Abstract
Background
The yield of screening for acute HIV infection among general medical patients in resource-scarce settings remains unclear. Our objective was to evaluate a strategy of pooled HIV plasma RNA to diagnose acute HIV infection in patients with negative or discordant rapid HIV antibody tests in Durban, South Africa.
Methods
We prospectively enrolled patients with negative or discordant rapid HIV antibody tests from a routine HIV screening program in an outpatient department in Durban with an HIV prevalence of 48%. Study participants underwent venipuncture for pooled qualitative HIV RNA, and if positive, quantitative RNA, enzyme immunoassay and Western Blot (WB). Patients with negative or indeterminate WB and positive quantitative HIV RNA were considered acutely infected. Those with chronic infection (positive RNA and WB) despite negative or discordant rapid HIV tests were considered false negative rapid antibody tests.
Results
Nine hundred ninety-four participants were enrolled with either negative (N=976) or discordant (N=18) rapid test results. Eleven (1.1%, 95% CI: 0.6–2.0%) had acute HIV infection. Of the 994 patients, an additional 20 (2.0%, 95% CI: 1.3–.3.1%) had chronic HIV infection (false negative rapid test).
Conclusions
One percent of outpatients with negative or discordant rapid HIV tests in Durban, South Africa had acute HIV infection readily detectable through pooled serum HIV RNA screening. Pooled RNA testing also identified an additional 2% of patients with chronic HIV infection. HIV RNA screening has the potential to identify both acute and chronic HIV infections that are otherwise missed by standard HIV testing algorithms.
Keywords: Rapid HIV test, acute HIV, Africa, HIV seropositivity, HIV screening
Introduction
Acute HIV infection – the period following initial HIV infection prior to antibody seroconversion – is the time of peak virus concentration in blood and genital fluids.1–4 The presence of a symptomatic acute viral syndrome is variable, estimated to occur in 40–90% of patients, though rates appear lower in African cohorts documenting acute infection with non-clade B virus.5–8 Because symptoms are nonspecific and overlap with many common syndromes, acute HIV infection is difficult to detect on clinical grounds alone.
However, detection of acute HIV infection is critical for both individual and public health. Transmission is highest in the period following infection, with transmission from acutely infected partners nearly 12-fold higher than in prevalent discordant couples.9, 10 Detecting cases during primary infection would allow for counseling and other prevention strategies that could help decrease transmission and link the HIV-infected individual to care.11
Though the HIV RNA assay is the most sensitive test for the diagnosis of acute infection,12 its expense and technical requirements limit its utility for screening large volumes of samples quickly, particularly in resource-limited settings. Pooled sera for detection of viral RNA is more labor intensive, but is a much more economically efficient method of estimating HIV incidence 13 and has been used successfully in sexually transmitted disease clinics in resource-limited settings.14–17
The objective of this study was to evaluate the yield of screening for acute HIV infection using pooled HIV RNA testing in a general medical outpatient population in Durban, South Africa. In addition, we compared rapid HIV testing to gold standard serologic tests for the diagnosis of chronic HIV infection.
Methods
Study setting and participants
Subjects were prospectively enrolled from the outpatient department HIV testing site at McCord Hospital, Durban, South Africa from March through November 2007. McCord Hospital is a state-aided urban hospital which serves a predominantly African Zulu-speaking population; the outpatient department sees 150–200 patients daily with general medical complaints. During the study period, most patients tested for HIV following physician referral; patients could also self-refer for testing without prior outpatient department registration. The outpatient department HIV counselors test approximately 300–400 patients per month using rapid HIV test kits as per the South African and World Health Organization testing guidelines.18, 19 The HIV counselors are lay English and/or Zulu speakers trained in testing techniques and observed for several months prior to working independently. During the study period the HIV prevalence for adults tested was 48%.
Study eligibility and procedures
All adult patients (≥ 18 years) who had undergone HIV testing during weekday business hours in the outpatient department and had a negative or discordant rapid HIV test were eligible for this study. We excluded patients who were too ill to understand the counseling session or to provide informed consent, and patients known to be pregnant. Pregnant women were excluded because they are HIV tested in a physically different location at the hospital. Eligible patients who consented to participate in the study underwent venipuncture for HIV RNA, enzyme immunoassay (EIA), and Western Blot (WB) on the same day as the rapid HIV test and were asked to return for their results in 10 days. Study personnel contacted subjects found to be HIV-infected with the venipuncture specimen who did not return in 10 days by telephone and advised them to return for test results.
The project was approved by the McCord Hospital Ethics Committee [Durban, South Africa] and the Partners Human Subjects Committee (Protocol # 2006-P-001379/8) [Boston, MA].
Rapid HIV antibody testing
During the 9-month study period, testing kits and procedures changed in the outpatient department due to changes in hospital policy and provincial Department of Health manufacturer tenders which were beyond the control of the study. The test kits included: Determine HIV 1/2 Test (Abbott Laboratories, Abbott Park, IL), SmartCheck HIV 1& 2 (World Diagnostic Inc, FL), Sensa Tri-line HIV 1/2/0 (Hitech Healthcare LTD, China), and SD Bioline (Standard Diagnostics Inc, Korea). Initially, there was a period of serial testing (March–August 2007), followed by a period of parallel testing (September–November 2007).
During the serial testing period, a positive rapid screening test was confirmed by a second rapid test using a kit made by a different manufacturer. A single negative rapid HIV test was reported as negative. During the parallel testing period, two rapid tests were performed simultaneously for each patient. A rapid HIV test was reported negative if a patient had two parallel negative tests and positive if a patient had two parallel positive tests. Patients with one positive and one negative rapid test were considered “discordant” but were included in the study because of a previously described association of discordant rapid HIV tests with acute HIV infection.15, 20
HIV RNA testing and confirmatory antibody testing
To ensure no evolution of serologic response between rapid testing and WB, venipuncture specimens were collected in edetic acid (EDTA) tubes on the same day that the rapid HIV test was performed. Plasma was removed from the whole blood specimens and stored daily. Each week, plasma was manually pooled using a modification of a previously described protocol13, 15, 16: 167μl aliquots from individual patient specimens were pooled into groups of six. Pools were initially screened qualitatively with Roche COBAS AmpliScreen HIV-1 Test version 1.5 (Roche Molecular Systems, Branchburg, NJ, USA). Quantitative RNA testing on individual positive specimens was then performed using Roche COBAS Amplicor HIV-1 version 1.5.
All patients with a positive individual quanitative HIV RNA screen underwent antibody testing with HIV EIA (Vironostika HIV-1 Microelisa System, Biomérieux or HIV-1 rLAV EIA, Bio-Rad, Bio-Rad Laboratories, Richmond, WA, USA) and HIV WB (Bio-Rad). Patients were defined as having acute HIV infection if they had an HIV RNA level >10,000 copies/ml with either negative HIV antibody testing or HIV EIA positivity with a negative or indeterminate WB.16 Patients were defined as having chronic HIV infection if they had both positive EIA and WB in the presence of elevated HIV RNA (>5,000 copies/ml);21 these patients were considered to have “false negative” rapid test results. The highest HIV RNA among chronically infected patients was reported as >750,000 copies/ml (the upper limit of the assay); this was considered 750,000 copies/ml for the purpose of the analysis. One patient had a positive qualitative RNA screen but had neither WB nor HIV RNA available and was excluded from further analysis.
Response to initial false negative rapid HIV test results
Within the first month of the study, there were several patients identified via RNA testing with chronic infection and false negative rapid tests results. After the first three false negative rapid test results, the HIV testing protocol in the outpatient department was evaluated. Because the issue initially appeared to involve false negatives from a single lot of confirmatory test kits (i.e. the second rapid test performed in series to confirm an initial positive test), that test lot was promptly discarded (SmartCheck). The local Department of Health was notified of the findings and a new confirmatory rapid test kit was adopted (SD Bioline). The counselors performing the rapid HIV tests were re-trained in testing technique by representatives from one of the HIV test kit manufacturers. Counselors’ offices were already air conditioned in an effort to control temperature and humidity for optimal test integrity. During the last 3 months of the study period, when false negatives continued to occur, the hospital adopted a parallel rapid testing algorithm as described above.
Statistical analysis and evaluation of rapid HIV test performance
The main study outcome was the proportion of subjects with acute HIV infection among patients with negative or discordant rapid tests. The second study outcome was the proportion of patients with chronic HIV infection among patients with negative or discordant rapid tests (false negative rapid test). Ninety-five percent confidence intervals around prevalence estimates of acute and chronic HIV infection were calculated using the binomial distribution.
We evaluated the rapid HIV test performance compared to serologic tests performed on venipuncture samples. We calculated the sensitivity of the rapid tests for detecting chronic HIV infection with 95% confidence intervals. We calculated the negative predictive value of a negative or discordant rapid test. Because patients with two rapid positive tests were considered HIV-infected, without verification by an independent serologic test, we were unable to calculate the specificity of the rapid HIV tests from this study. We therefore calculated the negative predictive value assuming 100% specificity (as reported by some of the individual rapid test kit manufacturers) and then performed a sensitivity analysis incorporating published specificity results (90.4%) from a Ugandan study of rapid test diagnostic accuracy.22
Results
During the 9-month study period, 1,005 patients enrolled in the study with rapid HIV test negative or discordant results from the outpatient department. Eleven patients either did not complete the venipuncture or had an inadequate specimen. The remaining 994 patients had qualitative HIV RNA screen data available and were considered for the analysis (Figure 1, Table 1). Fifty-eight percent of the enrolled cohort was female; the median age was 36 years. The results of background HIV testing during the study period were: 1,294 patients had reactive rapid HIV tests (53% female, median age 34 years); 1,429 subjects overall had negative rapid HIV tests (56% female, median age 38 years).
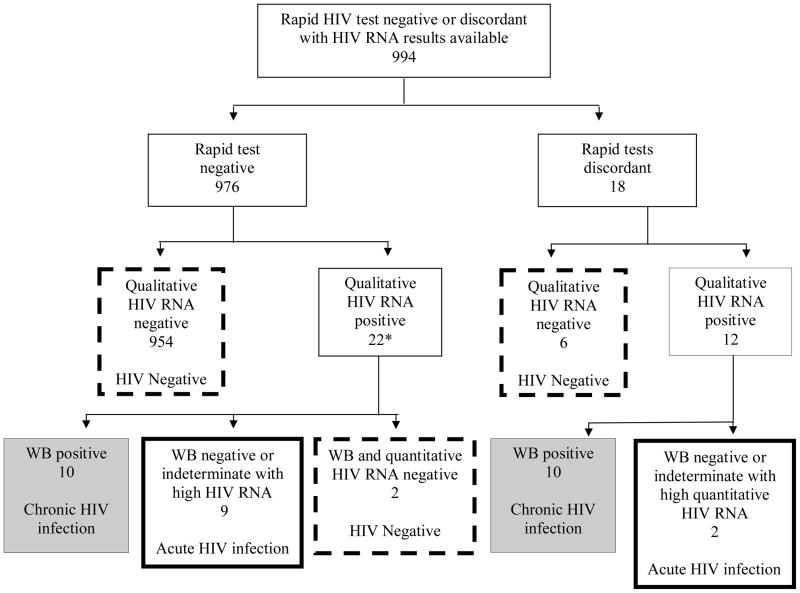
Figure 1. Flow chart of study population, McCord Hospital outpatient department Acute HIV screening study, Durban, South Africa, 2007.
The flow diagram shows the results of rapid HIV tests (1st and 2nd rows), qualitative HIV RNA results (3rd row), and then quantitative HIV RNA and WB results (4th row). The solid bolded boxes outline patients with acute HIV infection, the gray shading highlights those with chronic HIV infection, and the dashed boxes show HIV negative patients. WB, Western Blot.
*No WB or HIV RNA available for 1 patient
Table 1.
Cohort demographic information for an acute HIV screening study in Durban, South Africa.
| Total Cohort | HIV Negative | Acute Infection† | Chronic Infection |
||
|---|---|---|---|---|---|
| Negative Rapid Test | Discordant Rapid Test | ||||
| N | 994* | 962 | 11 | 10 | 10 |
| Age (median, IQR) | 36 (26–47) | 36 (26–47) | 34 (21–50) | 39 (28–50) | 34 (26–44) |
| Female Gender (%) | 572 (58%) | 555 (58%) | 7 (64%) | 4 (40%) | 6 (60%) |
| Median HIV RNA (Log10copies/ml)‡ | N/A | N/A | 5.88 | 5.59 | 5.45 |
One patient had positive HIV RNA but did not have an adequate specimen for EIA/WB and was not further categorized.
Includes one patient without WB data, with viral load 22,200,000 copies/ml, classified as acutely infected based on consensus of 5 HIV clinical experts.
HIV RNA reported as >750,000 copies/ml were considered 750,000 copies/ml for calculations.
Thirty-four patients (22 with rapid test negative and 12 with rapid test discordant) had a positive qualitative HIV RNA screen. Two patients had negative rapid HIV tests with a positive qualitative RNA screen, but had undetectable quantitative HIV RNA and negative serum antibody tests; these patients were considered HIV negative. One subject had a positive qualitative HIV RNA screen but had no WB or HIV RNA available (Figure 1).
Acute HIV infection
Of the 994 patients, eleven had acute HIV infection, for a prevalence of 1.1% (95% CI 0.6–2.0%, Table 1). Seven of the acutely infected patients (64%) were women, and the median age was 34 years (Table 1). All of the participants with acute HIV infection had HIV RNA >750,000 copies/ml (range 750,000–22,200,000 copies/ml). One patient had two concordant negative rapid HIV tests (parallel testing period), a positive EIA and insufficient specimen available for a WB. However, her quantitative HIV RNA was 22,200,000, compatible with acute infection; she was included among the 11 acutely infected patients based on the unanimous consensus of 5 clinical HIV experts who were consulted to assist with classifying this case. Two of the 11 acutely infected cases (one male and one female) had discordant rapid HIV tests; the other 9 had negative rapid HIV tests.
“False negative” rapid HIV tests or chronic HIV infection
Rapid HIV test negative
Of 976 patients who had a negative rapid test and underwent qualitative RNA screening, 954 (98%) were confirmed to be HIV negative by qualitative HIV RNA testing (Figure 1, left side). Twenty-two patients with negative rapid HIV tests had positive qualitative HIV RNA testing. Ten of these 22 patients were found to have chronic HIV infection with positive serologic antibody tests (EIA and/or WB). These ten patients had “false negative” rapid HIV tests (10 chronically infected/976 with negative rapid tests, 1.0%, false negative, 95% CI: 0.6–1.9%).
Rapid HIV test discordant
Of 18 patients who had discordant rapid HIV tests (11 men and 7 women) and underwent qualitative RNA screening, 6 (all men) were confirmed to be HIV negative by qualitative HIV RNA testing (Figure 1, right side). Twelve patients with discordant rapid HIV tests had positive qualitative HIV RNA testing. Ten of these 12 patients were found to have chronic HIV infection with positive EIA and/or WB (10 chronically infected/18 with discordant rapid tests, 56% false negative, 95% CI: 34–76%).
Chronic HIV infection overall
In total, 20/994 (2.0%, 95% CI: 1.3–3.1%) patients with negative or discordant rapid HIV test results were confirmed to have chronic HIV infection with subsequent serologic testing (Figure 1, shaded gray boxes). False negative rapid tests occurred with all 3 of the trained counselors, and with all different rapid testing kit schemes employed during the study period (Table 2).
Table 2.
Patients with negative or discordant rapid HIV tests who had chronic HIV infection in Durban, South Africa by testing protocol.
| Dates of Testing protocols (all in 2007) | Testing Protocol | Rapid Test Negative | Rapid Test Discordant | Rapid Test Negative or Discordant (Total) | |||
|---|---|---|---|---|---|---|---|
| Number Tested | True HIV Positive (%) (95%CI) | Number Tested | True HIV Positive (%) (95%CI) | Number Tested* | True HIV Positive (%) (95%CI) | ||
| Feb 27–Apr 15 | 1st Determine | 187 | 1 (0.5%) | 11 | 6 (54.5%) | 198 | 7 (3.5%) |
| 2nd SmartCheck | (0.1–2.9%) | (27.7–78.9%) | (1.8–7.1%) | ||||
| Apr 16– July 25 | 1st Determine | 250 | 1 (0.4%) | 1 | 1 (100%) | 251 | 2 (0.79%) |
| 2nd SD Bioline | (0.1–2.2%) | (20.7–100%) | (0.3–2.8%) | ||||
| July 26–Aug 29 | 1st Sensa | 155 | 7 (4.5%) | 2 | 1 (50%) | 157 | 8 (5.1%) |
| 2nd SD Bioline | (2.2–9.0%) | (9.5–90.6%) | (2.6–9.7%) | ||||
| Aug 30–Nov 30 | Parallel | 394 | 1 (0.25%) | 5 | 2 (40%) | 399 | 3 (0.75%) |
| Sensa & SD Bioline | (0.0–1.4%) | (11.8–76.9%) | (0.3–2.2%) | ||||
From 27 Feb–29 Aug 2007, rapid testing was done serially, first one test was performed, and if reactive, a second rapid test was performed. From 30 Aug–30 Nov 07, two simultaneous rapid tests were performed in parallel. See methods for further details.
Includes all patients tested with rapid HIV test, even if no HIV RNA result available.
Rapid HIV test performance
The sensitivity for detecting chronic HIV infection using the rapid test kits was 98.5% (95% CI: 97.8, 99.1%). The negative predictive value of a negative or discordant rapid test, assuming 100% specificity, was 97.9% (95% CI: 96.9, 98.7%). Using the rapid HIV test kit specificity published from Uganda 22, the negative predictive value dropped to 88.5% (95% CI 86.4, 90.3%). Including both acute HIV infections (N=11) and chronic HIV infections (N=20) discovered by the RNA screening program, a total of 3.1% (31/994) of patients who tested HIV rapid test negative or discordant in the outpatient department of the hospital had readily detectable and confirmed HIV infection.
Discussion
We found that ~3% of patients with negative or discordant rapid HIV tests in a medical outpatient department in South Africa had confirmed HIV infection using pooled HIV RNA serum screening. One percent of patients who had a negative or discordant rapid HIV test had acute HIV infection. In addition, standard rapid HIV testing missed 2% of patients who had chronic HIV infection.
Pooling serum for detection of acute infection in the setting of high HIV prevalence is feasible as long as polymerase chain reaction technology is available. In settings like this outpatient department in Durban, one percent of patients seeking medical care would otherwise receive a negative rapid HIV test result at the time when they are maximally infective.9 Diagnosis of acute HIV infection may prevent further HIV transmission by providing an opportunity for risk behavior counseling.11 Screening for acute HIV and linking patients to HIV care may be particularly important for infected individuals in settings such as Durban where CD4 count at the time of HIV diagnosis is typically <200/μl and the vast majority of patients are accessing care late in the course of their illness.23
Implementation of pooled RNA for acute HIV screening presents several challenges. The need to provide rapid turnaround of test results in a clinically meaningful time frame to ensure patient follow-up makes it difficult to accumulate a large number of specimens for pooling;15 this barrier may be overcome by pooling specimens from dried blood spots.24 Optimal pool size depends on the prevalence of acute HIV in the population and the skill of the laboratory personnel if a manual pooling technique is required. The failure of rapid HIV tests in this study to identify all chronic HIV cases led to an increased number of positive pools requiring additional testing that highlighted chronic rather than acute HIV cases.
Patients with a negative or discordant rapid HIV test had ~2% probability of having chronic HIV infection in this setting. From this study, we are unable to evaluate whether this relatively high false negative rate, higher than reported by the test kit manufacturers, was the result of operator error, faulty test kits/storage, or characteristics of the patient population. There was no apparent change during the study period in the rate of false negative results, despite retraining the HIV counselors and changing the test kits. A recently reported South African field study also noted challenges in HIV rapid test sensitivity compared to ELISA and pooled HIV RNA PCR. In this study, which also used the SD Bioline kit, 5% of participants, all of whom were pregnant, had false negative results.25 A high rate of false negative rapid tests was also reported in a study from South Africa among children on ART, however, the test kits evaluated were different from those used in the current study.26 Rapid test kits have had disappointing performance in other contexts22, 27, 28 suggesting that inaccurate rapid tests may not be a setting- or test-specific problem. Other than the Abbott Determine HIV 1/2 rapid test, none of the rapid kits used during the study period have been extensively validated against gold standard tests in Africa in published studies; the World Health Organization recommends that individual countries evaluate each assay used to determine its performance characteristics and suitability for use within a given setting.29, 30 To the extent that this is not practiced, many false negatives are likely occurring, since the settings using pooled HIV RNA are extremely limited.
Rapid HIV testing has been an essential part of improving diagnostic capacity and treatment opportunities for patients in resource-limited settings.32 It is important to counsel patients and providers, however, that there is a small but real risk of a false negative test due to both chronic and acute infection and to encourage retesting; countrywide guidelines should recommend a re-testing frequency to guide counselors’ efforts. It is also critical to implement quality control measures, such as periodic operator retraining and temperature/humidity control for test kits to ensure the integrity of the testing process. In addition, parallel rapid testing should be promoted, whenever feasible, with follow-up of discordant samples.
We included patients with discordant rapid HIV tests in this screening study for acute HIV infection since discordant rapid HIV tests had previously been strongly associated with acute HIV infection in a sexually transmitted disease clinic in Malawi.20 In our study, only 2 of 18 patients with discordant rapid tests and available HIV RNA results were acutely infected, but 10 of the 18 discordant patients had chronic HIV infection. This may reflect that our study used different test kits, which perhaps have different sensitivity to detect early infection. Much of the study was performed using serial rapid kits. For participants enrolled using the serial method, we could not account for subjects who had a negative first test but who might have had a positive second test if the kits were administered in parallel. In addition, the pre-test probability of HIV infection is lower in a general medical population than in a sexually transmitted disease clinic. Because over half of the patients with discordant rapid tests were chronically HIV-infected, in a setting of high prevalence, immediate testing with serum EIA is appropriate in all patients with discordant results to evaluate for chronic HIV infection.
This study has several limitations. The rapid test kits used for HIV diagnosis in the outpatient department changed several times due to hospital policies and changes in provincial tenders, so individual test protocols could not be evaluated. The kits may detect HIV antibodies at different time points in early infection, which makes the determination of test performance for any one kit or testing protocol difficult from these data. Because of the kit changes, we were unable to standardize the expected length of the window period; this would have been helpful for improving the acute HIV incidence estimate from using pooled RNA in this population.31 Because we do not have CD4 count data for patients who were found to be chronically HIV-infected, we cannot determine whether advanced immune suppression predicted a false negative rapid test. However, the HIV RNA levels of many of the patients with false negative rapid HIV tests may support this conclusion. In addition, because we have limited clinical data regarding the enrolled patients, we are unable to examine associations between acute HIV infection and signs and/or symptoms of an acute viral syndrome or a sexually transmitted infection. Pregnant women were excluded from this study; however, they may represent a high risk population worthy of consideration in future studies screening for acute HIV infection. We did not confirm positive rapid HIV tests with venipuncture serologic specimens, and therefore assumed perfect specificity when generating the negative predictive value of a rapid negative/discordant test. We performed a sensitivity analysis on this assumption with Ugandan data; the Ugandan data may not be fully generalizeable to South Africa because different test kits were used for diagnosis of a different HIV clade.22
Screening for acute HIV infection using pooled serum in a general medical population in a high prevalence setting is feasible and identifies patients who would not be recognized as HIV-infected with the current HIV testing algorithm. In addition, RNA screening revealed even more patients with chronic HIV infection who had been missed with standard rapid HIV test kits. The optimal HIV testing algorithm in high prevalence but resource-limited settings has yet to be defined. The results of this study should be confirmed in other settings; if they are, then routine pooling of sera from rapid HIV test negative and discordant patients in resource-scarce settings will identify substantial numbers of both acutely and chronically HIV-infected patients.
Acknowledgments
We would like to thank Slindile Mbhele and Kriebashnie Nair for their technical assistance. We are grateful to the HIV counselors in the McCord Hospital outpatient department for their outstanding work enrolling patients into the study: Esme Kelly Nkosi, Pepsi (Shamla) Pillay, and Sibongile Hadebe.
Supported in part by the National Institutes of Health: K23 AI 068458; R01 AI058736; K24 AI062476; R01 MH073445; R0I AI 067073; P30 AI42851 (Center for AIDS Research) and The Doris Duke Charitable Foundation, Clinical Scientist Development Award (RPW).
Footnotes
Presented in part at the 15th Conference on Retroviruses and Opportunistic Infections, February 3–6, 2008, Boston, MA, USA
No conflicts of interest exist concerning the authors or contents of this article.
References
- 1.Goulston C, McFarland W, Katzenstein D. Human immunodeficiency virus type 1 RNA shedding in the female genital tract. J Infect Dis. 1998;177:1100–3. doi: 10.1086/517404. [DOI] [PubMed] [Google Scholar]
- 2.Lavreys L, Baeten JM, Panteleeff DD, et al. High levels of cervical HIV-1 RNA during early HIV-1 infection. AIDS. 2006;20:2389–90. doi: 10.1097/QAD.0b013e328010f1e7. [DOI] [PubMed] [Google Scholar]
- 3.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 4.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–30. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–9. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 6.Lavreys L, Thompson ML, Martin HL, Jr, et al. Primary human immunodeficiency virus type 1 infection: clinical manifestations among women in Mombasa, Kenya. Clin Infect Dis. 2000;30:486–90. doi: 10.1086/313718. [DOI] [PubMed] [Google Scholar]
- 7.Morgan D, Mahe C, Whitworth J. Absence of a recognizable seroconversion illness in Africans infected with HIV-1. AIDS. 2001;15:1575–6. doi: 10.1097/00002030-200108170-00016. [DOI] [PubMed] [Google Scholar]
- 8.Sarr AD, Eisen G, Gueye-Ndiaye A, et al. Viral dynamics of primary HIV-1 infection in Senegal, West Africa. J Infect Dis. 2005;191:1460–7. doi: 10.1086/429409. [DOI] [PubMed] [Google Scholar]
- 9.Pinkerton SD. Probability of HIV Transmission during acute infection in Rakai, Uganda. AIDS Behav. 2008;12:677–84. doi: 10.1007/s10461-007-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 11.Pilcher CD, Eron JJ, Jr, Galvin S, et al. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004;113:937–45. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daar ES, Little S, Pitt J, et al. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001;134:25–9. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 13.Pilcher CD, McPherson JT, Leone PA, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002;288:216–21. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 14.Chen XS, Yin YP, Tucker JD, et al. Detection of acute and established HIV infections in sexually transmitted disease clinics in Guangxi, China: implications for screening and prevention of HIV infection. J Infect Dis. 2007;196:1654–61. doi: 10.1086/522008. [DOI] [PubMed] [Google Scholar]
- 15.Fiscus SA, Pilcher CD, Miller WC, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. J Infect Dis. 2007;195:416–24. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 16.Pilcher CD, Price MA, Hoffman IF, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18:517–24. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 17.Quinn TC, Brookmeyer R, Kline R, et al. Feasibility of pooling sera for HIV-1 viral RNA to diagnose acute primary HIV-1 infection and estimate HIV incidence. AIDS. 2000;14:2751–7. doi: 10.1097/00002030-200012010-00015. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Rapid HIV Tests: Guidelines for use in HIV testing and counselling services in resource-constrained settings. [Accessed 4 December 2009];2004 Available at: http://www.emro.who.int/aiecf/web28.pdf.
- 19.Operational plan for comprehensive HIV and AIDS care, management and treatment for South Africa. [Accessed 4 December 2009];2003 Available at: http://www.info.gov.za/issues/hiv/careplan.htm.
- 20.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS. 2007;21:2237–42. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 22.Gray RH, Makumbi F, Serwadda D, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. BMJ. 2007;335:188. doi: 10.1136/bmj.39210.582801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. JAIDS. 2007;46:181–6. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bebell L, Pilcher CD, Dorsey G, et al. Acute HIV-1 infection is highly prevalent in Ugandand outpatient suspected of malaria [abstract 700]. Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2008. [Google Scholar]
- 25.Black V, von Mollendorf C, Scott LE, et al. Field Validation of rapid HIV testing kits among pregnant women in an antenatal clinic in Johannesburg, South Africa [abstract CDB017]. International AIDS Society Meeting; Cape Town, South Africa. 2009. [Google Scholar]
- 26.Claassen M, van Zyl GU, Korsman SN, et al. Pitfalls with rapid HIV antibody testing in HIV-infected children in the Western Cape, South Africa. J Clin Virol. 2006;37:68–71. doi: 10.1016/j.jcv.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Aghokeng A, Dimodi H, Atem-Tambe A, et al. Inaccurate HIV diagnosis in developing countries: an unresolved issue [abstract 1051]. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 28.Walensky RP, Arbelaez C, Reichmann WM, et al. Revising expectations from rapid HIV tests in the emergency department. Ann Intern Med. 2008;149:153–60. doi: 10.7326/0003-4819-149-3-200808050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Guidelines for Appropriate Evaluations of HIV Testing Technologies in Africa. [Accessed 4 December 2009];2002 Available at: http://wwwn.cdc.gov/dls/pdf/HIV%20Test%20Guidelines%20Africa.pdf.
- 30.Plate DK. Evaluation and implementation of rapid HIV tests: the experience in 11 African countries. AIDS Res Hum Retrovirus. 2007;23:1491–8. doi: 10.1089/aid.2007.0020. [DOI] [PubMed] [Google Scholar]
- 31.Patel P, Mackellar D, Simmons P, et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA. Arch Intern Med. 2006–2008;170:66–74. doi: 10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. [Accessed 4 December 2009];The importance of simple/rapid assays in HIV testing. 1998 Available at: http://www.who.int/diagnostics_laboratory/publications/en/simple_rapid_wer.pdf.