Abstract
The purpose of this study was to investigate hemoglobin and iron handling after subarachnoid hemorrhage (SAH), examine the relationship between iron and neuroglial cell changes, and determine whether deferoxamine (DFX) can reduce SAH-induced injury. The SAH was induced in Sprague-Dawley rats (n=110) using an endovascular perforation technique. Animals were treated with DFX (100 mg/kg) or vehicle 2 and 6 hours after SAH induction followed by every 12 hours for 3 days. Rats were killed at 6 hours, Days 1 and 3 to determine nonheme iron and examine iron-handling proteins using Western blot and immunohistochemistry. 8-Hydroxyl-2′-deoxyguanosine and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining were performed to assess oxidative DNA damage and neuronal cell death. After SAH, marked heme-oxygenase-1 (HO-1) upregulation at Day 3 (P<0.01) was accompanied by elevated nonheme iron (P<0.01), transferrin (Tf) (P<0.01), Tf receptor (P<0.05), and ferritin levels (P<0.01). Deferoxamine treatment reduced SAH-induced mortality (12% versus 29%, P<0.05), brain nonheme iron concentration, iron-handling protein expression, oxidative stress, and neuronal cell death at Day 3 (P<0.01) after SAH. These results suggest that iron overload in the acute phase of SAH causes oxidative injury leading to neuronal cell death. Deferoxamine effectively reduced oxidative stress and neuronal cell death, and may be a potential therapeutic agent for SAH.
Keywords: deferoxamine, hemoglobin, iron, oxidative injury, subarachnoid hemorrhage
Introduction
Despite considerable improvements in treatment, the mortality rate within the first few days after subarachnoid hemorrhage (SAH) is still ∼35%. Thus, early brain injury represents the primary cause of mortality in SAH patients (Broderick et al, 1994). It is believed that early diagnosis and treatment of the underlying aneurysm are critical for potential reduction in mortality rate (Sehba and Bederson, 2006). Although the underlying injury mechanisms during this early period remain poorly understood, the combination of increased intracranial pressure (ICP) and decreased cerebral blood flow resulting in global ischemia is considered the leading cause of SAH-induced early brain injury (Sehba and Bederson, 2006). Although hemoglobin has been intensively studied as a potent factor for vasospasm in SAH studies (Macdonald and Weir, 1991), only a few studies have investigated the effect of hemoglobin and its major degradation product iron on cellular changes immediately after SAH (Turner et al, 1998). It is well known that the amount of blood released during SAH correlates with neurologic deficits and poor clinical outcome (Brouwers et al, 1993). Blood released into the subarachnoid space clots almost immediately and disappears within 3 days via clot lysis, which starts early after SAH (Nina et al, 2001). Recent evidence indicates that oxidative injury because of excessive hemoglobin and iron overload contributes significantly to brain damage after intracerebral hemorrhage (ICH; Xi et al, 2006). Iron, a major hemoglobin degradation product, also has a key role in neurodegeneration, for example in Alzheimer's disease and Parkinson's diseases (Benarroch, 2009). Thus, it seems likely that subarachnoid blood clot may additionally trigger cellular and molecular responses resulting in secondary brain injury.
After SAH, the brain is exposed to high concentrations of hemoglobin as erythrocytes lyse, especially at the basal surface of the brain (Lee et al, 2009a). Furthermore, it has been reported that subarachnoid blood distributes rapidly over the entire brain and penetrates easily into the deeper layers of the cortex within a few hours (Turner et al, 1998). Heme is degraded in brain by heme-oxygenase (HO) into carbon monoxide, biliverdin, and iron. Three HO isoforms have been identified in mammalian brain tissue (Wagner et al, 2003). Heme-oxygenase-1 is expressed primarily by glial cells and is induced by heat shock, heme, and a variety of oxidants. Heme-oxygenase-2 is constitutively expressed by neurons and endothelial cells (Xi et al, 2006). The role of HO-3 in brain remains to be clarified (Wagner et al, 2003).
Iron is an essential element needed for processes such as neuronal development, myelination and synthesis of neurotransmitters (Carbonell and Rama, 2007). However, free iron can react with H2O2 and O2− to form hydroxyl radicals (OH˙) in a sequence of Fenton or Haber–Weis reactions, which can inactivate or destroy biomolecules (Carbonell and Rama, 2007). Most of the nonheme iron in brain is bound to ferritin as Fe3+, and can only be released after being reduced to Fe2+. The reduction and release of iron from ferritin can be accomplished by superoxide, acidic pH, ascorbate, and catecholamines, all of which are abundant in the extracellular fluid of the brain, particularly during hypoxia ischemia (Carbonell and Rama, 2007). Moreover, changes in iron metabolism resulting in increased intracellular iron accumulation in the brain have been associated with iron-mediated neurotoxicity leading to greater brain damage in experimental cerebral ischemia or with early neurologic deterioration and excitotoxicity in patients with acute ischemic stroke (Dávalos et al, 2000). Several of our earlier studies have shown that iron deposition after ICH causes oxidative injury resulting in brain edema and neuronal cell death and delayed brain atrophy (Hua et al, 2006; Huang et al, 2002; Nakamura et al, 2004; Song et al, 2007). The degree of brain lesion correlates directly with regional iron concentration (Koeppen et al, 1995). Iron-induced oxidative damage in specific brain regions has also been found in Alzheimer's disease and Parkinson's disease (Benarroch, 2009). Iron accumulation may induce neuronal cell injury even after binding to ferritin. Iron can be released in its ferrous form under the acidic conditions present in extracellular fluid (Bishop and Robinson, 2001). Conditions such as SAH or hypoxia ischemia might further aggravate the potential for iron release from transferrin (Tf) or ferritin because of decreased pH (Carbonell and Rama, 2007) and the creation of an electrolyte imbalance within the brain extracellular fluid (Bishop and Robinson, 2001). Also, considering the amount of iron released after intracranial hemorrhage, it is likely that many of the mechanisms responsible for normal iron homeostasis may become saturated and that the excess iron present can induce significant neurotoxicity (Levy et al, 2002). Thus, studies have shown that iron overload can result in a large increase in the chelatable free iron pool, which is too large to be sequestered by cellular ferritin (Carbonell and Rama, 2007).
An iron chelator, deferoxamine (DFX), is used to treat iron toxicity in hemochromatosis (Prass et al, 2002). Previous studies from our laboratories found that DFX reduces hemoglobin-induced brain edema and decreases brain injury in experimental ICH (Nakamura et al, 2004; Song et al, 2007).
This study focuses on the role of subarachnoid hemoglobin and iron in brain injury during the acute phase of SAH and examines the effect of DFX on SAH-induced injury. Recently, we showed that the endovascular perforation SAH rat model is more suitable for studying the acute pathophysiology of SAH compared with a cisterna magna injection model, which yields smaller SAHs and less brain damage (Lee et al, 2009a). Therefore, this experimental model was used for SAH induction.
Materials and methods
Animal Preparation and Subarachnoid Hemorrhage Induction
The protocols for these animal studies were approved by the University of Michigan Committee on the Use and Care of Animals at the University of Michigan. A total of 145 adult male Sprague-Dawley rats weighing between 275 and 325 g were used. General anesthesia was induced with 5% isoflurane (Aerrane; Baxter Healthcare Corp., Deerfield, IL, USA). After intubation and initiation of mechanical ventilation, isoflurane was titrated between 2.25% and 2.75% to maintain a mean arterial pressure between 80 and 120 mm Hg. Blood was obtained from the catheter for analysis of pH, Pa2, Pa2, hematocrit, and blood glucose. Body temperature was maintained at 37°C, with use of a feedback-controlled heating pad.
In supine position and after a left paramedian incision of the ventral neck, the left external carotid artery was identified under a surgical microscope, transected distally, and reflected caudally in line with the internal carotid artery. A 3-0 nylon monofilament suture with rounded tip to prevent endothelial injury was inserted into the stump of external carotid artery past the common carotid artery bifurcation and into the internal carotid artery. The external carotid artery was tightened around the suture to prevent blood loss. The filament was advanced distally into the intracranial internal carotid artery and carefully perforated under ICP monitoring. The SAH was confirmed by a sudden rise in ICP. The suture was then withdrawn producing hemorrhage. Common carotid artery was temporarily occluded for 2 minutes to limit the hemorrhage volume. Sham-operated control rats underwent an identical procedure except that the suture was not advanced beyond the point of resistance. The suture was withdrawn 5 minutes after insertion.
After recovery, the animals were maintained in an air-conditioned room at 20°C, with ad libitum to food and water. Neurologic evaluation was performed daily for 5 minutes in their normal environment (cage) as previously described (Lee et al, 2009a).
Study Design and Experimental Groups
Before SAH induction, the rats were randomly assigned to three groups: sham, and SAH without and with DFX (100 mg/kg) treatment. Deferoxamine was administered intraperitoneally 2 and 6 hours after hemorrhage followed by every 12 hours for 3 days. Rats were killed at 6 hours, Days 1 and 3 after SAH induction. Biochemical and immunohistological studies were performed focusing on the neuroglial changes in the left frontobasal brain tissue next to arterial perforation site as a consequence of excessive hemoglobin and iron accumulation. After removal, the brain was processed for immunohistochemical studies or an ∼4 mm thick coronal slice around optic chiasm was separated and the left frontobasal cortex sampled for determination of nonheme brain iron content and Western blot analysis.
The study was performed in three parts. Part 1 examined nonheme brain tissue iron content at 6 hours, Days 1 and 3 after hemorrhage (n=5 per time point and group). Part 2 investigated HO-1, Tf, Tf receptor (TfR), and ferritin levels by Western blot analysis (n=7 per time point and group). Part 3 examined HO-1 and ferritin expression by immunohistochemistry and immunofluorescent double labeling (n=5 per time point and group), and 8-hydroxyl-2′-deoxyguanosine (8-OHdG) and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining were performed to assess oxidative cell injury and neuronal cell death (n=5 per time point and group). Sham-operated rats were killed at 6 hours, Days 1 and 3 (n=5 each). Wherever possible, the investigators were masked to the treatment applied.
Intracranial Pressure
After positioning in a stereotactic frame (Kopf Instrument, Tujunga, CA, USA), a small scalp incision was made right frontal paramedian, and the ICP transducer (TSD175A, Samba Sensors) was inserted through a burr hole (3 mm lateral to midline and 1 mm rostral to coronal suture). The ICP was measured using a digital ICP monitor (MPMS100A-1; Biopac System, Goleta, CA, USA).
Grading of Subarachnoid Hemorrhage
In all animals, the extent of SAH was evaluated using a modified grading system of Sugawara et al (2008) as described previously (Lee et al, 2009b). The basal brain including brainstem was divided into six segments (anterior and posterior Circle of Willis on both sides and left and right brainstem). Each segment was assigned to grade from 0 to 3 depending on the amount of subarachnoid blood clot as follows: Grade 0, no subarachnoid blood; Grade 1, minimal subarachnoid blood; Grade 2, moderate blood clot with recognizable arteries; and Grade 3, blood clot covering the cerebral arteries. The blood distribution in ventrolateral brainstem was also considered Grades 2 and 3 depending on blood amount.
Nonheme Brain Tissue Iron Determination
Rats were killed at 6 hours, Days 1 and 3 after SAH. The brains were extensively perfused with 0.1 mol/L phosphate-buffered saline (PBS) (pH 7.4) before decapitation. After brain removal, the remaining subarachnoid blood was carefully removed from the basal surface of the brain. A coronal slice of basal brain ∼4 mm thick around optic chiasm (between −2.8 and 1.2 mm in relation to the Bregma) was cut. Ipsilateral frontobasal cortex was separated and weighed. Nonheme brain tissue iron concentration was determined as reported previously (Wu et al, 2003). Briefly, brains were homogenized and 1 mL of 8.5 mol/L HCl added. Brain samples were hydrolyzed at 90°C for 60 minutes. After cooling, 2 mL of 20% trichloroacetic acid was added to precipitate the proteins, and the supernatant was collected after centrifugation. The supernatant was then run through an acid-washed filter and the precipitate was washed with 1 mL of 4.25 mol/L HCl plus 20% trichloroacetic acid (1:1). The supernatant was collected and 4 mL of 1 mol/L sodium citrate was added. The pH was adjusted to 3.1 and the final volume was adjusted to 25 mL. The total nonheme iron concentration was assayed by a spectrophotometer with ferrozine as color reagent.
Western Blot Analysis
Rats were anesthetized and underwent intracardiac perfusion with 0.1 mol/L PBS (pH 7.4). Brains were removed and a 3-mm-thick coronal slice was cut at the level of optic chiasm, and the ipsilateral frontobasal cortex separated. Western blot analysis was performed as described previously (Lee et al, 2009b). Briefly, brain samples were sonicated in Western blot lysis buffer. Protein concentration was determined using a Bio-Rad Laboratories (Hercules, CA, USA) protein assay kit. Protein samples (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a hybond-C pure nitrocellulose membrane (Amersham, Piscataway, NJ, USA). Membranes were blocked in Carnation nonfat milk and probed with primary and secondary antibodies. Primary antibodies were the following: polyclonal rabbit anti-horse spleen ferritin (Sigma, St Louis, MO, USA; 1:250 dilution), polyclonal rabbit anti-human Tf (DAKO, Carpinteria, CA, USA; 1:1000 dilution), monoclonal mouse anti-human TfR (ZYMED Laboratories Inc, San Francisco, CA, USA; 1:2000 dilution), or polyclonal rabbit anti-rat HO-1 (StressGen, San Diego, CA, USA; 1:2500 dilution). Antigen–antibody complexes were visualized with the ECL chemiluminescence system (Amersham) and exposed to Kodak X-OMAT film. Relative band densities were analyzed with NIH Image program (Version 1.61).
Immunohistochemistry and Histochemistry
Rats underwent intracardiac perfusion with 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4). Brains were removed and kept in 4% paraformaldehyde for 12 hours, then immersed in 25% sucrose for 3 days at 4°C. Brains were then placed in embedding compound and sectioned (18 μm) on a cryostat. Immunohistochemical staining was performed using the avidin–biotin complex technique. Primary antibodies were polyclonal rabbit anti-rat HO-1 (StressGen; 1:400 dilution) and rabbit anti-human ferritin (DAKO; 1:100 dilution). Normal rabbit IgG (Vector Laboratories, Burlingame, CA, USA) was used as a negative control.
Immunohistochemistry for 8-OHdG was used to examine oxidative DNA injury and performed as previously described (Nakamura et al, 2004). Sections were incubated in 1:10 horse serum (Vector Laboratories) for 30 minutes, rinsed, and incubated overnight with primary antibody (mouse anti-8-OHdG monoclonal antibody, 10 μg/mL; Oxis International Inc, Portland, OR, USA). Normal mouse IgG (Vector Laboratories) was used as a negative control. The secondary antibody was anti-mouse IgG antibody (1:150; Vector Laboratories).
To detect DNA fragmentation, sections were processed for TUNEL using an in situ cell death detection kit (Intergen, Burlington, MA, USA). In brief, sections were dried, rehydrated in PBS, and fixed in paraformaldehyde for 10 minutes. After permeabilization for 5 minutes with ethanol 95%–acetic acid (2:1) at −20°C, sections were incubated with a mixture of terminal deoxynucleotidyl transferase and fluorescein-conjugated deoxyuridine triphosphate for 1 hour at 37°C. The reaction was stopped by PBS washing.
Cell counting was conducted in the basal cerebral cortex on × 40 microscopic images using a CK-2 Olympus microscope. Positive cells were counted in three separate fields in three different slices cut at the level of the optic chiasm. Five animals per group were analyzed.
Immunofluorescent Double Labeling
For immunofluorescent double labeling, the primary antibodies were rabbit anti-human ferritin (DAKO; 1:100 dilution), mouse anti-rat OX-42 (Serotec, Raleigh, NC, USA; 1:100 dilution), goat anti-glial fibrillary acid protein (Santa Cruz, Santa Cruz, CA, USA; 1:100 dilution), and mouse anti-neuronal nuclei (NeuN; Chemicon, Billerica, MA, USA; 1:100 dilution). Rhodamine-conjugated goat anti-rabbit (Vector Laboratories, 1:100 dilution), fluorescein isothiocyanate-labeled horse anti-mouse and rabbit anti-goat antibodies (Chemicon; 1:100 dilution) were used as secondary antibodies.
To assess colocalization with ferritin, TUNEL staining was followed by incubation with PBS containing 10% normal goat serum, 1% bovine serum albumin, and 0.3% Triton X-100 to prevent nonspecific conjugate binding. Sections were then incubated overnight at 4°C with primary anti-ferritin antibody (DAKO). After washing, sections were incubated with secondary antibody for 2 hours.
Statistical Analysis
Values are presented as means±s.d. Statistical comparisons between groups were performed using one-way analysis of variance followed by either a Dunnett's or a Tukey's post hoc test, the former for comparisons to a single control group, the latter to compare across multiple groups. Mortality was compared with a χ2 test. A probability of P<0.05 was considered to be statistically significant.
Results
Physiological parameters including mean blood pressure, blood pH, blood gases, hematocrit, and blood glucose were recorded and were controlled within normal ranges at the onset of SAH.
Subarachnoid Hemorrhage Extent and Mortality Rate
No sham-operated control animals died. The SAH induction caused a sudden increase in ICP from 8±2 to 65±13 mm Hg. The mortality rate in the SAH group without DFX treatment was 29% (21 of 72 rats). Deferoxamine treatment decreased the mortality rate to 12% (7 of 58, P<0.05). All animals in both groups had extensive SAH. This was particularly pronounced on the ipsilateral side, around the Circle of Willis, and along the ventral brainstem (Figure 1). A SAH score was used to assess the degree of hemorrhage. At 24 hours after hemorrhage, the score was 14±3 and 13±2 out of a possible 18 in the SAH groups without and with DFX treatment, respectively. Despite the rapid clearance of subarachnoid blood, a significant amount of blood clot could still be observed on Day 3 (Figure 1).
Figure 1.
(A) Time course of the subarachnoid hemorrhage (SAH) caused by endovascular perforation of the internal carotid artery. Severe SAH was seen around the Circle of Willis and along the ventral brainstem 6 hours after hemorrhage (B). Then, SAH diminished slowly over 3 days after hemorrhage (D). The extent of SAH was assessed 24 hours after hemorrhage (C). The basal surface of the brain was divided into six segments and each segment rated for hemorrhage (3: massive hemorrhage covering the cerebral arteries; 2: moderate blood clot with recognizable arteries; 1: minimal subarachnoid blood; 0: no blood).
Increased Heme-Oxygenase-1 Expression After Subarachnoid Hemorrhage
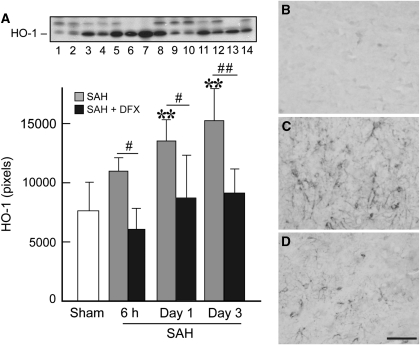
The HO-1 expression as assessed by Western blot was low in brain tissue from sham-operated rats and few HO-1-positive cells were found by immunohistochemistry (Figure 2). In vehicle-treated SAH rats, a marked increase in HO-1 protein level was observed on Day 1 (13,529±1802 versus 7634±2409 pixels in controls, P<0.01) after SAH, reaching a maximum at Day 3 (15,242±2720, P<0.01; Figure 2). Accordingly, HO-1 immunoreactivity was evident as early as 6 hours after SAH induction, and markedly induced on Days 1 and 3 compared with sham-operated controls. The HO-1-positive cells were detected in the cortical area of the basal brain, and the immunoreactivity was predominantly localized to cells with small, rounded cell bodies with well-defined processes, consistent with microglia (Figure 2). Treatment with DFX greatly reduced the induction of HO-1 by SAH.
Figure 2.
(A) Western blot analysis showing significant increase in heme-oxygenase-1 (HO-1) expression 24 hours (lanes 5 and 6) and 72 hours after hemorrhage (lanes 7 and 8) compared with sham-operated control group (lanes 1 and 2). Deferoxamine (DFX) treatment resulted in significant reduced HO-1 expression 6 hours (lanes 9 and 10), 24 hours (lanes 11 and 12), and 72 hours (lanes 13 and 14) after subarachnoid hemorrhage (SAH) induction. MW of HO-1 is ∼32 kDa. Values are mean±s.d. **P<0.01 versus sham. # and ##P<0.05 and P<0.01 versus vehicle-treated SAH group, respectively. The HO-1 immunoreactivities in the left basal brain after a sham operation (B) or 3 days after SAH induction without (C) and with DFX treatment (D). Scale bar=50 μm.
Nonheme Iron Concentration in the Brain Tissue
Brain nonheme iron levels increased progressively in vehicle-treated SAH rats, reaching a maximum 3 days after SAH (101±16 versus 43±10 μg/g brain tissue in the sham-operated control group, P<0.01). This increase in nonheme iron after SAH was markedly reduced by systemic DFX treatment (64±17 μg/g brain tissue at Day 3, P<0.05; Figure 3).
Figure 3.
Nonheme iron concentration in left basal brain tissue after subarachnoid hemorrhage (SAH) in vehicle-treated and deferoxamine (DFX)-treated rats and in sham-operated rats. Values are mean±s.d. **P<0.01 versus sham; #P<0.05 versus vehicle-treated SAH group.
Time Course of Iron-Handling Proteins
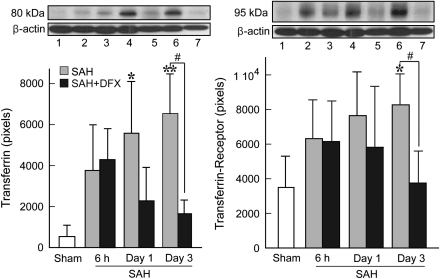
Transferrin is involved in the transport of iron into cells through binding to its receptor TfR. After SAH induction in vehicle-treated rats, Tf and TfR protein levels increased rapidly, reaching a maximum at Day 3 (6540±2567 versus 538±555 pixels in controls for Tf, P<0.01 and 8275±1785 versus 3498±1811 pixels in controls for TfR, P<0.05; Figure 4). Treatment with DFX markedly reduced Tf and TfR expression (1561±671 pixels, P<0.05 and 3724±1885 pixels, P<0.05 for Tf and TfR, respectively).
Figure 4.
Western blot analyses showing time course of transferrin (Tf) (left) and transferrin receptor (TfR) (right) protein levels in the left basal brain tissue at 6 hours (lanes 2 and 3), Day 1 (lanes 4 and 5), and Day 3 (lanes 6 and 7) after subarachnoid hemorrhage (SAH) induction without (lanes 2, 4, and 6) and with deferoxamine (DFX) treatment (lanes 3, 5, and 7). Lane 1: sham-operated control group. Values are mean±s.d. *P<0.05 and **P<0.01 versus sham. #P<0.05 versus vehicle-treated SAH group.
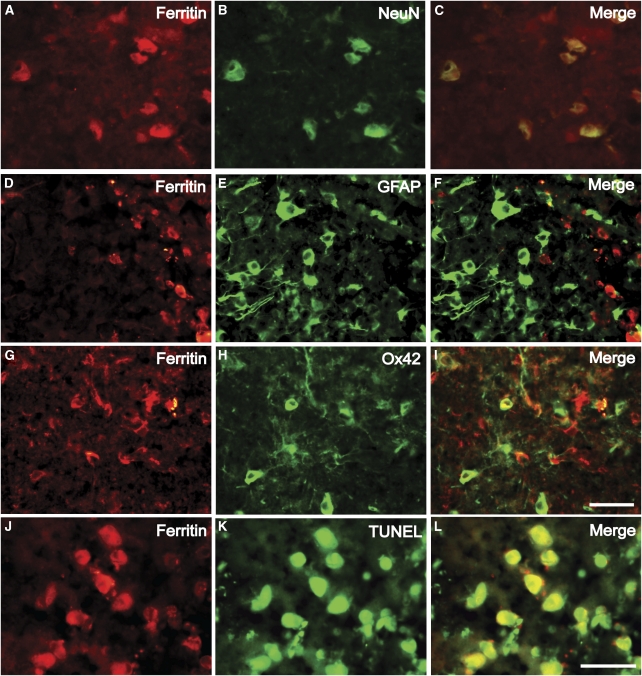
Ferritin is a key iron-storage protein in the brain. Western blot analysis showed that ferritin levels were low 6 and 24 hours after SAH in vehicle-treated rats and did not differ significantly compared with sham-operated rats. However, levels increased significantly on Day 3 after SAH (6693±1596 versus 1474±797 pixels in sham-operated controls, P<0.01; Figure 5). By immunohistochemistry, few ferritin-positive cells were found in normal brain. However, after SAH, the number of ferritin-positive cells progressively increased over 3 days after hemorrhage (data not shown). Double labeling showed that most ferritin-positive cells were either microglial cells or neurons (Figures 6A–6I). Western blot analysis showed that systemic DFX administration significantly reduced ferritin expression (3074±557, P<0.01).
Figure 5.
Representative Western blot showing ferritin expression at 6 hours (lanes 2 and 3), Day 1 (lanes 4 and 5), and Day 3 (lanes 6 and 7) after subarachnoid hemorrhage (SAH) induction without (lanes 2, 4, and 6) and with deferoxamine (DFX) treatment (lanes 3, 5, and 7). Lane 1: sham-operated control group. Values are mean±s.d. **P<0.01 versus sham. ##P<0.01 versus vehicle-treated SAH group.
Figure 6.
Double immunofluorescent labeling for ferritin and NeuN (A–C), glial fibrillary protein (GFAP; D–F), and OX-42 (G–I), showing that ferritin-positive cells are neurons and microglia. Moreover, ferritin-positive cells exhibit DNA damage, indicating apoptotic cell death (J–L). Scale bar=50 μm.
To detect the relationship between ferritin- and TUNEL-positive cells, double staining was performed. Most TUNEL-positive cells were ferritin positive and neuron like (Figures 6J–6L).
Neuronal Cell Death and Oxidative DNA Injury
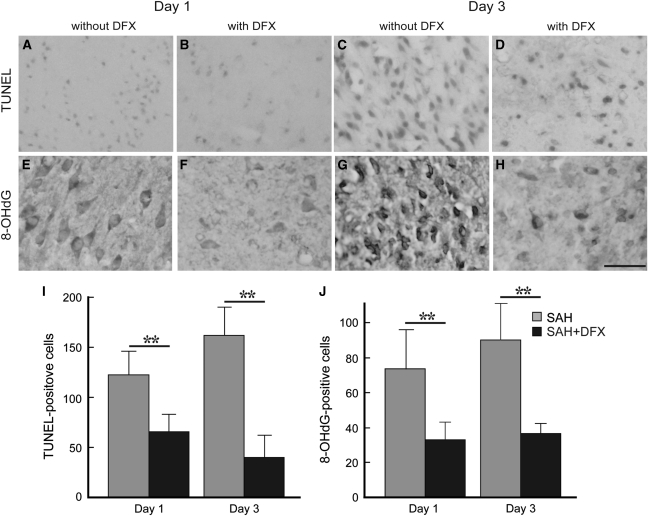
DNA damage in brain was detected by TUNEL staining at Days 1 and 3 after SAH in vehicle-treated rats. The TUNEL-positive cells showed apoptotic characteristics, such as chromatin condensation and fragmented nuclei. Numerous TUNEL-positive cells were found in the cortical and subcortical area of the basal brain at Day 1 and further increased at Day 3 after hemorrhage. Deferoxamine treatment caused a significant reduction in TUNEL-positive cells at Day 1 (122±23 and 65±16, P<0.01) and at Day 3 (162±28 and 40±22, P<0.01, n=5; Figures 7A–7D and 7I).
Figure 7.
Photomicrographs showing terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL; A–D) and 8-hydroxyl-2′-deoxyguanosine (8-OHdG; E–H) immunoreactivity in the ipsilateral basal brain on Days 1 (A, B, E, F) and 3 (C, D, G, H) after subarachnoid hemorrhage (SAH) without (A, C, E, G) and with deferoxamine (DFX; B, D, F, H) treatment. Quantification of the TUNEL (I) and 8-OHdG (J) results. Values are mean±s.d., n=5. **P<0.01.
To examine oxidative DNA damage, 8-OHdG immunohistochemistry was performed. In vehicle-treated SAH rats, extensive 8-OHdG immunoreactivity was found at Days 1 and 3 (Figure 7). The cortical and subcortical distribution in the basal brain was similar to that found with TUNEL staining. The number of 8-OHdG-positive cells was significantly reduced by DFX treatment at Day 1 (33±10 versus 74±23 cells with vehicle treatment, P<0.01) and Day 3 (36±6 versus 90±21 cells with vehicle treatment, P<0.01; Figures 7E–7H and 7J).
Discussion
This study determined changes in brain nonheme iron and proteins related to iron handling (HO-1, Tf, TfR, and ferritin) in the acute phase after SAH and examined the effects of an iron chelator, DFX, on those changes and SAH-induced brain injury. The SAH induced marked increases in brain nonheme iron and iron-handling proteins. Systemic DFX treatment largely prevented those changes and reduced SAH-induced cell damage (oxidative DNA damage and TUNEL staining). Together, these results suggest that iron may be a target for reducing acute brain injury after SAH.
Nonheme Iron and Iron-Handling Proteins After Subarachnoid Hemorrhage
After SAH, a large amount of hemoglobin is released into the subarachnoid space (Lee et al, 2009a). Heme-oxygenase is a key enzyme for heme degradation (Wagner et al, 2003; Xi et al, 2006). In this study, HO-1 expression rapidly increased in the basal brain at Day 1 after SAH, and peaked at Day 3. Immunohistochemistry showed that HO-1 was mainly localized to microglia in cortical area, in accordance with previous studies (Turner et al, 1998; Wu et al, 2003). This response might be the result of intraparenchymal hemoglobin overload after lysis of subarachnoid erythrocytes and subsequent penetration of hemoglobin into adjacent brain tissue (Turner et al, 1998). Heme is a potent inducer of HO-1 (Wagner et al, 2003). However, HO-1 can be induced by a wide variety of the factors including hydroxyl radicals and inflammatory mediators (Syapin, 2009). Our finding that DFX blunted SAH-induced HO-1 upregulation suggests that iron-mediated free radical production contributes to the upregulation or that DFX can interfere with induction of HO-1 by heme.
Heme-oxygenase-1 degrades hemoglobin into carbon monoxide, biliverdin, and iron. Thus, the increase in HO-1 along with its substrate, heme, after SAH is very likely responsible for the increase in brain nonheme iron found in this study. The block of SAH-induced HO-1 upregulation by DFX may contribute to the lower nonheme iron levels in DFX-treated rats.
Whether HO-1 is beneficial or detrimental in hemorrhagic injury is still unclear (Wagner et al, 2003). Although overexpression of HO-1 protects neurons from oxidative injury (Chen et al, 2000), inhibition of HO has been associated with attenuation of perihematomal brain edema (Huang et al, 2002).
This study showed a progressive increase in brain nonheme iron with peak at Day 3 after SAH, paralleling the rise in HO-1 expression. Nonheme tissue iron was measured to avoid the influence of hemoglobin from the hematoma (Wu et al, 2003). The increased iron deposition was associated with a considerable upregulation of iron-handling proteins, Tf and TfR. Transferrin, an 80-kDa iron-transporting glycoprotein, binds iron in the extracellular space and cerebrospinal fluid (Wagner et al, 2003). Cellular uptake of Tf-bound iron occurs by binding to TfR, which appears to be localized to neuron-like cells in perihematomal area after ICH (Wagner et al, 2003). By transporting iron into cells, they probably limit the damage that would be induced by an increase in extracellular iron during hematoma lysis. Brain Tf and TfR levels are also altered in various neurodegenerative disorders (Benarroch, 2009; Wagner et al, 2003).
Excess intracellular iron not used for metabolic purposes is normally stored in the cytosol bound to ferritin, protecting cells from oxidative damage (Wagner et al, 2003). Fully assembled ferritin consists of 24 subunits of H- and L-subunits, and has been found mainly in glial cells (Connor et al, 1994). The brain can produce ferritin, and hemoglobin degradation products such as iron and heme are strong ferritin inducers through iron regulatory proteins (Eisenstein et al, 1991). After SAH, ferritin expression was increased and the time course paralleled the increase in nonheme brain tissue iron, suggesting that regulation of ferritin synthesis after SAH is mainly iron mediated, as in ICH (Wu et al, 2003). In ICH, most ferritin- and iron-positive cells are microglia (Wu et al, 2003). However, in this study, ferritin was localized in neurons as well as microglia after SAH. This observation is supported by Nakamura et al (2004), who reported that Perl's positive cells were neuron like in the acute phase after ICH.
Oxidative DNA Injury and Acute Neuronal Cell Death
Because of high iron content after SAH and abundance of oxidizable lipids in neuronal membranes, the generation of toxic hydroxyl radicals may present a significant problem for the brain (Carbonell and Rama, 2007). Recently, significantly increased O2− production after SAH has been shown, suggesting oxidative stress has a significant role in acute brain injury (Endo et al, 2007). DNA is vulnerable to oxidative stress, and based on the observation that 8-OHdG is formed in cellular DNA on treatment with various oxygen radical-producing agents, it has been used as a marker of oxidative DNA injury (Kasai et al, 1986; Nakamura et al, 2004). The TUNEL staining is an additional sensitive marker of DNA damage (Chen et al, 1997). DNA damage can result either from endonuclease-mediated DNA fragmentation (apoptosis) or oxidative injury (Graham and Chen, 2001). In the current study, TUNEL- and 8-OHdG-positive cells increased progressively on Days 1 and 3 after SAH induction, suggesting that DNA damage may be an important injury mechanism after SAH. Such DNA damage was markedly reduced by DFX treatment, indicating a potential role for iron in that damage.
Colocalization studies of TUNEL with ferritin indicated that the majority of dying cells were ferritin positive and neuron like. In vitro studies have shown differential cellular responses to excess iron (Kress et al, 2002) and hemoglobin (Regan and Panter, 1993) with severe toxicity in neurons but not glial cells. Furthermore, prolonged ferritin elevation within neurons can lead to neurodegeneration and neurotoxicity (Kaur et al, 2007).
Recently, increased autophagic changes in neurons, which may potentially lead to cell death, have been shown in the same basal structures during the acute phase of SAH (Lee et al, 2009b). This phenomenon was pronounced after injection of iron into the basal ganglia (He et al, 2008) and could partly be explained by activation of autophagic pathways for removal of large amount of ferritin after binding of intracellular redox-active iron (Kurz and Brunk, 2009).
Deferoxamine
Deferoxamine is clinically used for treatment of acute iron intoxication and chronic iron overload because of transfusion-dependent anemia. Deferoxamine rapidly penetrates the blood–brain barrier after intraperitoneal injection and accumulates in brain at significant concentrations (Palmer et al, 1994). Deferoxamine chelates free iron forming a stable complex preventing iron from entering into further chemical reactions. On the basis of previous studies from our laboratories, DFX was injected intraperitoneally at 100 mg/kg beginning 2 and 6 hours after hemorrhage and then every 12 hours. Deferoxamine has been shown to reduce hemoglobin-induced brain edema, to ameliorate ICH-induced oxidative DNA damage and neuronal toxicity (Nakamura et al, 2004; Song et al, 2007). Moreover, DFX increases levels of APE/Ref-1, which is involved in DNA repair (Nakamura et al, 2004). In this study, DFX markedly reduced brain nonheme iron and, probably as a consequence, also lowered the expression of iron-handling proteins. Moreover, oxidative injury was ameliorated and TUNEL-labeled cell death reduced. These findings suggest that DFX effectively diminished hydroxyl radical formation and oxidative stress by sequestering redox-active iron. However, DFX may have other effects. Thus, it exerts inflammatory process modulating effects by stimulating cyclooxygenase (Tanji et al, 2001). It can also induce brain tolerance via hypoxia-inducible factor-1, which regulates a number of neuroprotective genes, including erythropoietin (Prass et al, 2002).
The current study has some limitations. The mechanism(s) involved in HO-1 induction after SAH requires further examination. Heme-oxygenase-1 expression can be induced by inflammatory processes (Syapin, 2009) and significant upregulated tumor necrosis factor-α or nuclear factor-κB upregulation has been shown after SAH (Simard et al, 2009). Moreover, HO-1 induction seems to be able to control the high-mobility group box-1 translocation and extracellular secretion (García-Arnandis et al, 2010), which has an important role in the inflammatory process in the brain, and its downregulation causes a dramatic reduction in infarct size in a stroke model (Yang et al, 2010). The relative role of Fe3+ and Fe2+ in SAH-induced injury also merits further investigation. Deferoxamine has a much greater affinity for Fe3+ than Fe2+. Therefore, iron-mediated neuronal cell injury might be more effectively reduced with an additional Fe2+ chelator. Horky et al (1998) have shown that 2,2′-dipyridyl, a Fe2+ chelator, can prevent vasospasm.
Conclusion
In the acute phase after SAH, subarachnoid hemoglobin and a marked HO-1 upregulation lead to increased iron release and production of proteins involved in iron transport and storage in the basal part of the brain. Moreover, large amounts of iron arising from hemoglobin degradation might participate in the generation of free radicals leading to increased oxidative injury and DNA damage. Systemic administration of DFX reduced iron level and iron-handling proteins and significantly ameliorated oxidative injury and neuronal cell death. Thus, increased iron deposition in the brain tissue after subarachnoid hemoglobin degradation may be a therapeutic target for patients with SAH.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflict of interest.
References
- Benarroch EE. Brain iron homeostasis and neurodegenerative disease. Neurology. 2009;72:1436–1440. doi: 10.1212/WNL.0b013e3181a26b30. [DOI] [PubMed] [Google Scholar]
- Bishop GM, Robinson SR. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res. 2001;907:175–187. doi: 10.1016/s0006-8993(01)02303-4. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- Brouwers PJ, Dippel DW, Vermeulen M, Lindsay KW, Hasan D, van Gijn J. Amount of blood on computed tomography as an independent predictor after aneurysm rupture. Stroke. 1993;24:809–814. doi: 10.1161/01.str.24.6.809. [DOI] [PubMed] [Google Scholar]
- Carbonell T, Rama R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Curr Med Chem. 2007;14:857–874. doi: 10.2174/092986707780363014. [DOI] [PubMed] [Google Scholar]
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- Connor J, Boeshore K, Benkovic S, Menzies S. Isoforms of ferritin have a specific cellular distribution in the brain. J Neurosci Res. 1994;37:461–465. doi: 10.1002/jnr.490370405. [DOI] [PubMed] [Google Scholar]
- Dávalos A, Castillo J, Marrugat J, Fernández-Real JM, Armengou A, Cacabelos P, Rama R. Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology. 2000;54:1568–1574. doi: 10.1212/wnl.54.8.1568. [DOI] [PubMed] [Google Scholar]
- Eisenstein R, Garcia M, Pettingell W, Munro H. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci USA. 1991;88:688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3β survival signaling. J Cereb Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arnandis I, Guillén MI, Castejón MA, Gomar F, Alcaraz MJ. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology. 2010;49:854–861. doi: 10.1093/rheumatology/kep463. [DOI] [PubMed] [Google Scholar]
- Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- He Y, Wan S, Hua Y, Keep RF, Xi G. Autophagy after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2008;28:897–905. doi: 10.1038/sj.jcbfm.9600578. [DOI] [PubMed] [Google Scholar]
- Horky LL, Pluta RM, Boock RJ, Oldfiled EH. Role of ferrous iron chelator 2,2′-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 1998;88:298–303. doi: 10.3171/jns.1998.88.2.0298. [DOI] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- Huang F, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kaur D, Rajagopalan S, Chinta S, Kumar J, Di Monte D, Cherny RA, Andersen JK. Chronic ferritin expression within murine dopaminergic midbrain neurons result in a progressive age-related neurodegeneration. Brain Res. 2007;1140:188–194. doi: 10.1016/j.brainres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci. 1995;134:102–112. doi: 10.1016/0022-510x(95)00215-n. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Dineley KE, Reynolds IJ. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–5855. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz T, Brunk UT. Autophagy of HSP70 and chelation of lysosomal iron in a non-redox-active form. Autophagy. 2009;5:93–95. doi: 10.4161/auto.5.1.7248. [DOI] [PubMed] [Google Scholar]
- Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G. Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res. 2009b;1287:126–135. doi: 10.1016/j.brainres.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Sagher O, Keep R, Hua Y, Xi G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009a;65:331–343. doi: 10.1227/01.NEU.0000345649.78556.26. [DOI] [PubMed] [Google Scholar]
- Levy YS, Streifler JY, Panet H, Melamed E, Offen D. Hemin-induced apoptosis in PC12 and neuroblastoma cells: implications for local neuronal death associated with intracerebral hemorrhage. Neurotox Res. 2002;4:609–616. doi: 10.1080/1029842021000045624. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Nina P, Schisano G, Chiappetta F, Luisa Papa M, Maddaloni E, Brunori A, Capasso F, Corpetti MG, Demurtas F. A study of blood coagulation and fibrinolytic system in spontaneous subarachnoid hemorrhage. Correlation with Hunt-Hess grade and outcome. Surg Neurol. 2001;55:197–203. doi: 10.1016/s0090-3019(01)00402-5. [DOI] [PubMed] [Google Scholar]
- Palmer C, Roberts RL, Bero C. Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke. 1994;25:1039–1045. doi: 10.1161/01.str.25.5.1039. [DOI] [PubMed] [Google Scholar]
- Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U, Meisel A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Flow Blood Metab. 2002;22:520–525. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Regan RF, Panter SS. Neurotoxicity of hemoglobin in cortical cell culture. Neurosci Lett. 1993;153:219–222. doi: 10.1016/0304-3940(93)90326-g. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Meth. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2009;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Imaizumi T, Matsumiya T, Itaya H, Fujimoto K, Cui X, Toki T, Ito E, Yoshida H, Wakabayashi K, Satoh K. Desferrioxamine, an iron chelator, upregulates cyclooxygenase-2 expression and prostaglandin production in a human macrophage cell line. Biochim Biophys Acta. 2001;1530:227–235. doi: 10.1016/s1388-1981(01)00089-0. [DOI] [PubMed] [Google Scholar]
- Turner CP, Bergeron M, Matz P, Zegna A, Noble LJ, Panter SS, Sharp FR. Heme oxygenase-1 (HO-1) is induced in glia throughout the brain by subarachnoid hemoglobin. J Cereb Blood Flow Metab. 1998;18:257–273. doi: 10.1097/00004647-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yang Q-W, Wang J-Z, Li J-C, Zhou Y, Zhong Q, Lu F-L, Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:243–254. doi: 10.1038/jcbfm.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]