Abstract
Physiological dependence and associated withdrawal episodes are thought to constitute a motivational force that sustains alcohol use and abuse and may contribute to relapse in dependent individuals. Although no animal model duplicates alcoholism, models for specific factors, like withdrawal, are useful for identifying potential genetic and neural determinants of liability in humans. Previously, we identified a quantitative trait locus (QTL) and gene (Mpdz, which encodes the multi-PDZ domain protein) on chromosome 4 with a large effect on alcohol withdrawal in mice. Using congenic mice that confirm this QTL and c-Fos expression as a high-resolution marker of neuronal activation, we report that congenic mice demonstrate significantly less neuronal activity associated with alcohol withdrawal in the rostroventral caudate putamen (rvCP), but not other parts of the striatum, compared with background strain mice. Moreover, bilateral rvCP lesions significantly increase alcohol withdrawal severity. Using retrograde (fluorogold) and anterograde (Texas Red conjugated dextran amine) tract tracing, we found that ~25% of c-Fos immunoreactive rvCP neurons project to caudolateral substantia nigra pars reticulata (clSNr), which we previously found is crucially involved in withdrawal following acute and repeated alcohol exposure. Our results expand upon work suggesting that this QTL impacts alcohol withdrawal via basal ganglia circuitry associated with limbic function, and indicate that an rvCP-clSNr projection plays a critical role. Given the growing body of evidence that the syntenic region of human chromosome 9p and MPDZ are associated with alcohol abuse, our results may facilitate research on alcohol dependence and associated withdrawal in clinical populations.
Keywords: Seizure, c-Fos, striatum, lesion, Mpdz, MUPP1
Introduction
Alcohol (ethanol) abuse and alcoholism are serious public health concerns throughout the world. Alcoholism is the second most prevalent axis I diagnosis among individuals aged 15–44 (Manderscheid and Henderson, 2002), and is one of the most highly heritable addictive disorders (Goldman et al., 2005). More than 18 million Americans (8.5% of the population 18 and older) have been estimated to meet the diagnostic criteria of alcohol abuse or dependence (Grant et al., 2004). Withdrawal is a hallmark of alcohol physiological dependence, and constitutes a motivational force that perpetuates alcohol use and abuse (Little et al., 2005). Unfortunately, due to its complexity the genes and neural networks that contribute to genetic differences in risk for alcohol dependence and associated withdrawal are largely unknown. This has hindered treatment and resulted in a lack of alternatives for dependent individuals.
Although no animal model duplicates clinically defined alcoholism, models for specific factors, including withdrawal, are useful for identifying potential neural and genetic determinants of liability in humans. Previously, we identified quantitative trait loci (QTLs) on chromosome 4 with large effects on predisposition to alcohol withdrawal in mice (Buck et al., 1997, Fehr et al., 2002), and a gene (Mpdz) underlying the phenotypic effect of this QTL on withdrawal (Fehr et al., 2002, Shirley et al., 2004). Using c-Fos as a high-resolution histological marker of neuronal stimulation (Herdegen & Leah, 1998, Morgan et al., 1987), we identified a distinct activation pattern associated with acute alcohol withdrawal (Kozell et al., 2005) and impacted by chromosome 4 QTL(s) status (Chen et al., 2008). Lesion studies also indicate the involvement of specific brain regions in ethanol withdrawal (Chakravarty & Faingold, 1998, Kostowski & Trzaskowska, 1980, Ripley et al., 2004), and the integration of c-Fos expression studies and focused lesions proved to a powerful approach to suggest that this QTL impacts ethanol withdrawal via basal ganglia circuitry associated with limbic function, and that the caudolateral substantia nigra pars reticulata (clSNr) plays a critical role in withdrawal following acute and repeated alcohol exposure (Chen et al., 2008, submitted).
The aim of the present studies is to further elucidate neural circuitry associated with alcohol withdrawal and impacted by the chromosome 4 QTL, focusing on potential projections to the clSNr. The SNr is inhibited by the striatum via direct striatonigral projections, and excited via indirect striatopallidal projections involving globus pallidus (external) and the subthalamus (Kreitzer & Malenka, 2008). Because previous analyses suggested that the subthalamic nucleus is not directly involved in alcohol withdrawal (Chen et al., 2008), here we assessed the role of the striatum in ethanol withdrawal as well as a putative striatal-clSNr projection in mice.
Methods
Animals
All of the animals used in these studies were bred in our colony in the Department of Comparative Medicine at Oregon Health & Science University. DBA/2J (D2) strain breeders were purchased from the Jackson Laboratory (Bar Harbor, ME). The chromosome 4 QTL (Alcw2) congenic strain (with the QTL interval from the B6 donor strain introgressed onto a uniform D2 background) was developed in our laboratory (ISCS1) (Fehr et al., 2002). A total of 40 naïve adult (60–90 days old) male congenic and background strain (D2) mice were used for the c-Fos expression analyses. An additional 42 male D2 mice were used for lesion, tract tracing, and other immunohistochemical analyses, and were 60–70 days old at the time of surgery.
Retrograde and anterograde tract tracing
Retrograde tract tracing was performed as in previous work (Chen et al., 2006). Adult mice were anesthetized with mouse cocktail (50 mg/10 ml xylazine, 500 mg/10 ml ketamine, and 10 mg/10 ml acepromazine in 0.9% saline), then placed in a Cartesian stereotaxic. A pulled filament pipette with a tip size of 30–50 μm was placed for unilateral pressure microinjection of fluorogold (FG) into the clSNr, a region crucially involved in ethanol and barbiturate withdrawal (Chen et al., 2008, submitted). The coordinates for clSNr were estimated based on coordinates for the B6 strain (Paxinos & Franklin, 2001) and determined empirically for D2 strain mice as follows: 3.2 mm caudal to bregma (AP = −3.2), 1.6 mm lateral to midsaggital suture (ML = ±1.6), 4.5 mm deep from the skull surface (DV = −4.5). The pipette was withdrawn 7 min after injection. Approximately 7 days post-surgery, the FG-injected mice were deeply anesthetized and transcardially perfused with 50 ml of 0.9% saline followed by 50 ml of 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS, pH 7.4). FG labeling was visualized using excitation wavelengths 340–380 nm. In order to assess acute ethanol withdrawal associated target of the rvCP. The coordinates for clSNr were estimated based upon coordinates for the B6 strain (Paxinos & Franklin, 2001) and determined empirically for D2 mice. c-Fos induction, half of the FG-injected mice received ethanol (4 g/kg, i.p) 7 hr prior to anesthesia and perfusion, and the other half received saline.
For anterograde tract tracing, Texas Red conjugated dextran amine (TRDA; 10,000 MW; Vector Laboratories) was unilaterally injected into the rvCP. The coordinates used for rvCP in D2 mice were as followed: AP = 1.0, ML = ±2.3, DV = −3.8. Approximately 7 days post-surgery, the TRDA-injected mice were deeply anesthetized and transcardially perfused with 50 ml of 0.9% saline followed by 50 ml of 4% paraformaldehyde in 0.1 M PBS. TRDA labeling was visualized using excitation wavelengths 555–655 nm.
Immunohistochemistry
c-Fos immunohistochemical analyses were performed as described in our previous work (Chen et al., 2008). In order to avoid potential confounds of evoked convulsions on c-Fos expression, none of the mice used were tested for withdrawal convulsions. Briefly, animals were administered a hypnotic dose of ethanol (4 g/kg, 20% v/v in saline, i.p.) or an equivalent volume of vehicle (sterile 0.9% saline) and returned to their home cage and left undisturbed for 7 hr. Withdrawal convulsions are most severe ~6–7 hr post-ethanol exposure in chromosome 4 congenic and background strain (D2) mice (Fehr et al., 2002). The mice were killed by cervical dislocation. Brains were coronally sectioned (30 μm) on a freezing microtome. Within an experiment, all of the experimental groups were processed at the same time. After blocking in 10 mM PBS containing 3% normal goat serum and 0.3% Triton-100, the sections were incubated with rabbit anti-c-Fos antibody (1:10,000; Oncogene Science, Cambridge, MA) for 72 hr at 4°C. The sections were subsequently incubated with biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) in 10 mM PBS, followed by an Avidin-Biotin Complex (ABC) staining procedure. The chromatic reaction was completed with diaminobenzidine (50 mg/100 ml of 0.05 M Tris, Sigma) in the presence of 0.01% nickel ammonium sulfate solution and 0.035% hydrogen peroxide. Omission of the primary antibody to the sections was used as a staining control. Thorough rinses are carried out between incubation steps. For quantitative morphometric analysis of c-Fos immunoreactive cells, an Olympus BX60 light microscope and LEICA DFC 480 imaging system were used to obtain a permanent record of cell distribution. Images of sections for rvCP (plates 22–24, from Paxinos and Franklin, 2001) were taken at 10X and signals were quantified using Image Pro Plus (Media Cybernetics, Bethesda, MD). Standardized brain region templates based on established anatomical markers were employed. Standardized threshold parameters (160, light intensity range from 0 to 255) were employed to identify and quantify individual c-Fos immunoreactive neurons. Sample size estimates were based on previous studies (Chen et al., 2008, Hitzemann & Hitzemann, 1997, Kozell et al., 2005). The density of c-Fos immunoreactive cells (cells/mm2) was calculated as the number of c-Fos labeled cells divided by the rvCP template area. The experimenter was blind to the experimental condition for each subject.
For immunofluorescent staining, brain sections were incubated in blocking solution (10 mM PBS containing 5% normal donkey serum and 0.3% Triton-100) for 2 hr at room temperature. The brain sections were then incubated with one or two of the following primary antibodies in blocking solution at 4°C for 24–48 hr: sheep anti-MPDZ antibody (1:200, Upstate Inc., Billerica, MA), rabbit anti-c-Fos antibody (1:2000, Oncogene Science), rabbit anti-SynGAP antibody (1:1000, Millipore, Billerica, MA), rabbit anti-5-HT2C antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) and goat anti-c-Fos antibody (1:1000, Santa Cruz Biotechnology). The sections were incubated for 90 min with one or two of the following: donkey anti-sheep IgG conjugated with Alexa 488 (1:500), donkey anti-rabbit IgG conjugated with Alexa 594 (1:500), donkey anti-rabbit IgG conjugated with Alexa 488 (1:500), and/or donkey anti-goat IgG conjugated with Alexa 594 (1:500) (from Invitrogen, Carlsbad, CA). Thorough rinses are carried out between incubation steps. Omissions of the primary or secondary antibodies were used as staining controls. We performed unbiased quantification of immunofluorescent cells retrogradely labeled with FG and immunoreactive for c-Fos or MPDZ using an optical dissector technique (West et al., 1991; Coggeshall and Lekan, 1996; Chen et al., 2006). Using this approach, we also performed unbiased quantitation of cells immunoreactive for c-Fos as well as MPDZ, 5-HT2C, or SynGAP. For each animal, the number of cells immunostained in the rvCP was determined for every third section. Cells in the outermost focal plane were not included in order to avoid counting a cell more than once. Assessment of single and double cells was performed within 325 μm × 250 μm counting frames. The percentage of double-labeled cells (i.e., the number of double labeled cells/total number of cells labeled with FG, or the number of double labeled cells/total number of c-Fos immunoreactive cells) was calculated and expressed as the mean ± SEM.
Striatal rvCP lesions
Animals were anesthetized by i.p. injection of 0.05 ml of mouse anesthetic cocktail. An insulated 0.1 mm O.D. tungsten wire electrode with only a conductive tip was lowered to the lesion site. Bilateral lesions were performed using a 0.4 mA current for 4 seconds. The coordinates for the rvCP were estimated based on coordinates for the B6 strain (Paxinos & Franklin, 2001) and determined empirically for D2 strain mice as follows: AP = 1.0, ML = ±2.3, DV = −3.8. The procedure for sham-lesioned animals was identical except that no current was passed. Thionin staining was used to confirm lesion locations.
Ethanol withdrawal severity
Physiological dependence is operationally defined as the manifestation of physical disturbances (withdrawal syndrome) after alcohol administration is suspended. McQuarrie and Fingl (1958) first demonstrated a state of withdrawal CNS hyperexcitability after acute alcohol administration. Alcohol withdrawal severity was examined in rvCP-lesioned, sham-lesioned, and naïve (no-surgery) D2 mice by monitoring handling-induced convulsions (HICs) associated with withdrawal, which is a sensitive index of ethanol withdrawal severity. Details of the acute ethanol withdrawal procedure have been published (Metten et al., 1998). Individual baseline HICs were measured the day before surgery. 7–10 days post-surgery, baseline (pre-ethanol) HICs were measured immediately before administration of ethanol (4 g/kg, i.p., 20% v/v in saline), and HIC testing continued hourly between 2 and 12 hr post-ethanol administration. In order to create an index of ethanol withdrawal response that is independent of individual differences in baseline HIC scores and reflects differences in withdrawal convulsion severity, post-ethanol HIC scores were corrected for the individual’s average baseline HIC score as in previous work (Metten et al., 1998). Ethanol withdrawal severity scores were calculated as the area under the curve (AUC; the summed, corrected HIC scores) over the full time-course post-ethanol. Individual ethanol withdrawal severity scores correspond to these AUC values.
Statistics
For comparisons of ethanol withdrawal associated c-Fos expression in congenic and background strain mice as well as the comparison of the ethanol withdrawal severity between lesioned and control animals, the data were not normally distributed based on Shapiro-Wilks tests. Therefore, these data were analyzed using non-parametric Kruskal-Wallis one-way analyses of variance (ANOVA), which generate a Mann-Whitney U statistic for a comparison of two groups, or an H statistic for a comparison of more than two groups. All data were analyzed using Systat 12 statistical software (Systat Statistical Inc., Chicago, IL). The significance level was p<0.05 (two-tailed). All data are shown as means ± S.E.M.
Results
c-Fos induction in rvCP of Alcw2 congenic and background strain mice
In order to isolate Alcw2’s influence from that of other alcohol withdrawal QTLs elsewhere in the genome (Buck et al., 1997, 2002), neuronal activation associated with ethanol withdrawal was compared in chromosome 4 congenic and background strain (D2) mice. c-Fos was selected because ethanol withdrawal is associated with a distinct pattern of neuronal activation as assessed by its induction (Kozell et al., 2005 and references therein); while expression of other immediate early gene products is generally insensitive to ethanol withdrawal, although its expression is induced in the central amygdala (Borlikova et al., 2006). None the animals used in these analyses were tested for ethanol withdrawal convulsions, and none exhibited spontaneous convulsions in their home cage post-ethanol. This mitigates the potential confound of evoked convulsions on c-Fos immunoreactivity.
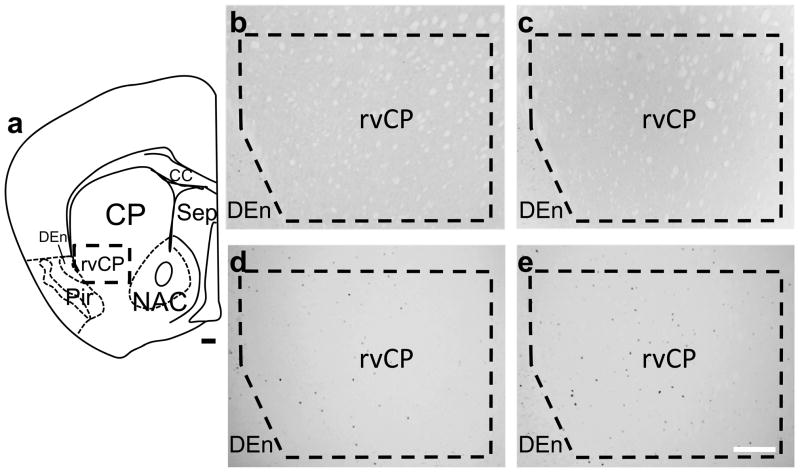
Representative c-Fos immunohistochemical photomicrographs for the rvCP are shown in Figure 1. As expected, no c-Fos expression was detected in the rvCP in saline control congenic or background strain animals (n=5 per group). c-Fos-immunoreactive cells were apparent in the rvCP in all ethanol withdrawn animals, but at a significantly lower density in congenic (29 ± 7 cells/mm2, n=14) compared with background strain mice (52 ± 4 cells/mm2, n=16) (U(1,30)=54.5, p<0.05). In contrast, no such genotype dependent c-Fos induction was detected in other striatal regions, i.e., nucleus accumbens (core and shell) and dorsal (dorsomedial and dorsolateral) caudate putamen (Chen et al., 2008).
Figure 1.
Photomicrographs of c-Fos expression in the rvCP in ethanol withdrawn and saline control Alcw2 congenic and background strain mice. The region of interest (ROI) for the rvCP is delineated within the thick dashed lines. (a) Schematic diagram of a representative brain section depicting the location of the rvCP. No c-Fos-immunoreactivity was detected within the rvCP in (b) control (saline) congenic or (c) background strain mice, respectively. (d) Ethanol withdrawn congenic mice had significantly fewer c-Fos immunoreactive cells within the rvCP than (e) background strain mice. Additional abbreviations: cc, corpus callosum; CP, caudate putamen; DEn, dorsal endopiriform nucleus; NAC, nucleus accumbens; Pir, piriform cortex; Sep, septum; Scale bar = 100 μm.
rvCP lesions
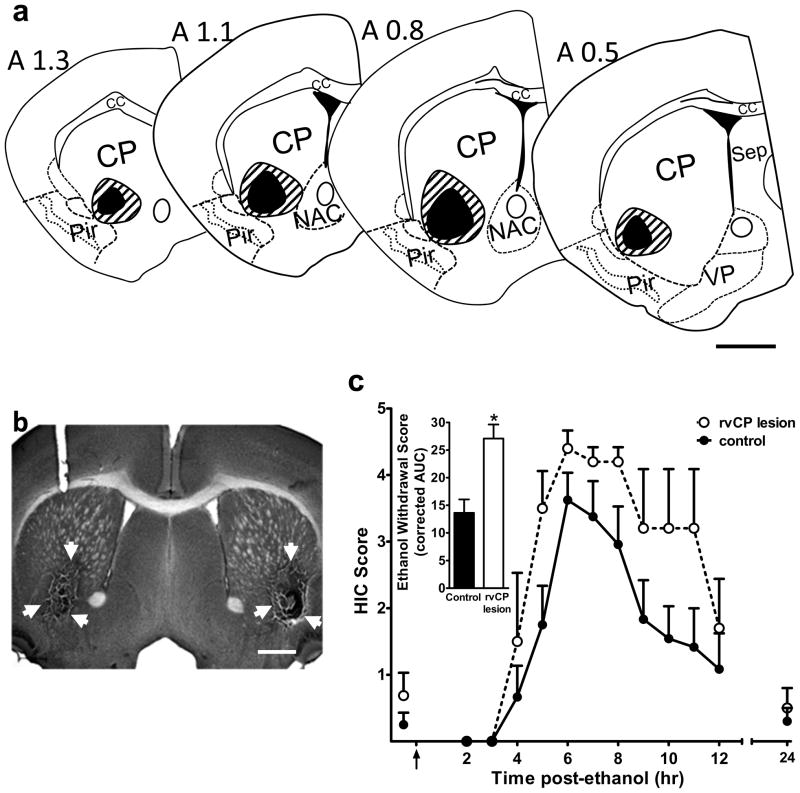
A total of 24 animals were tested, including rvCP-lesioned, sham-lesioned, and naïve (no surgery) controls. Confirmed lesions of the rvCP extended from 1.3 to 0.4 mm anterior to Bregma, and occasionally extended into the adjacent dorsal CP and nucleus accumbens (Fig 2a). Animals with lesions that extended to the adjacent piriform cortex were excluded from the analysis, as piriform cortex has been implicated in having an opposing role in CNS hyperexcitibility (Turski et al., 1989). A representative photomicrograph of the rvCP lesions is shown in Fig 2b.
Figure 2.
rvCP lesions. (a) Schematic representations of the minimal (black) and maximal (striped) extent of the rvCP lesions. Coordinates of the coronal sections are indicated with reference to Bregma according to the stereotaxic atlas of Paxinos and Franklin (2001) from 1.3 to 0.5 mm anterior to Bregma. (b) Low magnification (2X) photomicrograph of a representative brain section showing bilateral rvCP lesions. White arrows indicate the lesioned area. Scale bar = 500 μm. (c) Bilateral rvCP lesions exacerbate acute ethanol withdrawal. Bilateral lesioned (n=5) and control (n=12) D2 mice were scored for baseline handling-induced convulsions (HICs) immediately before administration of 4 g/kg ethanol (the arrow marks ethanol injection at time 0), and hourly between 2 and 12 hr post-ethanol administration. Data represent the mean raw scores ± SEM for baseline and post-ethanol HICs. Alcohol administration initially lowered HIC scores (0–3 hr). Later, convulsion scores increased above baseline, indicating a state of withdrawal hyperexcitibility, which peaks approximately 6 hr post-ethanol administration. By hr 24, the HIC response return to baseline in both lesioned and control animals. Inset: rvCP lesions significantly increased ethanol withdrawal scores calculated as the area under the withdrawal curve (AUC, corrected for baseline HIC scores) compared to control animals (p<0.005). Additional abbreviations: VP, ventral pallidum. Scale bar = 500 μm.
Baseline HIC scores did not differ between sham-lesioned animals, assessed either pre-surgery or 7 days post-surgery, and naïve control animals (mean baseline HIC scores ± SEM = 0.4± 0.2, 0.3± 0.3 and 0.4±0.3, respectively; H(2,20)=0.15, p=0.93). Neither did these groups (sham-lesioned and naïve) subsequently differ in ethanol withdrawal severities (mean AUC values ± SEM = 13.6 ± 3.1 and 13.6 ± 5.3, respectively; U(1,12)=18.0, p=0.73), suggesting that the surgery procedure did not change HIC response pre- or post-ethanol. We therefore collapsed the data for the sham-lesioned and naive control mice into one control group in order to increase the statistical power of our analyses. The rvCP-lesioned and control animals did not differ in baseline (pre-ethanol) HIC scores (U(1,17)=31.5, p=0.85) (Fig 2c). However, bilateral lesions restricted to rvCP significantly increased ethanol withdrawal severity compared to control animals (U(1,17)=3.0, p=0.004) (Fig 2c).
Striatal rvCP projects to clSNr in mice
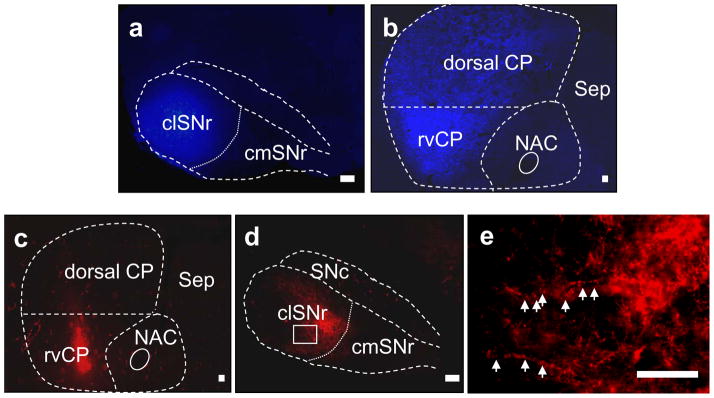
Although anatomical analyses using rats and primates suggest a direct projection from the rvCP to clSNr (Gerfen, 1985, Selemon & Goldman-Rakic, 1990), this projection had not been reported previously in mice. In the present studies, using both retrograde and anterograde tract tracing methods, we sought evidence for this projection in mice. Eight mice received a unilateral injection of retrograde tract tracer (FG). We confirmed that injection of this tracer was restricted to lateral SNr (Fig 3a) throughout the rostrocaudal extent in four of these animals, in which extensive FG-labeled neurons were apparent throughout the rostrocaudal, lateromedial and ventrodorsal extent of the CP; with little or no labeling detected in the nucleus accumbens. Within the CP, rvCP neurons showed a higher density of FG-labeled cells compared with the dorsal CP (Fig 3b). As expected, retrograde labeling was also detectable in other basal ganglia regions including the subthalamic nucleus, ventral pallidum, and external globus pallidus (not shown).
Figure 3.
Retrograde and anterograde tract tracing for the rvCP-SNr projection in mice. (a) Confirmed injection of retrograde tract tracer (FG) into the clSNr of a representative D2 mouse. (b) Retrogradely labeled neurons within the rostral striatum one-week post-FG injection. FG labeled neurons are concentrated within the rvCP, and are less dense within the dorsal CP. (c) Confirmed injection of anterograde tract tracer (TRDA) into the rvCP. (d) Anterograde terminal labeling within the SNr one week post-TRDA injection. Terminals within the clSNr are preferentially labeled, compared to the caudomedial SNr (cmSNr). (e) Higher magnification of labeled terminal varicosities (indicated by arrows) within the clSNr [from the area boxed in blue in panel d]. Additional abbreviations: IP, interpeduncular nucleus; ml, medial lemniscus; PAG, periaqueductal grey; SNc, substania nigra pars compacta. Scale bar = 100 μm.
To corroborate projection from the rvCP to clSNr, we injected an anterograde tract tracer (TRDA) unilaterally into the rvCP of seven mice. We confirmed that injection of this tracer was confined to the rvCP (Fig 3c) in three of these animals, in which we observed substantial TRDA-labeling of terminal arborizations and varicosities within the clSNr, with little labeling in the medial SNr (Fig 3d–e). The soma of a small number of cells within the SNr andSN pars compacta were labeled, which are likely dopaminergic nigrostriatal neurons (Fig 3d).
Taken together, these studies confirm that the clSNr preferentially receives afferent inputs from rvCP neurons in mice.
MPDZ and associated proteins in the rvCP
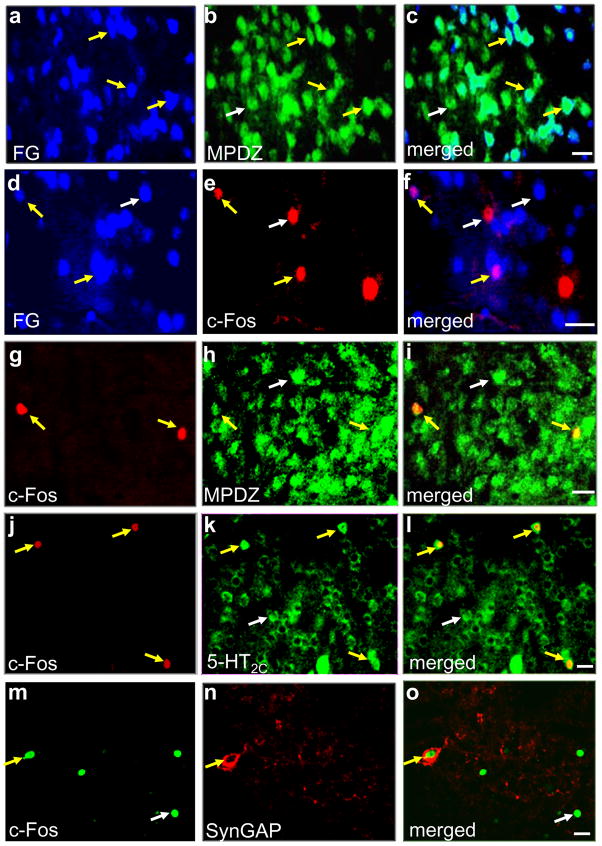
Mpdz, a quantitative trait gene (QTG) that underlies the phenotypic effects of an alcohol withdrawal QTL (Alcw2) on chromosome 4 (Shirley et al., 2004), encodes the multi-PDZ domain protein (MPDZ, also called MUPP1) (Becamel et al., 2001). We examined MPDZ expression in striatonigral projection neurons and c-Fos-immunoreactive cells in the rvCP and performed unbiased quantification of the labeled cells. Following FG microinjection into the clSNr, retrogradely labeled neurons were extensively distributed throughout the rvCP. Virtually all of the FG-labeled or c-Fos immunopositive neurons in the rvCP expressed MPDZ, whereas 50 ± 2% and 5 ± 1% of MPDZ-immunoreactive cells in the rvCP were also labeled with FG or c-Fos, respectively. 24 ± 3% of the c-Fos-immunoreactive neurons were also labeled with FG (Fig 4a–i).
Figure 4.
Representative photomicrographs of rvCP cells. Fluorescent staining with (a) FG retrogradely labeled from clSNr, (b) MPDZ, and (c) merged. Fluorescent staining with (d) FG retrogradely labeled from clSNr, (e) c-Fos, and (f) merged. Fluorescent staining for (g) c-Fos, (h) MPDZ, and (i) merged. Fluorescent staining for (j) c-Fos, (k) 5-HT2C receptor, and (l) merged. Fluorescent staining for for (m) c-Fos, (n) SynGAP, and (o) merged. White and yellow arrows point to single and dual labeled neurons, respectively, for each of the fields of view. Scale bar = 20 μm.
MPDZ has no apparent intrinsic activity and is thought to exert its effects by regulating the function of other proteins with which it interacts. MPDZ physically associates with the 5-HT2C receptor as well as postsynaptic density GTPase activating protein (SynGAP), which complexes with NMDA receptor 2B subunit/Ca+2-calmodulin kinase (MPDZ/NR2B/CaMKII) both in vitro and in vivo (Becamel et al., 2001, Kim et al., 2005, Krapivinsky et al., 2004). Using ethanol withdrawn animals, we assessed 5-HT2C receptor and SynGAP expression in c-Fos immunoreactive neurons within the rvCP. All c-Fos immunoreactive neurons detected in the rvCP also expressed the 5-HT2C receptor; while, conversely, 3 ± 1% of 5-HT2C immunoreactive cells expressed c-Fos (Fig 4k–l). In contrast, 26 ± 3% of the c-Fos-immunoreactive cells expressed SynGAP (Fig 4m–o). These results are consistent with the hypothesis that MPDZ may affect withdrawal by regulating the rate and/or fidelity of signaling mediated by proteins and receptors with which it associates. However, much future work (including co-immunoprecipitation analyses) will be needed in order to rigorously assess whether 5-HT2C receptors and/or SynGAP directly interact with MPDZ in the rvCP and the potential role of these interactions in withdrawal.
Discussion
The present studies are the first to implicate the rvCP in mediating the effects of an ethanol withdrawal QTL (Alcw2). Additionally, our study is the first to use focused lesions to assess the influence of the rvCP on ethanol withdrawal, and indicate that the rvCP plays a crucial role. Taken together with our previous work, our results suggest that Alcw2 impacts withdrawal behavior via its influence on basal ganglia circuitry associated with limbic function, and that the rvCP and clSNr are both critically involved. Moreover, the present studies are the first to demonstrate a direct rvCP-clSNr projection in mice.
In addition to its critical role in ethanol withdrawal convulsions, the rvCP has been implicated in controlling CNS hyperexcitibility (Turski et al., 1987). Additionally, because neuroimaging studies in humans and pharmacological microinjection analyses in nonhuman primates show that the striatal ventral putamen isassociated with depression, anxiety, apathy, stereotypy and hypoactivity (Hasler et al., 2007, Mah et al., 2007, Remy et al., 2005, Worbe et al., 2009), it is plausible that the rvCP may contribute to a variety of signs associated with the ethanol withdrawal syndrome beyond withdrawal convulsions. For example, preliminary analyses suggest that Alcw2 congenic animals exhibit less severe ethanol withdrawal associated depression-like behavior than background strain animals (L Milner and KJ Buck, unpublished data). While many signs of the ethanol withdrawal syndrome are genetically correlated with HIC severity (i.e., tremors, hypoactivity, emotionality; Belknap et al., 1987, Feller et al., 1994, Kosobud & Crabbe, 1986), others are not (i.e. tail stiffness; Kosobud & Crabbe, 1986). Thus, assessment of withdrawal HICs can inform analyses for correlated withdrawal signs, but represents only part of the complex syndrome of alcohol withdrawal (Wilson et al., 1984). Future studies will be needed to determine to what degree the rvCP may affect additional withdrawal signs. Neuroimaging evidence also suggests that the rvCP is associated with alcohol craving (Heinz et al., 2005, Olbrich et al., 2006). Moreover, the ventral CP receives projections from the amygdala (Russchen & Price, 1984), which has a prominent role in alcohol preference (Dhaher et al., 2008, Feng et al., 2007), and is also implicated in chronic ethanol withdrawal (Chen et al., 2009, Feng et al., 2007). Ethanol withdrawal and preference/consumption are significantly genetically correlated (Metten & Crabbe, 2005), and future studies will be important to assess the potential contribution of the rvCP to this inverse relationship.
Our results demonstrate a direct projection from the rvCP to clSNr in mice, in agreement with anatomical analyses in other species including primates and rats (Gerfen, 1985, Selemon & Goldman-Rakic, 1990). Previously, we found that bilateral lesions of the clSNr attenuate withdrawal severity following acute and repeated administration of ethanol (Chen et al., 2008, submitted). Based on current understanding of basal ganglia circuitry, the striatum directly inhibits the SNr via the striatonigral pathway and indirectly excites the SNr via the striatopallidal pathway. In this scenario, lesioning the rvCP would be expected to disrupt the inhibitory striatonigral projection and thus facilitate hyperactivity of the SNr. Lesioning the clSNr attenuates ethanol withdrawal severity (Chen et al., 2008, submitted); thus, SNr hyperactivity would be expected to result in more severe ethanol withdrawal, as was observed in the present studies following bilateral lesions of the rvCP. Lesioning the rvCP may also disrupt the indirect excitatory pathway, but this would be expected to mitigate ethanol withdrawal, which was not observed. Additionally, we previously found that bilateral lesions of the subthalamic nucleus within the indirect pathway do not influence ethanol withdrawal convulsions, suggesting that the indirect pathway is not crucially involved in ethanol withdrawal (Chen et al., 2008). Taken together, our results suggest that the rvCP affects alcohol withdrawal via its impact on SNr output, and that the direct striatonigral projection has a significant role.
Mpdz is a proven QTG for alcohol withdrawal in mice (Shirley et al., 2004, and unpublished in vivo data), and its human homolog (MPDZ) is also implicated in alcohol dependence (Karpyak et al., 2009, Tabakoff et al., 2009). Mpdz encodes the multi-PDZ domain protein (MPDZ; also called MUPP1) (Simpson et al., 1999, Ullmer et al., 1998), which is highly expressed in the rvCP. MPDZ is a member of a family of PDZ domain proteins, which has emerged as central organizers of protein complexes and contribute to the targeting, trafficking, and the fine-tuning of signaling properties of membrane-bound receptors (Nourry et al., 2003). We observed that MPDZ is expressed in virtually all c-Fos immunoreactive rvCP neurons. Interestingly, striatonigral projection neurons represent a subset of the c-Fos immunoreactive cells, suggesting that MPDZ may influence rvCP-clSNr projection neurons directly as well as indirectly via other neurons (e.g., interneurons) within the rvCP. Currently, little is known about the mechanism by which MPDZ affects withdrawal. MPDZ has no apparent intrinsic activity, and is thought to exert its effects by altering the rate/fidelity of signal transduction mediated by one or more of the proteins with which it associates. These include 5-HT2C receptors (Becamel et al., 2001, Parker et al., 2003) and SynGAP (Krapivinsky et al., 2004). Our results in ethanol withdrawn animals indicate that virtually all c-Fos immunoreactive cells in the rvCP also express 5-HT2C receptors. Selective blockade of 5-HT2C receptors changes neuronal activation within the striatum (De Deurwaerdere et al., 2009), so it is plausible that MPDZ expression influences neural activation associated with ethanol withdrawal in the rvCP via 5-HT2C receptor-mediated transmission. We also found that that ~30% of c-Fos-immunoreactive rvCP cells also express SynGAP. MPDZ physically associates with SynGAP and is integral to the NR2B subunit/SynGAP/CamKII complex (Kim et al., 2005, Krapivinsky et al., 2004). NR2B subunit containing NMDA receptors are abundant in the striatum (Kawakami et al., 2003), with preferential control over striatonigral neurons (Fantin et al., 2007, 2008). These observations raise the possibility that MPDZ may regulate NR2B receptor mediated transmission via SynGAP in rvCP striatonigral projection neurons.
Our results provide a framework for examining the potential role of MPDZ’s association with 5-HT2C receptors and/or SynGAP in withdrawal in greater detail, but there are some limitations. First, our results based on ethanol withdrawal convulsions, while an important first step, will need to be expanded in future studies assessing the potential role of the rvCP and its projection to the clSNr in additional signs of ethanol withdrawal (e.g., depression and anxiety-like behaviors). Moreover, because Mpdz is implicated in ethanol and barbiturate withdrawal (Shirley et al 2004), and it is well-established that there is common genetic influence on withdrawal from these two drugs as well as other sedative-hypnotics including inhalants and benzodiazepines (Belknap et al., 1988; Crabbe et al., 1991), future studies will be needed to assess the potential role of the rvCP in withdrawal from CNS depressant drugs beyond ethanol withdrawal convulsions. Assessment of pentylenetetrazol (PTZ)-enhanced convulsions in rvCP lesioned animals might also detect a broader role in CNS hyperexcitability, although this seems unlikely given that PTZ-enhanced convulsions are not affected in clSNr lesioned animals (Chen et al., 2008, submitted). Additionally, the congenic used contains a number of genes in the introgressed interval in addition to the QTL, so it is possible that additional genes influenced the c-Fos expression pattern. More definitive confirmation that QTL status influences neural activation in the rvCP will likely require verification using genetic models that specifically target Mpdz (e.g., transgenic, knockout, or RNA interference models). Finally, our lesion and tract tracing results, while an important first step toward defining the role of rvCP-clSNr projection in ethanol withdrawal, will need to be expanded upon in future studies. Selective inactivation of c-Fos expressing neurons is a promising new approach to target neurons of interest (Koya et al., 2009), and does not affect passing fibers that must be considered. Standard tract tracing methods are largely qualitative in nature, and future work using more quantitative methods will be needed in order to assess whether subtle (quantitative) differences exist between congenic and background strain animals in the rvCP-clSNr pathway. As more information becomes available on the rvCP-clSNr projection, and proven associations between MPDZ and other proteins within this projection, their role in withdrawal and the contribution of genetic variation in Mpdz will become apparent.
Acknowledgments
Supported by AA011114, AA10760, DA05228, and the VA. We are grateful to Drs. Laura Kozell, Robert Hitzemann, Charles Meshul, John Belknap, and John Crabbe for helpful discussions of these experiments and their comments on a draft of this manuscript.
References
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Laursen SE, Crabbe JC. Ethanol and nitrous oxide produce withdrawal-induced convulsions by similar mechanisms in mice. Life Sci. 1987;41:2033–2040. doi: 10.1016/0024-3205(87)90477-2. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Danielson PW, Lame M, Crabbe JC. Ethanol and barbiturate withdrawal convulsions are extensively codetermined in mice. Alcohol. 1988;5:167–71. doi: 10.1016/0741-8329(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Le Merrer J, Stephens DN. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology (Berl) 2006;185:188–200. doi: 10.1007/s00213-005-0301-3. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci affecting risk for pentobarbital withdrawal map near alcohol withdrawal loci on mouse chromosomes 1, 4, and 11. Mamm Genome. 1999;10:431–437. doi: 10.1007/s003359901018. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Rademacher BS, Metten P, Crabbe JC. Mapping murine loci for physical dependence on ethanol. Psychopharmacology (Berl) 2002;160:398–407. doi: 10.1007/s00213-001-0988-8. [DOI] [PubMed] [Google Scholar]
- Chen G, Bonder EM, Cheng MF. Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J Neurobiol. 2006;66:537–551. doi: 10.1002/neu.20247. [DOI] [PubMed] [Google Scholar]
- Chen G, Kozell LB, Hitzemann R, Buck KJ. Involvement of the limbic basal ganglia in ethanol withdrawal convulsivity in mice is influenced by a chromosome 4 locus. J Neurosci. 2008;28:9840–9849. doi: 10.1523/JNEUROSCI.1713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Buck KJ. Substantia nigra pars reticulata is crucially involved in barbiturate and ethanol withdrawal in mice. Beh Brain Res. submitted doi: 10.1016/j.bbr.2010.10.025. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 1991;550:1–6. doi: 10.1016/0006-8993(91)90397-e. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill C, Belknap JK. Acute dependence on depressant drugs is determined by common genes in mice. J Pharmacol Exp Ther. 1991;257:663–7. [PubMed] [Google Scholar]
- De Deurwaerdere P, Le Moine C, Chesselet MF. Selective blockade of serotonin(2C) receptor enhances Fos expression specifically in the striatum and the subthalamic nucleus within the basal ganglia. Neurosci Lett. 2009 doi: 10.1016/j.neulet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Demarest K, Hitzemann B, Mahjubi E, McCaughran J, Jr, Hitzemann R. Further evidence that the central nucleus of the amygdala is associated with the ethanol-induced locomotor response. Alcohol Clin Exp Res. 1998;22:1531–1537. [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Fantin M, Auberson YP, Morari M. Differential effect of NR2A and NR2B subunit selective NMDA receptor antagonists on striato-pallidal neurons: relationship to motor response in the 6-hydroxydopamine model of parkinsonism. J Neurochem. 2008;106:957–968. doi: 10.1111/j.1471-4159.2008.05439.x. [DOI] [PubMed] [Google Scholar]
- Fantin M, Marti M, Auberson YP, Morari M. NR2A and NR2B subunit containing NMDA receptors differentially regulate striatal output pathways. J Neurochem. 2007;103:2200–2211. doi: 10.1111/j.1471-4159.2007.04966.x. [DOI] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Belknap JK, Crabbe JC, Buck KJ. Congenic mapping of alcohol and pentobarbital withdrawal liability loci to a <1 centimorgan interval of murine chromosome 4: identification of Mpdz as a candidate gene. J Neurosci. 2002;22:3730–3738. doi: 10.1523/JNEUROSCI.22-09-03730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller DJ, Bassir JM, Crabbe JC, Le Fevre CA. Audiogenic seizure susceptibility in WSP and WSR mice. Epilepsia. 1994;35:861–867. doi: 10.1111/j.1528-1157.1994.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Yang L, Faingold CL. Role of the amygdala in ethanol withdrawal seizures. Brain Res. 2007;1141:65–73. doi: 10.1016/j.brainres.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of γ-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther. 1983;227:663–670. [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, O’Malley S, Anton R. COMBINE genetics study: the pharmacogenetics of alcoholism treatment response: genes and mechanisms. J Stud Alcohol Suppl. 2005:56–64. doi: 10.15288/jsas.2005.s15.56. discussion 33. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: Grading the withdrawal system. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP, Hettinger MK. Intranigral muscimol suppresses ethanol withdrawal seizures. Brain Res. 1984;298:163–166. doi: 10.1016/0006-8993(84)91162-4. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Hitzemann B, Hitzemann R. Genetics ethanol and the Fos response: a comparison of the C57BL/6J and DBA/2J inbred mouse strains. Alcohol Clin Exp Res. 1997;21:1497–1507. [PubMed] [Google Scholar]
- Karpyak VM, Kim JH, Biernacka JM, Wieben ED, Mrazek DA, Black JL, Choi DS. Sequence variations of the human MPDZ gene and association with alcoholism in subjects with European ancestry. Alcohol Clin Exp Res. 2009;33:712–721. doi: 10.1111/j.1530-0277.2008.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science. 2003;300:990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kosobud A, Crabbe JC. Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J Pharmacol Exp Ther. 1986;238:170–177. [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozell LB, Hitzemann R, Buck KJ. Acute alcohol withdrawal is associated with c-Fos expression in the basal ganglia and associated circuitry: C57BL/6J and DBA/2J inbred mouse strain analyses. Alcohol Clin Exp Res. 2005;29:1939–1948. doi: 10.1097/01.alc.0000187592.57853.12. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Stephens DN, Ripley TL, Borlikova G, Duka T, Schubert M, Albrecht D, Becker HC, Lopez MF, Weiss F, Drummond C, Peoples M, Cunningham C. Alcohol withdrawal and conditioning. Alcohol Clin Exp Res. 2005;29:453–464. doi: 10.1097/01.alc.0000156737.56425.e3. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Jr, Singh J, Duan YF, Luckenbaugh DA, Manji HK, Drevets WC. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Manderscheid RW, Henderson MJ. Mental Health, United States, 2002. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; Rockville, Maryland: 2002. [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Mura A, Murphy CA, Feldon J, Jongen-Relo AL. The use of stereological counting methods to assess immediate early gene immunoreactivity. Brain Res. 2004;1009:120–128. doi: 10.1016/j.brainres.2004.02.054. [DOI] [PubMed] [Google Scholar]
- Nourry C, Grant SG, Borg JP. PDZ domain proteins: plug and play! Sci STKE. 2003;2003:RE7. doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. The economic costs of drug abuse in the United States, 1992–2002. Executive Office of the President; Washington, DC: 2004. (Publication No. 207303) [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Aust N Z J Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Parker LL, Backstrom JR, Sanders-Bush E, Shieh BH. Agonist-induced phosphorylation of the serotonin 5-HT2C receptor regulates its interaction with multiple PDZ protein 1. J Biol Chem. 2003;278:21576–21583. doi: 10.1074/jbc.M210973200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; Orlando FL: 2001. [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Price JL. Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neurosci Lett. 1984;47:15–22. doi: 10.1016/0304-3940(84)90379-3. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Topographic intermingling of striatonigral and striatopallidal neurons in the rhesus monkey. J Comp Neurol. 1990;297:359–376. doi: 10.1002/cne.902970304. [DOI] [PubMed] [Google Scholar]
- Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci. 2004;7:699–700. doi: 10.1038/nn1271. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Suffolk R, Jackson IJ. Identification, sequence, and mapping of the mouse multiple PDZ domain protein gene, Mpdz. Genomics. 1999;59:102–104. doi: 10.1006/geno.1999.5853. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, Hubner N, Heinig M, Pravenec M, Mangion J, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders J, Grant B, Hoffman PL. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Cavalheiro EA, Calderazzo-Filho LS, Bortolotto ZA, Klockgether T, Ikonomidou C, Turski WA. The basal ganglia, the deep prepyriform cortex, and seizure spread: bicuculline is anticonvulsant in the rat striatum. Proc Natl Acad Sci U S A. 1989;86:1694–1697. doi: 10.1073/pnas.86.5.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Meldrum BS, Cavalheiro EA, Calderazzo-Filho LS, Bortolotto ZA, Ikonomidou-Turski C, Turski WA. Paradoxical anticonvulsant activity of the excitatory amino acid N-methyl-D-aspartate in the rat caudate-putamen. Proc Natl Acad Sci U S A. 1987;84:1689–1693. doi: 10.1073/pnas.84.6.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Figge A, Lubbert H. Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett. 1998;424:63–68. doi: 10.1016/s0014-5793(98)00141-0. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Erwin VG, DeFries JC, Petersen DR, Cole-Harding S. Ethanol dependence in mice: direct and correlated responses to ten generations of selective breeding. Behav Genet. 1984;14:235–256. doi: 10.1007/BF01065544. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, Feger J, Tremblay L. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex. 2009;19:1844–1856. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]