-regulation of COUP-TFII in the tumor environment. To assess if COUP-TFII regulates Ang1 expression in the tumor microenvironment in the current model, we isolated adjacent normal pancreatic tissue and pancreatic islet tumor, and compared Ang1 levels in control and mutant mice. Our results indicated that Ang1 expression was indeed decreased in the pancreatic tissue adjacent to tumor of COUP-TFII mutant mice (Supplementary Fig. 4). These results are consistent with our previous findings (15). As expected, the expression of Ang1 in islet tumors remains the same in control and mutant tumors due to the high expression of Ang1 elicited by β tumor cells.
Here, we provide compelling evidence that COUP-TFII controls VEGFR-2 signaling by directly suppressing the endothelial expression of VEGFR-1 and the sVEGFR-1 (27). Consistent with the results from the pancreatic islet tumor model, we found that VEGFR-1 expression is consistently up regulated in the Matrigel Plug model (Supplementary Fig. 5), indicating that regulation of VEGFR-1 expression by COUP-TFII is a general mechanism in both physiological and pathological neoangiogenesis process. In the RIP-Tag2 tumors, the results from genetic inactivation of VEGF alleles in pancreatic β cells (28, 29) have documented that VEGFR-2 signaling is a key driver for vascular growth. This notion is supported by the observation that sVEGF-R1 delivered by an adenovirus vector is a potent inhibitor of RIP-Tag2 tumor angiogenesis and tumor progression (30). Although it has been reported that VEGFR-1 is also expressed in many nonendothelial cell types, including macrophages and various tumor cells (31), in situ hybridization analysis revealed that VEGFR-1 expression is detectable only in the islet vasculature, at least in this tumor model (32–34). Therefore, we conclude that in vivo up regulation of VEGFR-1 expression in COUP-TFII mutant mice is primarily contributed by endothelial regulation by COUP-TFII.
Our results suggest that COUP-TFII is essential for tumor-induced neo-lymphangiogenesis. Intriguingly, we found that COUP-TFII did not regulate the transcription of VEGFR-1 in lymphatic endothelial cells (Supplementary Fig. 3). Therefore, lymphangiogenesis defects displayed by COUP-TFII mutant mice is unlikely due to VEGFR-1 regulation by COUP-TFII. However, we recently showed that COUP-TFII enhanced the pro-lymphangiogenic actions of VEGF-C by directly stimulating expression of neuropilin-2, a co-receptor for VEGF-C, to control lymphatic vessel sprouting (22). Loss of COUP-TFII significantly compromised VEGFR-3 signaling, which is a major receptor mediating VEGF signaling for lymphangiogenesis. Furthermore, COUP-TFII has been shown to physically and functionally interact with Prox1, a key regulator of lymphatic endothelial cell (19, 35, 36), raising the interesting possibility that COUP-TFII acts jointly with Prox1 to regulate tumor lymphangiogenesis. In this regard, COUP-TFII might exert its effect in tumor lymphangiogenesis through regulation of VEGFR3/Nrp2 signaling and interactions with Prox1.
In summary, our results reveal a central role for COUP-TFII in the pathological angiogenic and lymphangiogenic response, and support COUP-TFII as a promising new target for anti-angiogenic therapy.
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH (HL076448 to SYT, DK059820 to SYT and MJT, and DK45641 and HD17379 to MJT), and from a Duncan Cancer Center grant (to SYT and L-YY-L).
Reference
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 6.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 9.Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev. 1997;18:229–240. doi: 10.1210/edrv.18.2.0294. [DOI] [PubMed] [Google Scholar]
- 10.You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, et al. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci U S A. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 12.Takamoto N, You LR, Moses K, Chiang C, Zimmer WE, Schwartz RJ, et al. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development. 2005;132:2179–2189. doi: 10.1242/dev.01808. [DOI] [PubMed] [Google Scholar]
- 13.Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, et al. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol. 2004;24:10835–10843. doi: 10.1128/MCB.24.24.10835-10843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci U S A. 2010;107:3687–3692. doi: 10.1073/pnas.0914619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parangi S, O'Reilly M, Christofori G, Holmgren L, Grosfeld J, Folkman J, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci U S A. 1996;93:2002–2007. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, et al. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–1859. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 22.Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120:1694–1707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 25.Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 30.Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 2000;60:7163–7169. [PubMed] [Google Scholar]
- 31.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 32.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995;9:1760–1770. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- 34.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells. 2009;14:425–434. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.