Abstract
Neuropeptide S (NPS) is known to produce anxiolytic-like effects and facilitate extinction of conditioned fear. Catecholaminergic neurotransmission in the medial prefrontal cortex (mPFC) has been suggested to be crucially involved in these brain functions. In the current study we investigated the effect of NPS on the release of dopamine and serotonin in the mPFC by in vivo microdialysis in rats. Central administration of NPS dose-dependently enhanced extracellular levels of dopamine and its major metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), with maximal effects lasting up to 120 min. In contrast, no effect on serotonergic neurotransmission was detected. Dopamine release in the mPFC has been previously linked to modulation of anxiety states and fear extinction. The present results may thus provide a physiological and anatomical basis for the reported effects of NPS on these behaviors.
Keywords: neuropeptide, microdialysis, dopamine, serotonin, cortex, rat
Introduction
The medial prefrontal cortex (mPFC) is a major integrating structure of emotion-related learning and memory. Extensive evidence shows that the mPFC plays an important role in extinction of fear memory that requires plasticity in the mPFC (Morgan and LeDoux, 1995; Quirk et al., 2000). Lesions or inhibition of protein synthesis in the mPFC result in impaired retention of fear extinction (Morgan et al., 1993; Morgan and LeDoux, 1995; Quirk et al., 2000; Santini et al., 2004). Conversely, mPFC stimulation reduces conditioned fear and stimulation of the mediodorsal thalamic inputs to the mPFC is associated with extinction maintenance (Herry and Garcia, 2002, 2003). Furthermore, electrophysiological recording studies indicate that the ventromedial PFC neurons respond to a tone conditioned stimulus only during a delayed test of extinction, rather than fear acquisition (Milad and Quirk, 2002). Moreover, increased tone responsiveness in the prelimbic cortex has been correlated with failure to recall extinction (Burgos-Robles et al., 2009). Recently, extinction learning studies in humans using brain imaging were also suggestive of a role for the PFC in fear inhibition (Oschner et al., 2002; Phelps et al., 2004). Patients with posttraumatic stress disorder (PTSD) showed reduced mPFC activity during exposure to traumatic pictures and sounds (Bremner et al., 1999).
Catecholamine neurotransmitters in the mPFC are prominently involved in the regulation of anxiety-like behaviors and emotional memory. Within the mPFC, cognitive and emotional processes are strongly modulated by dopaminergic neurotransmission (Pezze et al., 2003; Laviolette et al., 2005; Lauzon et al., 2009). Prefrontal serotonin (5-HT) has been suggested to play an important role in the regulation of anxiety and stress (Pum et al., 2009; Savitz et al., 2009; Maki, 2001).
Neuropeptide S (NPS) is a recently identified peptide transmitter in the brain (Xu et al., 2004) that appears to modulate emotional and cognitive functions. Previous studies indicated that central administration of NPS reduces stress related anxiety behavior in mice (Xu et al, 2004; Okamura and Reinscheid, 2007; Leonard et al., 2008; Rizzi et al., 2008) while NPSR knockout mice display increased anxiety-like behaviors when compared to their wildtype littermates (Duangdao et al., 2009). Consistent with these results, intra-amygdala administration of NPS produces anxiolytic effects (Jüngling et al., 2008), blocks the expression of conditioned fear (Fendt et al., 2010), and facilitates extinction of conditioned fear responses (Jüngling et al., 2008), while animals treated with SHA 68, a selective NPSR antagonist (Okamura et al., 2008), showed anxiety-like behavior and attenuated fear extinction (Jüngling et al., 2008). Also, SHA 68 has been reported to antagonize NPS-induced anxiolytic effects (Ruzza et al., 2010). Additionally, local injections of NPS in the endopiriform nucleus attenuated expression of contextual fear (Meis et al., 2008). To date, however, the cellular mechanisms and substrates through which NPS modulates emotional responses and fear memory remain unclear.
In rat, NPS precursor mRNA is expressed discretely in a few brainstem areas (Xu et al., 2007). Particularly strong expression of NPS precursor mRNA is detected in a previously uncharacterized nucleus ventromedial to the noradrenergic locus coeruleus (LC), an important neural center for anxiety, stress, and arousal, but LC function has also been implicated in attention and learning and memory (Aston-Jones, 2005; Weiss et al., 1994; Tanaka et al., 2000). In contrast to the restricted distribution of NPS-producing neurons, NPSR mRNA is widely expressed throughout the brain, including cortex, thalamus, subiculum, multiple hypothalamic nuclei, amygdala, ventral tegmental area and olfactory nuclei (Xu et al., 2007). NPSR transcripts are, however, not detected in the mPFC, but multiple in- and output regions of the mPFC express NPSR mRNA, including subiculum, medial and cortical amygdaloid nuclei, paraventricular hypothalamic nucleus, posterior, dorsomedial and lateral hypothalamic area.
We speculated that the mPFC might be involved in the mechanisms by which NPS regulates anxiety-like behaviors and fear memory. Therefore, the aim of the present study was to determine whether NPS could affect release of dopamine and 5-HT in the mPFC in vivo. Indeed, a recent study reported NPS-dependent inhibition of 5-HT and noradrenaline release from synaptosomes isolated from mouse frontal cortex tissue (Raiteri et al., 2009). We used microdialysis followed by high-performance liquid chromatography (HPLC) with electrochemical detection to analyze NPS-induced changes in extracellular dopamine and serotonin, including their metabolites.
Materials and Methods
Chemicals
All chemicals were of analytical grade or higher quality. NPS was synthesized by the Peptide Proteomic Centre, Brain Research Centre, University of British Columbia (Vancouver, BC, Canada) or Bachem (Torrance, CA) and stock solutions were dissolved in water.
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA), weighing ~300 g, were used for in vivo microdialysis experiments. Animals were kept in a temperature and humidity controlled animal care facility on a 12-hour light/dark cycle (lights on at 7 AM (DOPAC/5-HIAA experiments) and 6 AM (dopamine and 5-HT experiments) with water and chow freely available. Animals had a minimum of 1 week adaptation in the animal care facility before experimental procedures. All animal care and experiments were according to the NIH Animal Care Guidelines and all experimental methods were approved by the NIAAA Animal Care and Use Committee or the local Institutional Animal Care and Use Committee of the University of California Irvine.
Microdialysis
Experiments for dopamine and 5-HT release were performed as previously described (Bonaventure et al., 2007) using dual guide cannulation (Eicom, Kyoto, Japan) with one cannula (used for dialysis) in the prefrontal cortex and the other cannula (used for injections) in the lateral ventricle (incisor bar, −3.5 mm; PFC: +3.2 mm anterior, 0.8 mm lateral and 1 mm ventral to bregma, LV: −1.08 mm posterior, 4.0 mm lateral, 2.2 mm ventral at an angle of 28.8° to bregma) (Paxinos and Watson, 1997). Animals received a single intracerebroventricular (i.c.v.) injection of NPS at 10, 1, 0.1 nmol or saline. Samples were collected every 15 minutes into a 96-well plate (Sarstedt, 96 well multiply PCR) maintained at 4°C containing 3.75 μl of stabilizer (0.1 M acetic acid, 1 mM oxalic acid and 3 mM L-cysteine in sterile water). Dialysis experiments were conducted between 8:00 AM and 2:00 PM in a controlled environment with the animals remaining in their home cage throughout experimentation. Dialysis probes (Eicom, 4 mm active membrane length) were perfused with artificial cerebrospinal fluid at a flow rate of 1 μl/min as described (Bonaventure et al., 2007) and implanted the afternoon prior to sample collection.
Experiments for DOPAC and 5-HIAA detection were performed as follows: On the surgery day, rats were anesthetized with ketamine (100 mg/kg, Phoenix Pharmaceuticals, St. Joseph, MO) and xylazine (10 mg/kg, Lloyd Laboratories, Shenandoah, IA) and placed in a stereotaxic frame. Holes were drilled in the skull for implantation of microdialysis guide cannula (CMA/Microdialysis AB, North Chelmsford, MA) and a second cannula (Plastics One, Roanoke, VA) for NPS injection. The guide cannula was stereotaxically implanted into the brain to position the microdialysis probe tip in the medial prefrontal cortex (coordinates: 3.2 mm anterior to bregma; 0.8 mm lateral; 2.0 mm ventral to dura). The cannula for i.c.v. injection was implanted into the lateral ventricle (coordinates: 0.9 mm posterior to bregma; 1.4 mm lateral; 3.3 mm ventral to dura). After surgery, animals were allowed to recover for 2 days before microdialysis. Rats were slightly anesthetized with isoflurane (Sigma, St. Louis, MO) and the microdialysis probe (CMA 11 MD probe with membrane length 4 mm) was inserted into the guide cannula. Rats were allowed to move freely in the chamber during microdialysis. To collect dialysate for 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindoleacetic acid (5-HIAA) detection, the probes were perfused with artificial cerebrospinal fluid (aCSF; 125 mM NaCl, 2.5 mM KCl, 0.5 mM NaH2PO4, 5 mM Na2HPO4, 1 mM MgCl2 * 6 H2O, 1.2 mM CaCl2 * 2 H2O, 20 mM ascorbic acid) at a flow rate of 2 μl/min during the experiment. Samples were collected into vials (Sigma, 250 μl polypropylene insert) at 4°C every 20 minutes over 3 hours before and 4 hours after NPS (1 nmol) or saline i.c.v. administration. Sample tubes contained 1 μl of stabilizer (0.1 mM perchloric acid, 0.4 mM Na2S2O5) to protect the dialysate from oxidative degradation.
After all samples were collected, 0.1 nmol angiotensin II was i.c.v. injected and animals were expected to show immediate water intake, which indicated that the cannula was implanted correctly to target the lateral ventricle. Methyleneblue was injected into the guide cannula to test for correct positioning of the microdialysis probe. Rats were euthanized and brains were removed and sectioned to confirm cannula positioning. Animals with misplaced microdialysis probes or i.c.v. cannula were excluded from data analysis.
HPLC analysis
Dialysis samples were analyzed for dopamine and 5-HT by HPLC coupled with electrochemical detection (Eicom, San Diego, CA) as previously described (Bonaventure et al., 2007). Dialysis samples for DOPAC and 5-HIAA detection were analyzed by HPLC with electrochemical detection (Coularray System; ESA, Chelmsford, MA). Using an autosampler, 20μl of the dialysate were injected and loaded onto a C18 reversed-phase column (ESA HPLC column MD150 × 2 mm). Catecholamines and their metabolites were separated by isocratic elution with MD-TM mobile phase (ESA; 75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid sodium salt, 100μl/L triethylamine, 25μM EDTA, 10% acetonitrile, pH 3.0) followed by electrochemical detection (detector electrode at 120 mV). A series of catecholamine calibration standards were run under the same conditions before and after each set of microdialysis samples. Peak retention times were used to identify individual compounds and peak area served as a quantitative measure.
Statistical analysis
Peak areas of recovered catecholamines and their metabolites were quantified according to calibration standards. Baseline catecholamine output was determined from the average of the last four samples preceding drug injections and set as 100%. Data were then normalized as % of baseline, uncorrected for probe recovery. Two-way analysis of variance (ANOVA) with dose and time as variables was used to determine significant effects. When appropriate, data were further analyzed using post-hoc tests. The area under the curve (AUC) for each catecholamine was also determined following drug administration and analyzed by one-way ANOVA followed by Dunnett’s post-hoc test. GraphPad Prism 4.0 (GraphPad, San Diego, CA) was used for all statistical calculations. Results were considered significant when p < 0.05.
Results
Effect of NPS on dopamine and serotonin release in the mPFC
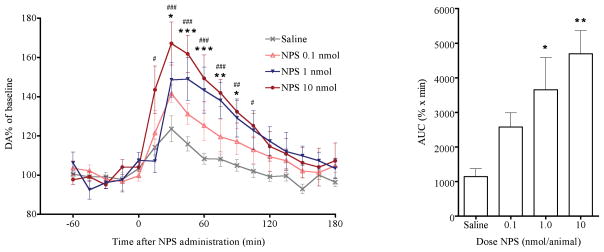
Basal levels of extracellular dopamine in the mPFC were 0.170 ± 0.006 pg/μl (not corrected for in vitro dialysis probe recovery). NPS dose-dependently increased extracellular dopamine concentrations in the mPFC (Fig 1a). Two-way ANOVA showed a significant effect of NPS dose [FDose(3,352) = 6.91; p = 0.0019] and time [FTime(16,352) = 35.86; p < 0.0001] with significant interaction [FDose × Time(48,352) = 3.51; p < 0.0001]. Bonferroni post-tests showed significant increases in dopamine release between 30 – 90 min after 1 nmol NPS administration and slightly stronger effects between 15 – 105 min after 10 nmol NPS administration, compared with saline-treated animals. One-way ANOVA showed a significant dose-dependent increase of extracellular dopamine in the area under the curve between 0 and 120 min after NPS administration (AUC) [F(3,24) = 9.626, p = 0.0002], with Dunnett’s post-hoc test demonstrating significant increases at 1.0 nmol and 10 nmol NPS compared to saline-injected animals (Fig 1b).
Fig. 1.
Dose-dependent effects of i.c.v. NPS on extracellular levels of dopamine in the mPFC. NPS significantly increased extracellular dopamine levels. (a) Time course of extracellular dopamine levels following central administration of saline or increasing doses of NPS. Dopamine levels were normalized to the average of four baseline samples. * p < 0.05, ** p < 0.01, *** p < 0.001, 1 nmol NPS versus saline; # p < 0.05, ## p < 0.01, ### p < 0.001, 10 nmol NPS versus saline; Bonferroni post-hoc test after significant treatment effect in two-way ANOVA. (b) Cumulative dopamine levels between 0 and 120 min post injection, calculated as area under the curve (AUC), ** p < 0.01, *** p < 0.001 versus saline; Dunnett’s post-hoc test after significant one-way ANOVA.
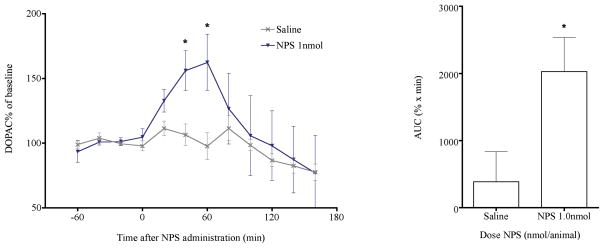
In a separate experiment, we also determined extracellular levels of 3,4-dihydroxyphenylacetic acid (DOPAC), a major metabolite of dopamine, in the mPFC before and after NPS administration. Two-way ANOVA indicated that 1 nmol NPS significantly increased DOPAC release [FTreatment(1,107) = 4.81, p = 0.0304; FTime(11,107) = 2.25, p = 0.0168, FTreatment × Time(11,107) = 0.88, p > 0.5] in the mPFC. DOPAC levels at 40 and 60 min after NPS administration were significantly higher [t-test; 40 min: t(8) = 2.844, p = 0.0217; 60 min: t(9) = 2.512, p = 0.0332] than in saline-treated animals (Fig. 2a) and the AUC of extracellular DOPAC was also significantly larger in NPS-treated rats compared to saline-treated controls [t-test, t(7) = 2.426, p = 0.0457; Fig. 2b].
Fig. 2.
Central administration of 1 nmol NPS significantly increased extracellular levels of DOPAC in the mPFC. (a) Time course of extracellular DOPAC as a percentage of basal levels. * p < 0.05 versus saline, t-test after significant treatment effect in two-way ANOVA. (b) Cumulative DOPAC levels between 0 and 120 min post injection, calculated as area under the curve (AUC), * p < 0.05 versus saline, t-test.
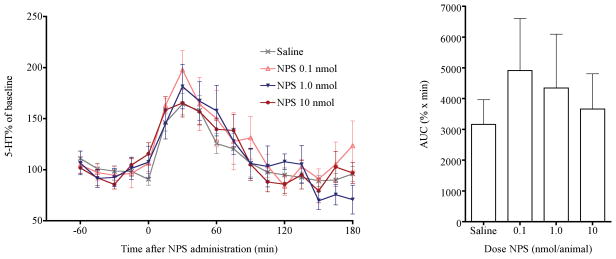
Average serotonin (5-HT) concentrations in the mPFC in all groups of animals before drug administration were 0.045 ±0.002 pg/μl. NPS showed little effect on 5-HT release during 3 hr after i.c.v. injection (Fig. 3) [FDose(3, 368) = 0.38, p = 0.7668]. However, extracellular 5-HT levels in the mPFC increased significantly in both saline and NPS- treated animals. [FTime(16,368) = 25.74, p < 0.0001; FDose × Time(48,368) = 0.79, p = 0.8378]. The significant increase of 5-HT release was possibly due to stress produced by the i.c.v. injections. Cumulative 5-HT release in the mPFC was very similar between NPS and saline-treated groups [F(3,23) = 0.3921, p = 0.7598].
Fig. 3.
NPS administration had no significant effects on extracellular levels of 5-HT. (a) 5-HT percentage of basal levels in the mPFC after saline or NPS i.c.v. administration. (b) Cumulative 5-HT levels between 0 and 120 min post injection, calculated as area under the curve (AUC).
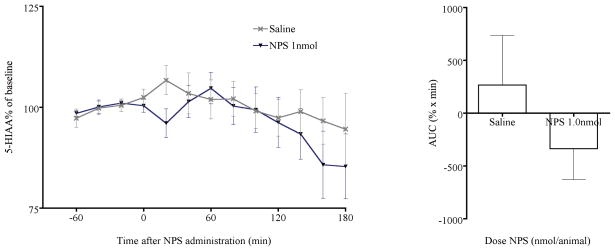
Similarly, extracellular levels of 5-hydroxyindoleacetic acid (5-HIAA), a primary metabolite of 5-HT, were not affected by NPS administration and cumulative 5-HIAA levels did not differ between NPS and saline-treated animals (Fig. 4). Although NPS administration appeared to reduce 5-HIAA levels within the first 20 min following administration, data did not reach significance. The reason for the acute reduction of 5-HIAA at 20 min after NPS administration might be a transient change in turnover rate of 5-HT or enzymatic activity of monoamine oxidase (MAO, EC 1.4.3.4), which cannot be determined in the present study.
Fig. 4.
NPS administration had no effect on extracellular levels of 5-HIAA. (a) 5-HIAA percentage of basal levels in the mPFC after saline or NPS i.c.v. administration. (b) Cumulative 5-HIAA levels between 0 and 120 min post injection, calculated as area under the curve (AUC).
Discussion
Our study aimed at investigating the effect of NPS on catecholamine neurotransmission in the mPFC. As dopaminergic and serotonergic neurotransmission in the mPFC is crucial for extinction of fear memory and anxiety modulation, and NPS appears to regulate these behaviors, our hypothesis was that NPS might affect release of dopamine or 5-HT in the mPFC. Our results show that NPS administration dose-dependently and significantly increased release of dopamine in the mPFC and the extracellular levels of its metabolite, DOPAC. The stimulatory effect of NPS on dopamine release in the mPFC lasted as long as 120 min and extracellular levels of DOPAC reached a peak at 60 min. However, NPS seemed to have little effects on 5-HT and 5-HIAA release. DOPAC is a major metabolite of dopamine, converted from dopamine by monoamine oxidase (MAO) and aldehyde dehydrogenase (AD, EC 1.2.1.3). As both MAO and AD are intracellular enzymes, extracellular levels of DOPAC are dependent on the amount of cytoplasmic dopamine. Therefore, extracellular DOPAC is closely related to the amount of dopamine synthesized and stored in the presynaptic neuron while extracellular dopamine levels are closely related to the rate of dopamine release. The stimulatory effect of NPS on extracellular DOPAC levels suggests that NPS might increase dopamine synthesis in the presynaptic neurons, or simply increase dopamine turnover including the release and reuptake of dopamine.
Our results seem to be contrary to the inhibitory effects of NPS on 5-HT release, as reported recently (Raiteri et al., 2009). In this in vitro study, purified cortical synaptosomes were used to determine NPS effects on 5-HT release, which is a highly artificial model compared to in vivo microdialysis in freely moving animals. The effects of NPS on dopamine and 5-HT release in the mPFC reported in this study are very likely caused by NPS-mediated stimulation of afferent inputs into the mPCF, since there is currently no evidence for NPSR expression within the rat mPFC (Xu et al., 2007). It is possible that the synaptosomal preparation used by Raiteri et al (2009) contained significant parts of orbitofrontal cortex tissue where NPSR transcripts are expressed. However, we cannot rule out extremely low levels of NPSR expression in these cortical areas that evaded detection in our previous in situ hybridization studies (Xu et al., 2007). Additional in vivo microdialysis studies in other cortical areas may be able to explain the discrepancies.
Based on the findings that expression of NPSR has been detected in VTA (Xu et al., 2007) and VTA dopamine neurons project to the mPFC, it is possible that the NPS-induced enhanced dopaminergic neurotransmission in the mPFC might be caused by activation of the mesolimbic dopaminergic pathways via NPSR expressed in the VTA. In support of this hypothesis, Mochizuki et al (2010) recently reported that local injection of NPS into the VTA significantly elevated extracellular levels of dopamine metabolites in the nucleus accumbens shell, another important area which receives projections from the VTA. Obviously, further microdialysis studies are required to verify this hypothesis.
Extensive evidence shows that the mesocortical dopamine system, originating in the ventral tegmental area (VTA), plays an important role in fear extinction learning. Depletion of dopaminergic innervation in the mPFC has been found to impair extinction learning without affecting the acquisition of conditioned fear (Morrow et al., 1999; Fernandez Espejo, 2003) and dopamine D4 receptors in the mPFC are thought to be involved in encoding fear extinction (Pfeiffer and Fendt, 2006). The role of prefrontocortical dopamine in the expression of conditioned fear has also received much attention. Pezze et al. (2003, 2004) reported that either stimulation or blockade of dopaminergic neurotransmission in the mPFC reduces the expression of conditioned fear, suggesting an inverted U-shaped relation between expression of conditioned fear and dopaminergic activity in the mPFC. As stated earlier, NPS has been reported to enhance fear extinction and reduce fear expression, therefore, we hypothesized that the stimulatory effect of NPS on dopamine release in the mPFC might be functionally connected to these effects. As improper processing of fear memory may result in anxiety disorders, prefrontocortical dopamine has been considered to produce anxiolytic and protective effects by modulating mPFC activity in times of stress (Thierry et al., 1976; Deutch and Roth, 1990; Finlay et al., 1995; Sullivan, 2004). For instance, in the elevated plus maze depletion of dopamine within the mPFC induces anxiogenic-like effects in rats (Espejo, 1997; Fernandez Espejo, 2003). In contrast, in vivo microdialysis and voltametric recording studies showed increased extracellular levels of prefrontocortical dopamine in response to stress, suggesting a facilitating role of dopamine for processing of anxiety-like behaviors in the mPFC (Abercrombie et al, 1989; Doherty and Gratton, 1996; Sorg and Kalivas, 1993; Feenstra, 2000). Additionally, other studies that examined the effects of anti-anxiety treatments on dopaminergic activity in the mPFC, demonstrated that attenuation of dopaminergic activation contributes to the anxiolytic effects of the drugs (Dazzi et al., 2001a,b; Deutch and Roth, 1990; Feenstra et al., 1995; Petty et al., 1997; Matsumoto et al., 1998; Wedzony et al., 1996; Beaufour et al., 2001). A plausible explanation for these controversial roles of dopamine in the mPFC could be that dopamine might have adaptive and protective effects to dampen excessive stress reactivity after stress-induced activation of the VTA dopaminergic system. When anti-anxiety treatments act to reduce the activity of several central stress circuits, the general stress perception is reduced, hence resulting in a reduced need for compensatory dopaminergic modulation in the mPFC. An emerging consensus to interpret these apparently contradictory findings postulates that dopaminergic activity in the mPFC is necessary to maintain response adaptability to stressful events and may itself signal subjective intensities of perceived stress (Sullivan, 2004). Our results indicate that NPS enhances basal levels of dopamine in the mPFC, although it is unknown how NPS may affect levels of dopamine in presence of a stressor. Additionally, previous studies indicated that intra-amygdala injection of NPS reduce anxiety-like behaviors, suggesting that the amygdala might be a substrate for NPS-induced anxiolytic effects. Our current results of NPS-mediated enhancement of dopamine release in the mPFC suggest that, in addition to the amygdala, the anxiolytic-like effects of NPS might be also mediated through modulation of dopaminergic activity in the mPFC. .
In conclusion, the current study provides evidence for a stimulatory effect of NPS on dopamine release in the mPFC, without affecting serotonergic neurotransmission. The adaptive and protective role of NPS in extinction of conditioned fear and stress-induced anxiety, as well as its antipsychotic profile, might be related to the NPS-mediated enhancement of dopamine release in the mPFC.
Acknowledgments
We would like to thank Dr. Frances Leslie for technical support. These studies were in part supported by a grant from the National Institute of Mental Health (MH-71313 to RKR). WS, NO, SDC and RKR declare no competing interest. LA, IF, SWS and PB are full-time employees of Johnson & Johnson Pharmaceutical Research and Development, LLC.
Abbreviations used
- NPS
neuropeptide S
- NPSR
neuropeptide S receptor
- mPFC
medial prefrontal cortex
- aCSF
artificial cerebrospinal fluid
- AUC
area under the curve
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- 5-HT
serotonin
- 5-HIAA
5-hydroxyindoleacetic acid
- MAO
monoamine oxidase
- LC
locus coeruleus
- VTA
ventral tegmental area
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Brain structures and receptors involved in alertness. Sleep Med. 2005;6 (Suppl 1):S3–S7. doi: 10.1016/s1389-9457(05)80002-4. [DOI] [PubMed] [Google Scholar]
- Beaufour CC, Le Bihan C, Hamon M, Thiébot M. Extracellular dopamine in the rat prefrontal cortex during reward-, punishment- and novelty-associated behaviour. Effects of diazepam. Pharmacol Biochem Behav. 69:133–142. doi: 10.1016/s0091-3057(01)00492-0. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, Miller K, Atack J, Lovenberg TW, Dugovic C. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. 2007;321:690–698. doi: 10.1124/jpet.107.119404. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi L, Serra M, Spiga F, Pisu MG, Jentsch JD, Biggio G. Prevention of the stress-induced increase in frontal cortical dopamine efflux of freely moving rats by long-term treatment with antidepressant drugs. Eur Neuropsychopharmacol. 2001a;11:343–349. doi: 10.1016/s0924-977x(01)00105-5. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Spiga F, Pira L, Ladu S, Vacca G, Rivano A, Jentsch JD, Biggio G. Inhibition of stress- or anxiogenic-drug-induced increases in dopamine release in the rat prefrontal cortex by long-term treatment with antidepressant drugs. J Neurochem. 2001b;76:1212–1220. doi: 10.1046/j.1471-4159.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Duangdao DM, Clark SD, Okamura N, Reinscheid RK. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav Brain Res. 2009;205:1–9. doi: 10.1016/j.bbr.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo EF. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 1997;762:281–284. doi: 10.1016/s0006-8993(97)00593-3. [DOI] [PubMed] [Google Scholar]
- Feenstra MG. Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Prog Brain Res. 2000;126:133–163. doi: 10.1016/S0079-6123(00)26012-3. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, van Uum JF. Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: inhibition by diazepam. Neurosci Lett. 1995;189:81–84. doi: 10.1016/0304-3940(95)11456-7. [DOI] [PubMed] [Google Scholar]
- Fendt M, Imobersteg S, Bürki H, McAllister KH, Sailer AW. Intra-amygdala injections of neuropeptide S block fear-potentiated startle. Neurosci Lett. 2010;474:154–157. doi: 10.1016/j.neulet.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Fernandez Espejo E. Prefrontocortical dopamine loss in rats delays long-term extinction of contextual conditioned fear, and reduces social interaction without affecting short-term social interaction memory. Neuropsychopharmacology. 2003;28:490–498. doi: 10.1038/sj.npp.1300066. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Garcia R. Behavioral and paired-pulse facilitation analyses of long-lasting depression at excitatory synapses in the medial prefrontal cortex in mice. Behav Brain Res. 2003;146:89–96. doi: 10.1016/j.bbr.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon NM, Bishop SF, Laviolette SR. Dopamine D1 versus D4 receptors differentially modulate the encoding of salient versus nonsalient emotional information in the medial prefrontal cortex. J Neurosci. 2009;29:4836–4845. doi: 10.1523/JNEUROSCI.0178-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, et al. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Maki Y. A behavioral and neurochemical study on the mechanism of the anxiolytic effect of monoamine oxidase inhibitors. Hokkaido Igaku Zasshi. 2001;76:133–142. [PubMed] [Google Scholar]
- Matsumoto M, Yoshioka M, Togashi H, Mori K, Ueno K, Saito H. Effects of idazoxan on dopamine release in the prefrontal cortex of freely moving rats. Eur J Pharmacol. 1998;343:165–170. doi: 10.1016/s0014-2999(97)01544-6. [DOI] [PubMed] [Google Scholar]
- Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PLoS One. 2008;3:e2695. doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Kim J, Sasaki K. Microinjection of neuropeptide S into the rat ventral tegmental area induces hyperactivity and increases extracellular levels of dopamine metabolites in the nucleus accumbens shell. Peptides. 2010;31:926–931. doi: 10.1016/j.peptides.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:553–564. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Okamura N, Reinscheid RK. Neuropeptide S: a novel modulator of stress and arousal. Stress. 2007;10:221–226. doi: 10.1080/10253890701248673. [DOI] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Compact. 3. Academic Press; San Diego: 1997. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Petty F, Jordan S, Kramer GL, Zukas PK, Wu J. Benzodiazepine prevention of swim stress-induced sensitization of cortical biogenic amines: an in vivo microdialysis study. Neurochem Res. 1997;22:1101–1104. doi: 10.1023/a:1027309117349. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Bast T, Feldon J. Significance of dopamine transmission in the rat medial prefrontal cortex for conditioned fear. Cereb Cortex. 2003;13:371–380. doi: 10.1093/cercor/13.4.371. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobio. 2004;74:301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pfeiffer UJ, Fendt M. Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport. 2006;17:847–850. doi: 10.1097/01.wnr.0000220142.29413.6f. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pum ME, Huston JP, Müller CP. The role of cortical serotonin in anxiety and locomotor activity in Wistar rats. Behav Neurosci. 2009;123:449–454. doi: 10.1037/a0014478. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri L, Luccini E, Romei C, Salvadori S, Calò G. Neuropeptide S selectively inhibits the release of 5-HT and noradrenaline from mouse frontal cortex nerve endings. Br J Pharmacol. 2009;157:474–481. doi: 10.1111/j.1476-5381.2009.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzza C, Rizzi A, Trapella C, et al. Further studies on the pharmacological profile of the neuropeptide S receptor antagonist SHA 68. Peptides. 2010;31:915–925. doi: 10.1016/j.peptides.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1993;53:695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Hemispheric Asymmetry in Stress Processing in Rat Prefrontal Cortex and the Role of Mesocortical Dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic- pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Maćkowiak M, Fijał K, Gołembiowska K. Evidence that conditioned stress enhances outflow of dopamine in rat prefrontal cortex: a search for the influence of diazepam and 5-HT1A agonists. Synapse. 1996;24:240–247. doi: 10.1002/(SICI)1098-2396(199611)24:3<240::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor. Brain Res Bull. 1994;35:561–572. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]