Abstract
Background
There is a controversy regarding the association between QRS width and ventricular arrhythmias (VA). We hypothesized that predictive value of the QRS width could be improved if QRS width were considered in the context of the sum magnitude of the absolute QRST integral in three orthogonal leads (SAI QRST). We explored correlations between QRS width, SAI QRST, and VA in primary prevention ICD patients with structural heart disease.
Methods
Baseline orthogonal ECGs were recorded at rest in 355 patients with implanted primary prevention ICDs (mean age 59.5±12.4; 279 male [79%]). Patients were followed prospectively at least 6 months; appropriate ICD therapies due to sustained VA served as endpoints. The sum magnitude of the absolute QRST integral in three orthogonal leads (SAI QRST) was calculated.
Results
During a mean follow-up of 18 months, 48 patients had sustained VA and received appropriate ICD therapies. There was no difference in baseline QRS width between patients with and those without arrhythmia (114.9±32.8 vs. 108.9±24.7 ms, p=0.230). SAI QRST was significantly lower in patients with VA at follow-up than in patients without VA (102.6±27.6 vs. 112.0±31.9 mV*ms, p=0.034). Patients with SAI QRST ≤145 mV*ms had a3-fold higher risk of VT/VF (HR 3.25; 95% CI: 1.59–6.75, p=0.001). In the univariate analysis QRS width did not predict VT/VF. In the bivariate Cox regression model every 1 ms of incremental QRS widening with a simultaneous 1 mV*ms SAI QRST decrease raised the risk of VT/VF by 2% (HR 1.02; 95% CI 1.01–1.03, p=0.005).
Conclusion
QRS widening is associated with ventricular tachyarrhythmia only if accompanied by low SAI QRST.
Background
Controversy remains regarding the association between QRS width and sudden cardiac death (SCD). In the early analysis of the MADIT II study, a wide QRS >150 ms was associated with a more substantial benefit from ICD1 than was a narrow QRS <120 ms. Subsequent studies did not confirm the relation between QRS width and ventricular arrhythmia (VA) occurrence in primary and secondary prevention ICD patients with structural heart disease.2, 3 However, QRS width predicted SCD in medically treated MADIT II heart failure patients.3
We proposed a novel marker of susceptibility to VA, namely SAI QRST, measured as the sum magnitude of the absolute QRST integral on three orthogonal ECG leads. To study the predictive value of SAI QRST and to further elucidate associations between SAI QRST, QRS width, and VA, we undertook analysis of prospective observational cohort study (PROSE-ICD) patients with structural heart disease and routine indications for primary prevention single-chamber or dual-chamber ICD.
Methods
All patients gave written informed consent before entering the study. The study protocol was approved by the Johns Hopkins University IRB.
Study population
PROSE-ICD (NCT00733590) is an ongoing prospective observational multicenter cohort study of patients with either ischemic or non-ischemic cardiomyopathy, who have routine indications for ICD as primary prevention of sudden cardiac death (SCD). Patients were eligible for the study if the left ventricular (LV) ejection fraction (EF) was less than or equal to 35%, myocardial infarction was at least 4 weeks old, or non-ischemic cardiomyopathy was present for at least 9 months. Patients were excluded if the ICD was indicated for secondary prevention of SCD, if the patient had a permanent pacemaker or a Class I indication for pacing, if the patient had NYHA class IV, or if the patient was pregnant. Consecutive patients with indications for single-chamber or dual-chamber ICD, but not CRT-D, and followed up for at least 6 months were included in this report.
Surface ECG recording
Digital orthogonal ECG was recorded before ICD implantation during 5 minutes at rest, using the modified Frank orthogonal XYZ leads by PC ECG machine (Norav Medical Ltd, Thornhill, ON, Canada), with a 1000 Hz sampling frequency, high-pass filter 0.05 Hz and low-pass filter 350 Hz. QRS width on the clinical 12-lead ECG was measured automatically by the build-in algorithm (Marquette ECG 12SL & MUSE system, GE Healthcare Clinical Systems, Wauwatosa, Wisconsin, USA).
QRST integral measurement
All ECGs were analyzed by customized software in a robust automated fashion. QRS onset and end of T wave were identified by the user (L.G.T.). The first 15 beats were used by the algorithm to construct templates. The user viewed templates of all three orthogonal leads, selected the best quality lead (default lead X), and manually defined the onset of the QRS and end of T wave fiducial points. Then the algorithm measured absolute QRST integral of each beat and calculated the average value for the time epoch. Absolute QRST integral was measured as the arithmetic sum of areas under the QRST curve (absolute area under the QRST curve above baseline was added to the area below baseline), averaged during a 5-minute epoch. The sum magnitude of three orthogonal leads absolute QRST integral (SAI QRST) was calculated.
Endpoints
Appropriate ICD therapies [either shock or anti-tachycardia pacing (ATP)] for VA served as the primary endpoints for analysis. Programming of the ICD was based on the attending electrophysiologist’s clinical evaluation. The ICD device was interrogated during follow-up visits every 6 months. All ICD interrogation data were reviewed by an independent endpoints adjudication committee, blinded to the results of SAI QRST analysis.
Statistical analysis
Study participants were categorized according to their baseline SAI QRST value, with SAI QRST ≤69 mV*ms labeled low, SAI QRST 70–145 mV*ms labeled intermediate, and SAI QRST >145 mV*ms labeled high. Linear regression analysis was used to study the correlation between SAI QRST and QRS width. Adjusted by QRS width, Kaplan-Meier survival curves were constructed for subjects with low, intermediate, or high SAI QRST. The log-rank (Mantel-Cox) and Tarone-Ware statistics were computed to test the equality of survival distributions. Univariate, bivariate, and multivariate Cox proportional hazards regression analyses were performed. An interaction between SAI QRST and QRS duration was tested in the Cox model. STATA 10 software (StataCorp LP, College Station, TX) was used for calculations.
Results
Patient population
Baseline clinical characteristics of patients are presented in Table 1. Study participants (n=355) were predominantly males with ischemic cardiomyopathy, heart failure NYHA class II–III, and narrow QRS about 110 ms at baseline. There were no statistically significant differences between baseline clinical and ECG characteristics of patients with low, intermediate, and high SAI QRST, with the exception of QRS duration. QRS was incrementally wider in patients with low, intermediate, and high SAI QRST (99.8±18.0 vs. 111.2±24.3 vs. 138.9±30.9 ms, ANOVA p<0.0001). During a mean follow-up of 18.0±16.5 months, 48 of 355 patients (13.5% or 9.0% per person-year of follow-up) experienced sustained VTs and received appropriate ICD therapies. Monomorphic ventricular tachycardia with an average cycle length (CL) of 294±40 ms was present in 31 patients (63%). Polymorphic ventricular tachycardia (CL 228±18 ms) or VF (CL 194±24 ms) was documented in 17 patients (27%).
Table 1.
Clinical and baseline ECG characteristics of patients
| Characteristic | n=355 |
|---|---|
| Age±SD, y | 59.5±12.4 |
| Male sex, n(%) | 279(78.6) |
| Whites, n(%) | 238(67.0) |
| Ischemic cardiomyopathy, n(%) | 220(62.0) |
| Baseline LVEF ±SD, % | 22.96±9.19 |
| QRS, ms | 109.79±26.04 |
| Left ventricular diastolic diameter ± SD, cm | 5.87±0.93 |
| Body mass index ± SD | 28.67±6.00 |
| Beta blockers, n(%) | 336(94.6) |
| NYHA class I, n(%) | 78(22.0) |
| NYHA class II, n(%) | 110(31.0) |
| NYHA class III, n(%) | 167(47.0) |
| Inducible VT, n(%) | 92(25.9) |
| Heart rate±SD, bpm | 72.6±14.3 |
SAI QRST and QRS width
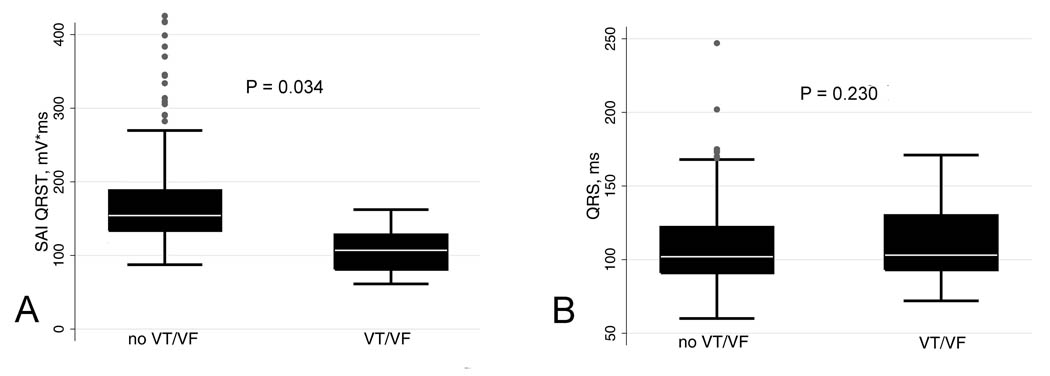
SAI QRST was significantly lower in patients with VA at follow-up than in patients without VA (102.6±27.6 vs. 112.0±31.9 mV*ms, p=0.034) [Figure 1A]. There was no difference in baseline QRS width between patients with and those without arrhythmia (114.9±32.8 vs. 108.9±24.7 ms, p=0.230) [Figure 1B].
Figure 1.
Boxplot of baseline SAI QRST and QRS width in patients with and without VT/VF at follow-up. Median (white horizontal line crossing the box) and interquartile range [IQR] (box) of SAI QRST (A) and QRS width (B). Whiskers specify the adjacent values, defined as the most extreme values within 1.5 IQR of the nearer quartile.
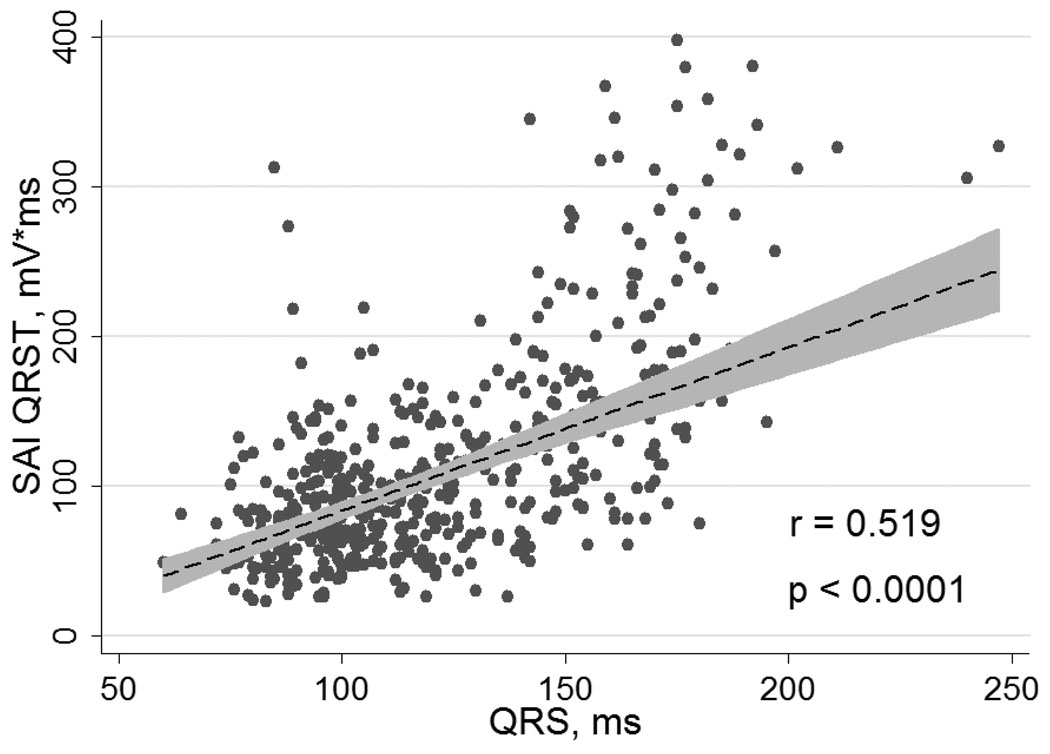
SAI QRST was regressed on QRS width, heart rate, and QT duration. These three predictors accounted for approximately one third of the variance in SAI QRST (R2 = 0.29; p<0.0001). QRS width was the most influential predictor of the SAI QRST variance (β=1.12; p<0.0001), followed by heart rate (β= − 0.56; p=0.025). For every 1 ms increase of QRS width, SAI QRST increased by 3 mV*ms. In bivariate linear regression analysis, QRS width accounted for 27% of the variance in SAI QRST value (R2 = 0.27; p<0.0001), heart rate alone accounted for 3% of the variance in SAI QRST value (R2 = 0.03; p=0.002), and QT duration alone accounted for 5% of the variance in SAI QRST value (R2 = 0.05; p<0.0001). Plot of linear regression of SAI QRST on QRS width is presented in Figure 2.
Figure 2.
Correlation between QRS width and SAI QRST. Linear regression fitted line of SAI QRST on QRS width (dashed line) with 95% CI (gray zone).
Survival analysis
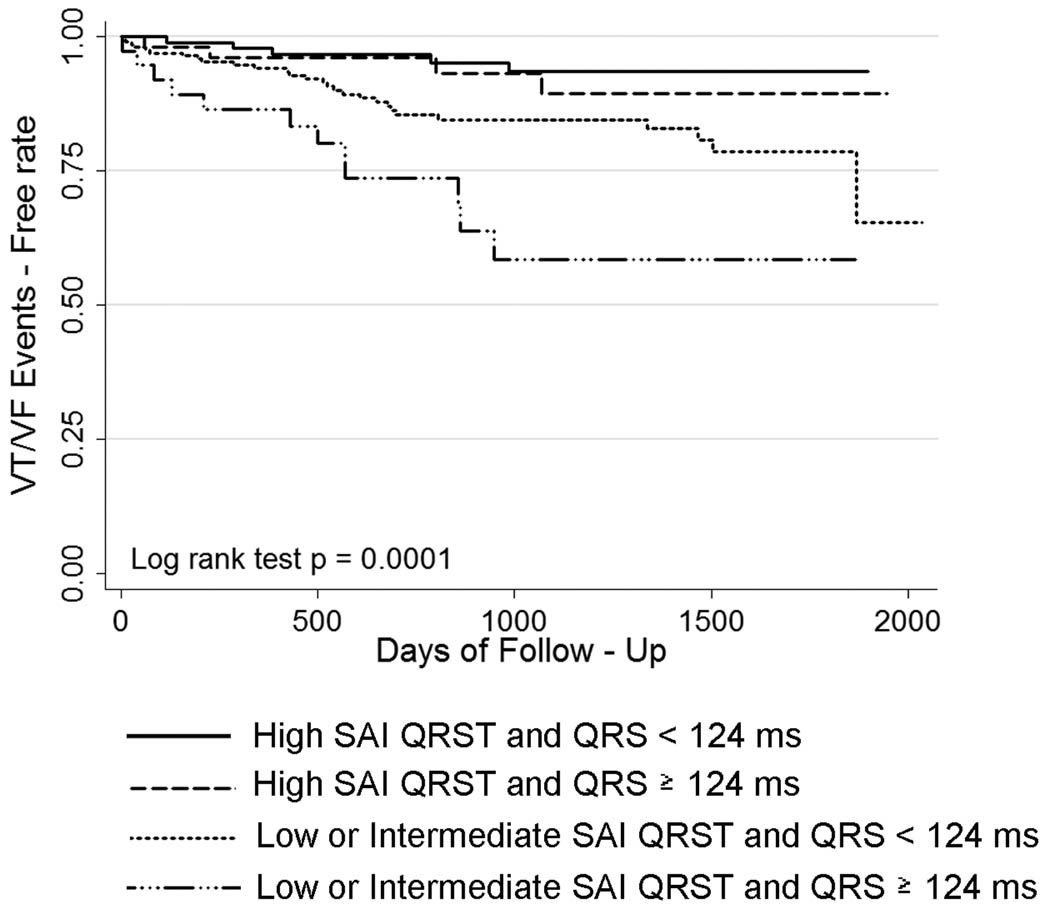
Patients with low and intermediate SAI QRST (≤145 mV*ms) had a 3-fold higher risk of VT/VF (HR 3.25; 95% CI: 1.59–6.75, p=0.001). In the univariate survival analysis, QRS width did not predict VA. Because the QRS width was different among SAI QRST groups, we adjusted our multivariate model of VT/VF prediction by QRS width. Kaplan-Meier survival analysis showed that the SAI QRST adjusted by QRS width above or below 124 ms was significantly predictive for freedom from VT/VF during follow-up (Log rank test p = 0.0001) [Figure 3]. To adjust our model for a different sample size at different times of follow-up, we also performed a weighted-by-sample-size Tarone-Ware test of equality of survival functions. The Tarone-Ware test confirmed a significant difference between the survival functions, p=0.0007. Cox regression analysis found no significant interaction between SAI QRST and QRS width, implying a similar predictive value of SAI QRST across any QRS width.
Figure 3.
Kaplan-Meier curves for freedom from ventricular arrhythmia events in patients with low, intermediate, and high SAI QRST, adjusted by QRS width.
Several variables were tested in the univariate Cox models as potential predictors of VA, including EF, NYHA class, left ventricular diastolic diameter, use of medications (beta-blockers, statins, angiotensin-converting enzyme-inhibitors or angiotensin receptor blockers, aldosterone antagonists), age, gender, race, diabetes mellitus, type of cardiomyopathy, QRS width, QT duration, and heart rate. However, only use of beta-blockers and SAI QRST in the univariate Cox regression were significant predictors of VA, and QRS width ≥ 124 ms was borderline significant (p=0.071). In our final multivariate model we included predictors that were significant in the univariate analysis (SAI QRST, QRS, use of beta-blockers) and type of cardiomyopathy as a clinically important variable. In the multivariate Cox regression analysis, SAI QRST ≤145 mV*ms remained a significant predictor of VA after adjustment for QRS width, type of cardiomyopathy, and use of beta-blockers (HR 4.06; 95% CI 1.91–8.65, p<0.0001). QRS width ≥124 ms was a significant predictor of VA events in this multivariate Cox regression model too (HR 2.58; 95% CI 1.37–4.86; p=0.003). In the bivariate Cox regression model every 1 ms of incremental QRS widening with simultaneous 1 mV*ms SAI QRST decrease raised the risk of VA by 2% (HR 1.02; 95% CI 1.01–1.03, p=0.005).
SAI QRST ≤ 145 mV*ms predicted VA with 82% sensitivity, 41% specificity, 14% positive predictive value, and 95% negative predictive value. Combined marker (SAI QRST ≤ 145 mV*ms and QRS ≥ 124ms) demonstrated 25% sensitivity, 90% specificity, 28% positive predictive value, and 88% negative predictive value for prediction of sustained VA with appropriate ICD therapies.
Discussion
The major finding of our study is that QRS width is associated with ventricular tachyarrhythmia only if accompanied by low SAI QRST.
Controversy around association of QRS duration with ventricular tachyarrhythmia
Several early studies of medically treated heart failure patients demonstrated that wide QRS is associated with increased all-cause mortality in heart failure patients,4 including an association between the QRS width and sudden cardiac death.5–7 Consistently, several major ICD trials, including MUSTT, MADIT II, and SCD-HeFT, showed the predictive value of QRS duration for all-cause mortality, SCD, and appropriate ICD shocks.6, 8, 9 Retrospective analysis of both primary and secondary prevention ICD patients also revealed that QRS duration ≥ 100 ms predicted appropriate ICD therapies during long-term follow-up.10 A recent prospective study of ICD patients showed that QRS duration > 100 ms predicted appropriate ICD shocks.11 However, a large prospective PainFREE Rx II study did not confirm predictive value of QRS duration for occurrence of VTs.2 Moreover, it showed that patients with left bundle branch block were less likely to experience sustained VT/VF than patients with narrow QRS < 120 ms. Subsequent post hoc analysis of MADIT-II data3 demonstrated that prolonged QRS ≥ 140 ms did not predict appropriate ICD therapy for rapid VT/VF in the ICD-treated arm, but did predict SCD in medically treated patients. This finding has become an important argument supporting the opinion that appropriate ICD therapy is not equivalent to SCD. Nonequivalence of SCD and ICD therapies cannot be tested in a randomized clinical trial for ethical reasons. Nonetheless, results of our study provide new data that suggest another possible explanation of the MADIT II findings.3 In our study prolonged QRS did not predict appropriate ICD therapy in univariate analysis, a finding consistent with the results of the PainFREE II Rx and MADIT II studies. However, bivariate Cox regression analysis showed that QRS width predicts VT/VF if it is accompanied by low SAI QRST. We revealed that every 1 ms of incremental QRS widening with a simultaneous 1 mV*ms SAI QRST decrease raised the risk of VT/VF by 2%. Patients with subsequent VT/VF were notably different in this regard, whereas in patients without arrhythmia every 1 ms of incremental QRS widening was accompanied by a simultaneous 3 mV*ms SAI QRST increase.
SAI QRST – novel predictor of ventricular tachyarrhythmia
In this manuscript we describe a novel marker of low VA risk – SAI QRST. Our study showed high 95% negative predictive value of this novel marker along with 82% sensitivity in predicting VA risk. The idea of our new metric arose from analysis of early works by Wilson et al.12 who proposed to calculate the time integral of the heart vector.13 Further studies showed that the QRST integral expresses the heterogeneity of the action potential (AP) morphology.14 It is important to emphasize, however, that our metric SAI QRST differs from the native QRST integral proposed by Wilson. First, we calculated sum absolute QRST integral to adjust our analysis to the modern requirements dictated by acquired filtered ECG signal. Doing so gave our metric the added benefit of less dependence on a precise definition of the isoelectric baseline position. Second, we utilized the advantages provided by orthogonal ECG leads, which permit assessment of the heart vector. Summation of absolute QRST integral of all three orthogonal ECG leads measures the magnitude of total cardiac electrical power and eliminates the bias of a single lead axis position. We speculate that the SAI QRST characterizes significant cancellation of forces as an important pre-existing condition that may facilitate sustained VA. Previous experiments showed that action potential gradients in different sites of the heart may have opposing directions and cancel out.15,16,17 Modeling studies showed that sum QRST integral was decreased if various values of action potential durations (APDs) were randomly assigned in the model to mimic APD heterogeneity in the heart.18 Importantly, random APD assignment made the model more susceptible to VF initiation. We speculate that decrease in the sum QRST integral (SAI QRST) due to cancellation of electrical forces coexists with the locally observed increase in APD gradients, as marker of a heterogeneity of repolarization or heterogeneity of AP morphology, as shown previously.19, 20 However, the precise electrophysiological meaning of SAI QRST remains to be elucidated.
Associations between SAI QRST, QRS width, and ventricular tachyarrhythmia
Patients included in this analysis predominantly had normal QRS width, as only patients with indications for ICD, but not CRT-D, were included in this analysis. The fact that very few patients had bundle branch block limited assessment of the predictive value of ventricular conduction abnormalities in this work. Previous studies showed that higher mortality, suggesting higher SCD rate, is associated with left bundle branch block and intraventricular conduction delay, but not with right bundle branch block.6, 21 However, a direct association between electrocardiographic conduction abnormalities as measured by QRS width and the development of VA in patients with structural heart disease has not been reported before. We believe that our results support this important association and underscore the fact that QRS width is not the perfect measure of conduction abnormalities. Indeed, the complex assessment provided by SAI QRST revealed links between intraventricular conduction, heterogeneity of ventricular action potential, and VA in the case of low SAI QRST.
It is important to emphasize that we did not study SAI QRST in a normal healthy population, and the range of SAI QRST values in healthy subjects is unknown at present. Results of our study should be interpreted carefully in populations other than patients with structural heart disease and systolic dysfunction (EF≤35%).
Conclusion
SAI QRST is a novel ECG marker for the assessment of VA risk in patients with structural heart disease. High SAI QRST is associated with low risk of sustained VA. QRS widening is associated with ventricular tachyarrhythmia only if accompanied by low SAI QRST.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Sweeney MO, Wathen MS, Josephson ME, Otterness MF, Hogan-Miller E, Stark AJ, Degroot PJ. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:310–316. doi: 10.1016/j.jacc.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 3.Dhar R, Alsheikh-Ali AA, Estes NA, III, Moss AJ, Zareba W, Daubert JP, Greenberg H, Case RB, Kent DM. Association of prolonged QRS duration with ventricular tachyarrhythmias and sudden cardiac death in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) Heart Rhythm. 2008;5:807–813. doi: 10.1016/j.hrthm.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 5.Kearney MT, Zaman A, Eckberg DL, Lee AJ, Fox KA, Shah AM, Prescott RJ, Shell WE, Charuvastra E, Callahan TS, Brooksby WP, Wright DJ, Gall NP, Nolan J. Cardiac size, autonomic function, and 5-year follow-up of chronic heart failure patients with severe prolongation of ventricular activation. J Card Fail. 2003;9:93–99. doi: 10.1054/jcaf.2003.15. [DOI] [PubMed] [Google Scholar]
- 6.Zimetbaum PJ, Buxton AE, Batsford W, Fisher JD, Hafley GE, Lee KL, O'Toole MF, Page RL, Reynolds M, Josephson ME. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–769. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 7.Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–1091. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Olshansky B, Poole JE, Johnson G, Anderson J, Hellkamp AS, Packer D, Mark DB, Lee KL, Bardy GH. Syncope predicts the outcome of cardiomyopathy patients: analysis of the SCD-HeFT study. J Am Coll Cardiol. 2008;51:1277–1282. doi: 10.1016/j.jacc.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 10.Stecker EC, Zargarian M, Dogra V, John BT, Kron J, McAnulty JH, Chugh SS. Native QRS duration predicts the occurrence of arrhythmic events in ICD recipients. Europace. 2006;8:859–862. doi: 10.1093/europace/eul090. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez-Candil J, Ruiz M, Herrero J, Leon V, Martin A, Morinigo J, Ledesma C, Martin-Luengo C. Relationship between the Duration of the Basal QRS Complex and Electrical Therapies for Ventricular Tachycardias among ICD Patients. Pacing Clin Electrophysiol. 2009 doi: 10.1111/j.1540-8159.2009.02648.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilson FN, Macleod AG, Barker PS, Johnston FD. The determination and the significance of the areas of the ventricular deflections of the electrocardiogram. Am Heart J. 1934:46–61. [Google Scholar]
- 13.Burger HC. A theoretical elucidation of the notion ventricular gradient. Am Heart J. 1957;53:240–246. doi: 10.1016/0002-8703(57)90211-9. [DOI] [PubMed] [Google Scholar]
- 14.Geselowitz DB. The ventricular gradient revisited: relation to the area under the action potential. IEEE Trans Biomed Eng. 1983;30:76–77. doi: 10.1109/tbme.1983.325172. [DOI] [PubMed] [Google Scholar]
- 15.Urie PM, Burgess MJ, Lux RL, Wyatt RF, Abildskov JA. The electrocardiographic recognition of cardiac states at high risk of ventricular arrhythmias. An experimental study in dogs. Circ Res. 1978;42:350–358. doi: 10.1161/01.res.42.3.350. [DOI] [PubMed] [Google Scholar]
- 16.Abildskov JA, Klein RM. Cancellation of electrocardiographic effects during ventricular excitation. Sogo Rinsho. 1962;11:247–251. doi: 10.1161/01.res.11.2.247. [DOI] [PubMed] [Google Scholar]
- 17.Burgess MJ, Millar K, Abildskov JA. Cancellation of electrocardiographic effects during ventricular recovery. J Electrocardiol. 1969;2:101–107. doi: 10.1016/s0022-0736(69)81005-8. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki O, Lux RL. Paradoxical QRST integral changes with ventricular repolarization dispersion. J Electrocardiol. 1999;32 Suppl:60–69. doi: 10.1016/s0022-0736(99)90045-6. [DOI] [PubMed] [Google Scholar]
- 19.Burton FL, Cobbe SM. Dispersion of ventricular repolarization and refractory period. Cardiovasc Res. 2001;50:10–23. doi: 10.1016/s0008-6363(01)00197-3. [DOI] [PubMed] [Google Scholar]
- 20.Gardner MJ, Montague TJ, Armstrong CS, Horacek BM, Smith ER. Vulnerability to ventricular arrhythmia: assessment by mapping of body surface potential. Circulation. 1986;73:684–692. doi: 10.1161/01.cir.73.4.684. [DOI] [PubMed] [Google Scholar]
- 21.Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol. 1987;10:73–80. doi: 10.1016/s0735-1097(87)80162-6. [DOI] [PubMed] [Google Scholar]